Variations in Theta/Beta Ratio and Cognitive Performance in Subpopulations of Subjects with ADHD Symptoms: Towards Neuropsychological Profiling for Patient Subgrouping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Evaluation
2.3. Working Memory Test
2.4. EEG Recording
2.5. Quantitative Analysis of EEG
2.6. Statistical Analysis
3. Results
3.1. Subjects
3.2. Analysis with Two Groups: ADHD and Controls
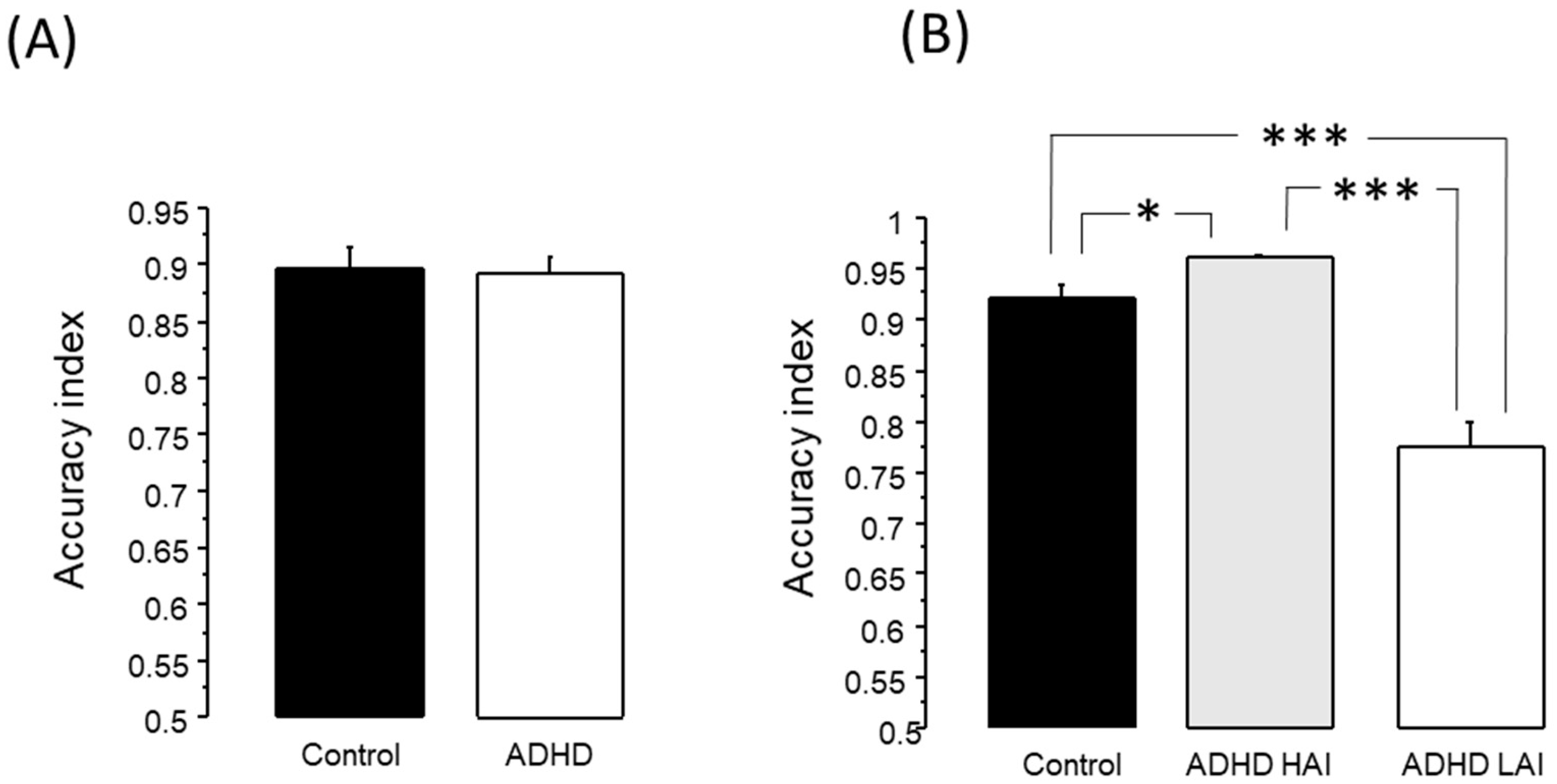
3.2.1. Working Memory Test
3.2.2. Analysis of Theta/Beta Ratio
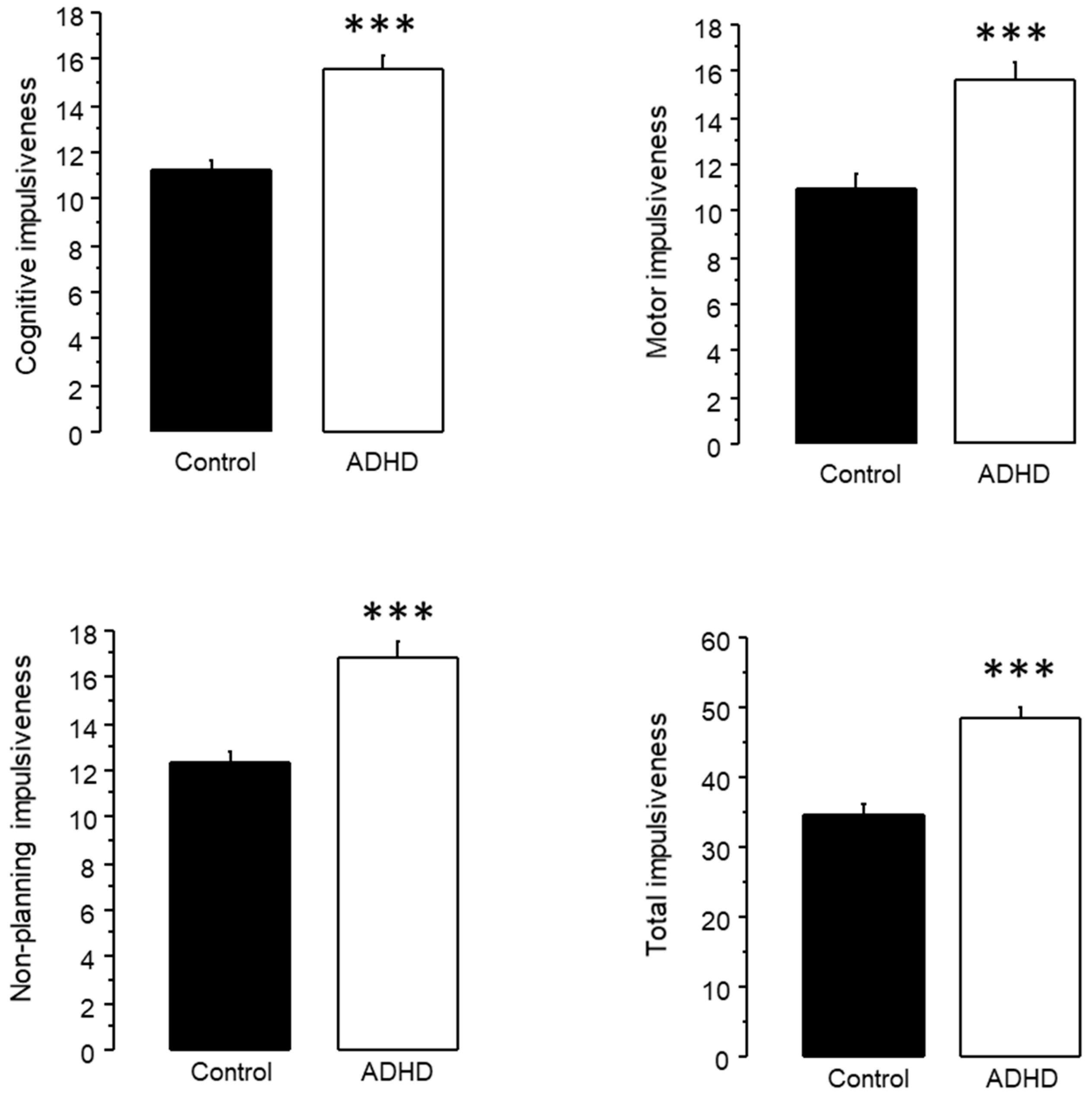
3.2.3. Analysis of Impulsiveness Scores
3.3. Analysis with Three Groups: ADHD HAI, ADHD LAI and Controls
3.3.1. Working Memory Test
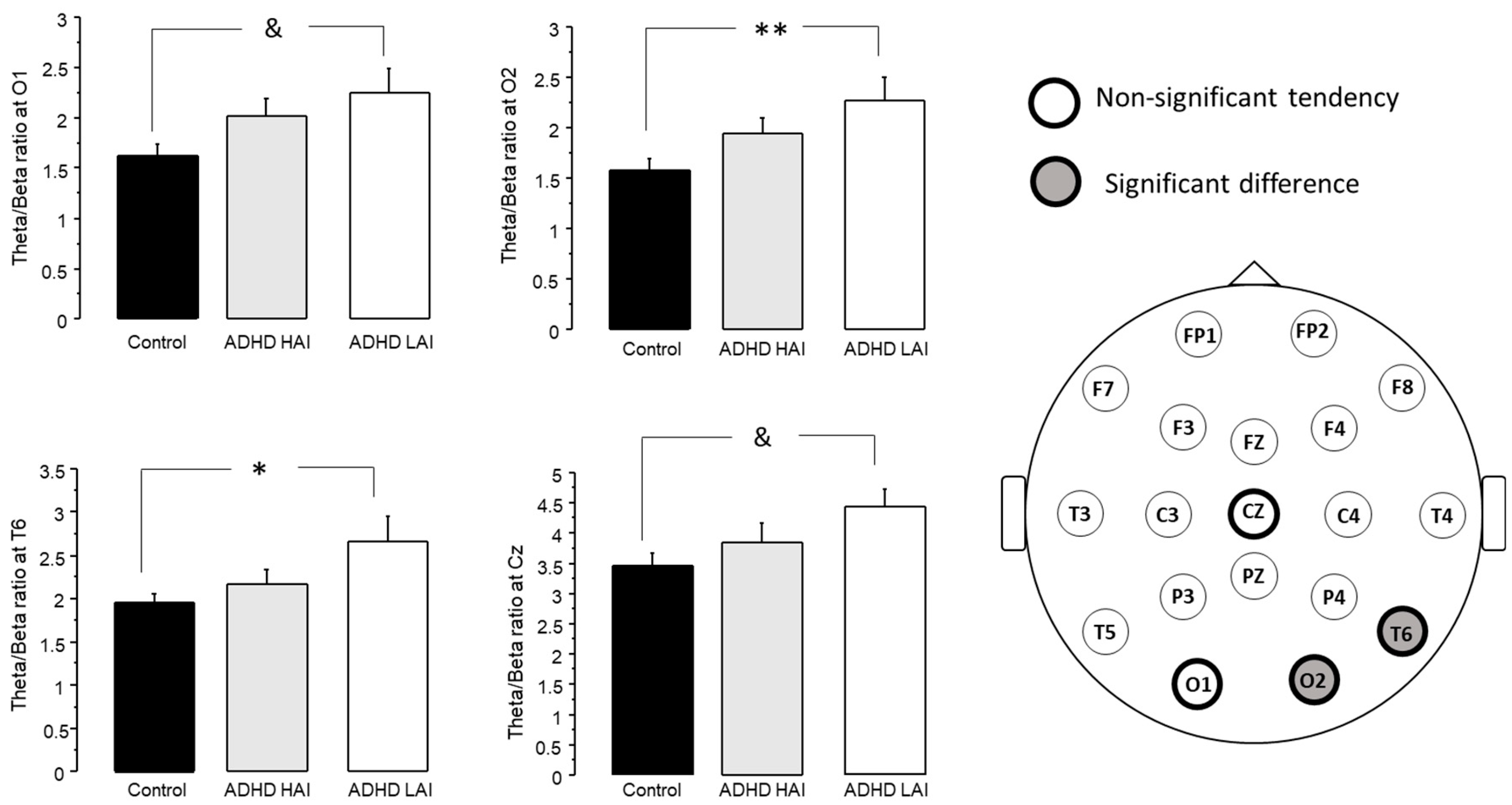
3.3.2. Analysis of Theta/Beta Ratio
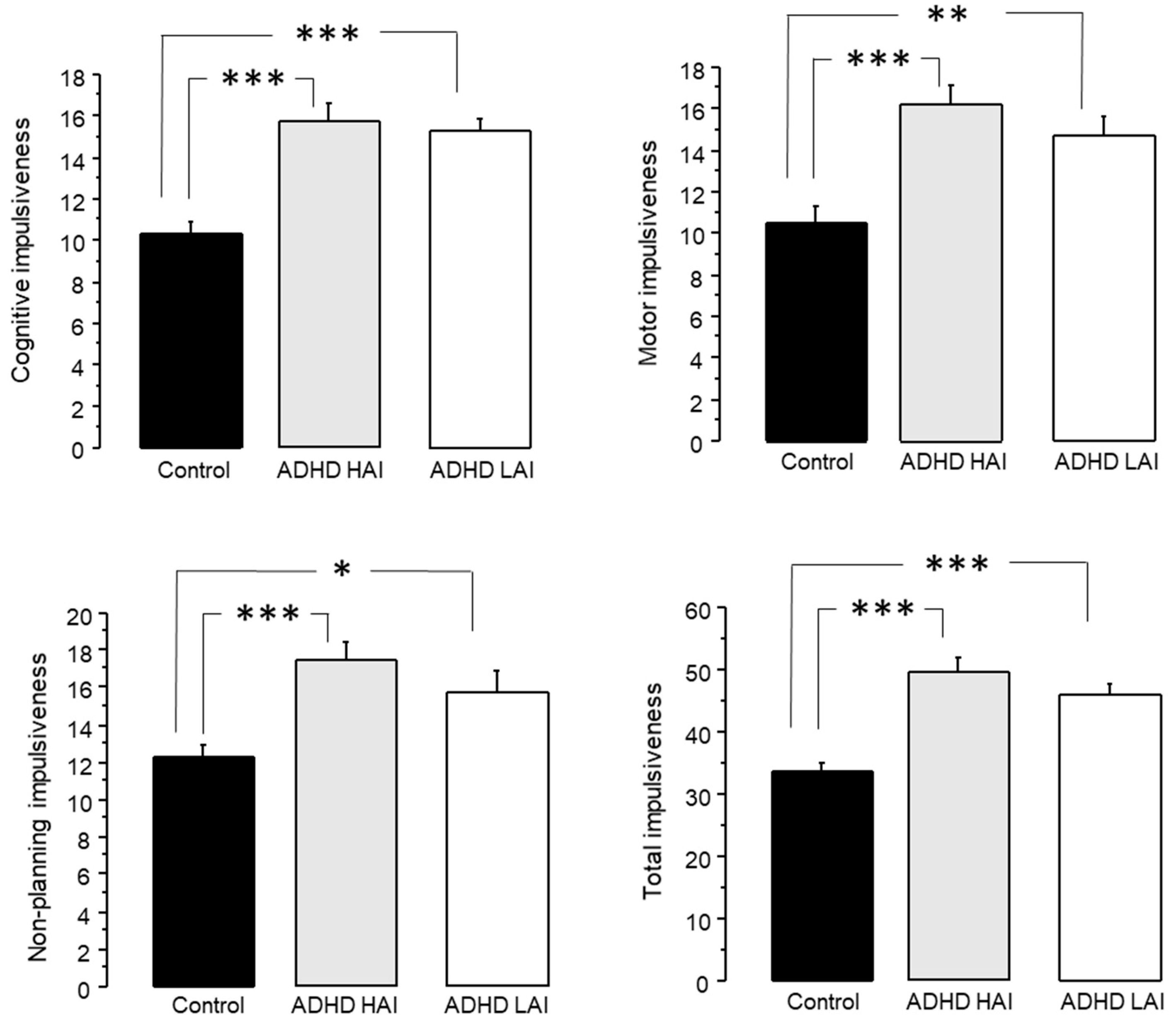
3.3.3. Analysis of Impulsiveness Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrak, S.; Wongsawat, Y. EEG brain mapping and brain connectivity index for subtypes classification of attention deficit hyperactivity disorder children during the eye-opened period. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 2013, 7400–7403. [Google Scholar] [CrossRef]
- Delgado-Mejia, I.D.; Palencia-Avendano, M.L.; Mogollon-Rincon, C.; Etchepareborda, M.C. Theta/beta ratio (NEBA) in the diagnosis of attention deficit hyperactivity disorder. Rev. Neurol. 2014, 58 (Suppl. 1), S57–S63. [Google Scholar]
- Huss, M.; Duhan, P.; Gandhi, P.; Chen, C.W.; Spannhuth, C.; Kumar, V. Methylphenidate dose optimization for ADHD treatment: Review of safety, efficacy, and clinical necessity. Neuropsychiatr. Dis. Treat. 2017, 13, 1741–1751. [Google Scholar] [CrossRef]
