Diagnostic Approach to Elevated Liver Function Tests during Pregnancy: A Pragmatic Narrative Review
Abstract