- Brown, K.A.; Samuel, S.; Patel, D.R. Pharmacologic management of attention deficit hyperactivity disorder in children and adolescents: A review for practitioners. Transl. Pediatr. 2018, 7, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo-Urbina, A.J.; Garcia-Hermoso, A.; Pardo-Guijarro, M.J.; Sanchez-Lopez, M.; Santos-Gomez, J.L.; Martinez-Vizcaino, V. The Effects of Long-Acting Stimulant and Nonstimulant Medications in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Meta-Analysis of Randomized Controlled Trials. J. Child Adolesc. Psychopharmacol. 2018, 28, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Johnston, A.; Husereau, D.; Kelly, S.E.; Eagles, C.; Charach, A.; Hsieh, S.C.; Bai, Z.; Hossain, A.; Skidmore, B.; et al. Pharmacologic treatment of attention deficit hyperactivity disorder in adults: A systematic review and network meta-analysis. PLoS ONE 2020, 15, e0240584. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.; Bedard, A.V.; Marks, D.; Gopalan, G.; Feirsen, N.; Uderman, J.; Chimiklis, A.; Heber, E.; Cornwell, M.; Anderson, L.; et al. Sequenced neurocognitive and behavioral parent training for the treatment of ADHD in school-age children. Child Neuropsychol. 2018, 24, 427–450. [Google Scholar] [CrossRef]
- Nimmo-Smith, V.; Merwood, A.; Hank, D.; Brandling, J.; Greenwood, R.; Skinner, L.; Law, S.; Patel, V.; Rai, D. Non-pharmacological interventions for adult ADHD: A systematic review. Psychol. Med. 2020, 50, 529–541. [Google Scholar] [CrossRef]
- Pan, M.R.; Huang, F.; Zhao, M.J.; Wang, Y.F.; Wang, Y.F.; Qian, Q.J. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019, 279, 23–33. [Google Scholar] [CrossRef]
- Sampedro Baena, L.; Fuente, G.A.C.; Martos-Cabrera, M.B.; Gomez-Urquiza, J.L.; Albendin-Garcia, L.; Romero-Bejar, J.L.; Suleiman-Martos, N. Effects of Neurofeedback in Children with Attention-Deficit/Hyperactivity Disorder: A Systematic Review. J. Clin. Med. 2021, 10, 3797. [Google Scholar] [CrossRef]
- Kuznetsova, E.; Veilahti, A.V.P.; Akhundzadeh, R.; Radev, S.; Konicar, L.; Cowley, B.U. Evaluation of Neurofeedback Learning in Patients with ADHD: A Systematic Review. Appl. Psychophysiol. Biofeedback 2023, 48, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Contini, V.; Victor, M.M.; Bertuzzi, G.P.; Salgado, C.A.; Picon, F.A.; Grevet, E.H.; Rohde, L.A.; Belmonte-de-Abreu, P.; Bau, C.H. No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J. Clin. Psychopharmacol. 2012, 32, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Bushe, C.; Sobanski, E.; Coghill, D.; Berggren, L.; De Bruyckere, K.; Leppamaki, S. Post Hoc Analysis of Potential Predictors of Response to Atomoxetine for the Treatment of Adults with Attention-Deficit/Hyperactivity Disorder using an Integrated Database. CNS Drugs 2016, 30, 317–334. [Google Scholar] [CrossRef]
- Arns, M.; Vollebregt, M.A.; Palmer, D.; Spooner, C.; Gordon, E.; Kohn, M.; Clarke, S.; Elliott, G.R.; Buitelaar, J.K. Electroencephalographic biomarkers as predictors of methylphenidate response in attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2018, 28, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.D.; Surman, C.B.H.; Elbe, D. Stimulant ‘rapid metabolizers’: Wrong label, real phenomena. Atten. Defic. Hyperact. Disord. 2018, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bussalb, A.; Congedo, M.; Barthelemy, Q.; Ojeda, D.; Acquaviva, E.; Delorme, R.; Mayaud, L. Clinical and Experimental Factors Influencing the Efficacy of Neurofeedback in ADHD: A Meta-Analysis. Front. Psychiatry 2019, 10, 35. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Smit, D.; Pimenta, M.G.; Arns, M. Neurofeedback as a Treatment Intervention in ADHD: Current Evidence and Practice. Curr. Psychiatry Rep. 2019, 21, 46. [Google Scholar] [CrossRef]
- Van Doren, J.; Arns, M.; Heinrich, H.; Vollebregt, M.A.; Strehl, U.; Loo, S.K. Sustained effects of neurofeedback in ADHD: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2019, 28, 293–305. [Google Scholar] [CrossRef]
- Cortese, S.; Ferrin, M.; Brandeis, D.; Holtmann, M.; Aggensteiner, P.; Daley, D.; Santosh, P.; Simonoff, E.; Stevenson, J.; Stringaris, A.; et al. Neurofeedback for Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Clinical and Neuropsychological Outcomes From Randomized Controlled Trials. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 444–455. [Google Scholar] [CrossRef]
- Razoki, B. Neurofeedback versus psychostimulants in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: A systematic review. Neuropsychiatr. Dis. Treat. 2018, 14, 2905–2913. [Google Scholar] [CrossRef]
- Núñez-Jaramillo, L.; Herrera-Solis, A.; Herrera-Morales, W.V. ADHD: Reviewing the Causes and Evaluating Solutions. J. Pers. Med. 2021, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Bernanke, J.; Luna, A.; Chang, L.; Bruno, E.; Dworkin, J.; Posner, J. Structural brain measures among children with and without ADHD in the Adolescent Brain and Cognitive Development Study cohort: A cross-sectional US population-based study. Lancet Psychiatry 2022, 9, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Makeig, S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: A research update. Neurotherapeutics 2012, 9, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Han, D.; Min, K.; Kim, D.; Lee, C. The utility of quantitative electroencephalography and Integrated Visual and Auditory Continuous Performance Test as auxiliary tools for the Attention Deficit Hyperactivity Disorder diagnosis. Clin. Neurophysiol. 2015, 126, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Bong, S.H.; Kim, J.W. The Role of Quantitative Electroencephalogram in the Diagnosis and Subgrouping of Attention-Deficit/Hyperactivity Disorder. J. Korean Acad. Child Adolesc. Psychiatry 2021, 32, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chatthong, W.; Khemthong, S.; Wongsawat, Y. Neuropsychological classification based on brain mapping performance in Thai children with and without ADHD. Appl. Neuropsychol. Child 2022, 11, 18–24. [Google Scholar] [CrossRef]
- Ji, Y.; Choi, T.Y.; Lee, J.; Yoon, S.; Won, G.H.; Jeong, H.; Kang, S.W.; Kim, J.W. Characteristics of Attention-Deficit/Hyperactivity Disorder Subtypes in Children Classified Using Quantitative Electroencephalography. Neuropsychiatr. Dis. Treat. 2022, 18, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Markovska-Simoska, S.; Pop-Jordanova, N. Quantitative EEG in Children and Adults With Attention Deficit Hyperactivity Disorder: Comparison of Absolute and Relative Power Spectra and Theta/Beta Ratio. Clin. EEG Neurosci. 2017, 48, 20–32. [Google Scholar] [CrossRef]
- Arns, M.; Conners, C.K.; Kraemer, H.C. A decade of EEG Theta/Beta Ratio Research in ADHD: A meta-analysis. J. Atten. Disord 2013, 17, 374–383. [Google Scholar] [CrossRef]
- Tombor, L.; Kakuszi, B.; Papp, S.; Rethelyi, J.; Bitter, I.; Czobor, P. Decreased resting gamma activity in adult attention deficit/hyperactivity disorder. World J. Biol. Psychiatry 2018, 20, 691–702. [Google Scholar] [CrossRef]
- Tombor, L.; Kakuszi, B.; Papp, S.; Rethelyi, J.; Bitter, I.; Czobor, P. Atypical resting-state gamma band trajectory in adult attention deficit/hyperactivity disorder. J. Neural. Transm. 2021, 128, 1239–1248. [Google Scholar] [CrossRef]
- Sibley, M.H.; Arnold, L.E.; Swanson, J.M.; Hechtman, L.T.; Kennedy, T.M.; Owens, E.; Molina, B.S.G.; Jensen, P.S.; Hinshaw, S.P.; Roy, A.; et al. Variable Patterns of Remission From ADHD in the Multimodal Treatment Study of ADHD. Am. J. Psychiatry 2022, 179, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Franke, B.; Michelini, G.; Asherson, P.; Banaschewski, T.; Bilbow, A.; Buitelaar, J.K.; Cormand, B.; Faraone, S.V.; Ginsberg, Y.; Haavik, J.; et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018, 28, 1059–1088. [Google Scholar] [CrossRef] [PubMed]
- de Quesada-Martinez, M.E.; Diaz-Perez, G.F.; Herrera-Ramos, A.; Tamayo-Porras, M.; Rubio-Lopez, R. Quantitative electroencephalography features and cognitive impairment in alcoholic patients. Rev. Neurol. 2007, 44, 81–88. [Google Scholar]
- Ramos-Quiroga, J.A.; Daigre, C.; Valero, S.; Bosch, R.; Gomez-Barros, N.; Nogueira, M.; Palomar, G.; Roncero, C.; Casas, M. Validation of the Spanish version of the attention deficit hyperactivity disorder adult screening scale (ASRS v. 1.1): A novel scoring strategy. Rev. Neurol. 2009, 48, 449–452. [Google Scholar] [PubMed]
- Reyes-Zamorano, E.; Garcia-Vargas, K.L.; Palacios-Cruz, L. Concurrent validity in Mexican college population of the adult ADHD self report scale. Rev. Investig. Clin. 2013, 65, 30–38. [Google Scholar] [PubMed]
- Shen, I.H.; Lee, D.S.; Chen, C.L. The role of trait impulsivity in response inhibition: Event-related potentials in a stop-signal task. Int. J. Psychophysiol. 2014, 91, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sutcubasi, B.; Metin, B.; Tas, C.; Krzan, F.K.; Sari, B.A.; Ozcimen, B.; Tarhan, N. The relationship between responsiveness to social and monetary rewards and ADHD symptoms. Cogn. Affect. Behav. Neurosci. 2018, 18, 857–868. [Google Scholar] [CrossRef]
- Kinumaki, S.; Miyauchi, E.; Kawasaki, M. Behavioral rhythm and EEG rhythm to determine timing deficits in attention deficit hyperactivity disorder symptoms. Heliyon 2020, 6, e04546. [Google Scholar] [CrossRef]
- Babor, T.; Higgins-Biddle, J.; Saunders, J.; Monteiro, M. The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care; World Health Organization: Genève, Switzerland, 2001.
- Herrera-Morales, W.V.; Ramírez-Lugo, L.; Santiago-Rodríguez, E.; Reyes-Lopez, J.V.; Núñez-Jaramillo, L. Hazardous alcohol consumption and risk of alcohol dependence present different neurophysiological correlates. Rev. Neurol. 2019, 68, 137–146. [Google Scholar]
- Knight, J.R.; Sherritt, L.; Harris, S.K.; Gates, E.C.; Chang, G. Validity of brief alcohol screening tests among adolescents: A comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol. Clin. Exp. Res. 2003, 27, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Jaramillo, L.; Vega-Perera, P.; Ramirez-Lugo, L.; Reyes-Lopez, J.V.; Santiago-Rodriguez, E.; Herrera-Morales, W.V. Quantitative electroencephalography analysis in university students with hazardous alcohol consumption, but not alcohol dependence. Neuroreport 2015, 26, 555–560. [Google Scholar] [CrossRef] [PubMed]
- John, E.R.; Prichep, L.S. Normative data banks and neurometrics: Basic concepts, methods and results of norm construction. In Handbook of Electroencephalography and Clinical Neurophysiology; Gevins, A.S., Remond, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Gudmundsson, S.; Runarsson, T.P.; Sigurdsson, S.; Eiriksdottir, G.; Johnsen, K. Reliability of quantitative EEG features. Clin. Neurophysiol. 2007, 118, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Tistarelli, N.; Fagnani, C.; Troianiello, M.; Stazi, M.A.; Adriani, W. The nature and nurture of ADHD and its comorbidities: A narrative review on twin studies. Neurosci. Biobehav. Rev. 2020, 109, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Katzman, M.A.; Bilkey, T.S.; Chokka, P.R.; Fallu, A.; Klassen, L.J. Adult ADHD and comorbid disorders: Clinical implications of a dimensional approach. BMC Psychiatry 2017, 17, 302. [Google Scholar] [CrossRef]
- Christiansen, L.; Beck, M.M.; Bilenberg, N.; Wienecke, J.; Astrup, A.; Lundbye-Jensen, J. Effects of Exercise on Cognitive Performance in Children and Adolescents with ADHD: Potential Mechanisms and Evidence-based Recommendations. J. Clin. Med. 2019, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Mechler, K.; Banaschewski, T.; Hohmann, S.; Hage, A. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol. Ther. 2022, 230, 107940. [Google Scholar] [CrossRef] [PubMed]
- Jalali, P.; Sho’ouri, N. Neurofeedback Training Protocol Based on Selecting Distinctive Features to Treat or Reduce ADHD Symptoms. Clin. EEG Neurosci. 2021, 52, 414–421. [Google Scholar] [CrossRef]
- Van Doren, J.; Heinrich, H.; Bezold, M.; Reuter, N.; Kratz, O.; Horndasch, S.; Berking, M.; Ros, T.; Gevensleben, H.; Moll, G.H.; et al. Theta/beta neurofeedback in children with ADHD: Feasibility of a short-term setting and plasticity effects. Int. J. Psychophysiol. 2017, 112, 80–88. [Google Scholar] [CrossRef]
- Martin, T.; Kero, K.; Pozar, R.; Giordani, B.; Kavcic, V. Mild Cognitive Impairment in African Americans Is Associated with Differences in EEG Theta/Beta Ratio. J. Alzheimers Dis. 2023, 94, 347–357. [Google Scholar] [CrossRef]
- Yang, J.G.; Thapa, N.; Park, H.J.; Bae, S.; Park, K.W.; Park, J.H.; Park, H. Virtual Reality and Exercise Training Enhance Brain, Cognitive, and Physical Health in Older Adults with Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2022, 19, 13300. [Google Scholar] [CrossRef] [PubMed]
- Baik, K.; Jung, J.H.; Jeong, S.H.; Chung, S.J.; Yoo, H.S.; Lee, P.H.; Sohn, Y.H.; Kang, S.W.; Ye, B.S. Implication of EEG theta/alpha and theta/beta ratio in Alzheimer’s and Lewy body disease. Sci. Rep. 2022, 12, 18706. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Barry, R.J.; Karamacoska, D.; Johnstone, S.J. The EEG Theta/Beta Ratio: A marker of Arousal or Cognitive Processing Capacity? Appl. Psychophysiol. Biofeedback 2019, 44, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, D.; Rask, O.; Welin, L.; Petersson, M.G.; Gustafsson, P.; Landgren, K.; Eberhard, S. The Winding Road to Equal Care: Attitudes and Experiences of Prescribing ADHD Medication among Pediatric Psychiatrists: A Qualitative Study. Int. J. Environ. Res. Public Health 2022, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kessel, E.; Lee, S.; Hong, S.; Raffanello, E.; Hulvershorn, L.A.; Margolis, A.; Peterson, B.S.; Posner, J. Causal effects of psychostimulants on neural connectivity: A mechanistic, randomized clinical trial. J. Child Psychol. Psychiatry 2022, 63, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Sari Gokten, E.; Tulay, E.E.; Beser, B.; Elagoz Yuksel, M.; Arikan, K.; Tarhan, N.; Metin, B. Predictive Value of Slow and Fast EEG Oscillations for Methylphenidate Response in ADHD. Clin. EEG Neurosci. 2019, 50, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ogrim, G.; Kropotov, J.; Brunner, J.F.; Candrian, G.; Sandvik, L.; Hestad, K.A. Predicting the clinical outcome of stimulant medication in pediatric attention-deficit/hyperactivity disorder: Data from quantitative electroencephalography, event-related potentials, and a go/no-go test. Neuropsychiatr. Dis. Treat. 2014, 10, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Ogrim, G.; Kropotov, J.D. Predicting Clinical Gains and Side Effects of Stimulant Medication in Pediatric Attention-Deficit/Hyperactivity Disorder by Combining Measures From qEEG and ERPs in a Cued GO/NOGO Task. Clin. EEG Neurosci. 2019, 50, 34–43. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Sayal, K.; Prasad, V.; Daley, D.; Ford, T.; Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 2018, 5, 175–186. [Google Scholar] [CrossRef]
- Rubia, K. Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front. Hum. Neurosci. 2018, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.A.; Mhizha-Murira, J.R.; Smith, L.; Potter, K.J.; Wong, D.; Evangelou, N.; Lincoln, N.B.; das Nair, R. Memory rehabilitation for people with multiple sclerosis. Cochrane Database Syst. Rev. 2021, 10, CD008754. [Google Scholar] [CrossRef]
- Nardo, T.; Batchelor, J.; Berry, J.; Francis, H.; Jafar, D.; Borchard, T. Cognitive Remediation as an Adjunct Treatment for Substance Use Disorders: A Systematic Review. Neuropsychol. Rev. 2022, 32, 161–191. [Google Scholar] [CrossRef] [PubMed]
- Nikolin, S.; Tan, Y.Y.; Schwaab, A.; Moffa, A.; Loo, C.K.; Martin, D. An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. J. Affect. Disord. 2021, 284, 1–8. [Google Scholar] [CrossRef]
- Guo, J.Y.; Ragland, J.D.; Carter, C.S. Memory and cognition in schizophrenia. Mol. Psychiatry 2019, 24, 633–642. [Google Scholar] [CrossRef]
- Goncalves, O.F.; Carvalho, S.; Leite, J.; Fernandes-Goncalves, A.; Carracedo, A.; Sampaio, A. Cognitive and emotional impairments in obsessive-compulsive disorder: Evidence from functional brain alterations. Porto Biomed. J. 2016, 1, 92–105. [Google Scholar] [CrossRef] [PubMed]

| Total (Age ± SEM) | F (Age ± SEM) | M (Age ± SEM) | |
|---|---|---|---|
| Controls | 66 (19.112 ± 0.176) | 36 (19 ± 0.14) | 30 (19.245 ± 0.351) |
| ADHD | 80 (19.387 ± 0.199) | 49 (19.495 ± 0.247) | 31 (19.216 ± 0.337) |
| Total (Age ± SEM) | F (Age ± SEM) | M (Age ± SEM) | |
|---|---|---|---|
| Controls | 40 (19.297 ± 0.272) | 20 (19.128 ± 0.189) | 20 (19.465 ± 0.514) |
| ADHD LAI | 30 (19.112 ± 0.19) | 19 (19.207 ± 0.244) | 11 (18.949 ± 0.308) |
| ADHD HAI | 50 (19.552 ± 0.297) | 30 (19.678 ± 0.373) | 20 (19.362 ± 0.498) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Morales, W.V.; Reyes-López, J.V.; Tuz-Castellanos, K.N.-H.; Ortegón-Abud, D.; Ramírez-Lugo, L.; Santiago-Rodríguez, E.; Núñez-Jaramillo, L. Variations in Theta/Beta Ratio and Cognitive Performance in Subpopulations of Subjects with ADHD Symptoms: Towards Neuropsychological Profiling for Patient Subgrouping. J. Pers. Med. 2023, 13, 1361. https://doi.org/10.3390/jpm13091361
Herrera-Morales WV, Reyes-López JV, Tuz-Castellanos KN-H, Ortegón-Abud D, Ramírez-Lugo L, Santiago-Rodríguez E, Núñez-Jaramillo L. Variations in Theta/Beta Ratio and Cognitive Performance in Subpopulations of Subjects with ADHD Symptoms: Towards Neuropsychological Profiling for Patient Subgrouping. Journal of Personalized Medicine. 2023; 13(9):1361. https://doi.org/10.3390/jpm13091361
Chicago/Turabian StyleHerrera-Morales, Wendy Verónica, Julián Valeriano Reyes-López, Karen Nicte-Ha Tuz-Castellanos, Desiree Ortegón-Abud, Leticia Ramírez-Lugo, Efraín Santiago-Rodríguez, and Luis Núñez-Jaramillo. 2023. "Variations in Theta/Beta Ratio and Cognitive Performance in Subpopulations of Subjects with ADHD Symptoms: Towards Neuropsychological Profiling for Patient Subgrouping" Journal of Personalized Medicine 13, no. 9: 1361. https://doi.org/10.3390/jpm13091361







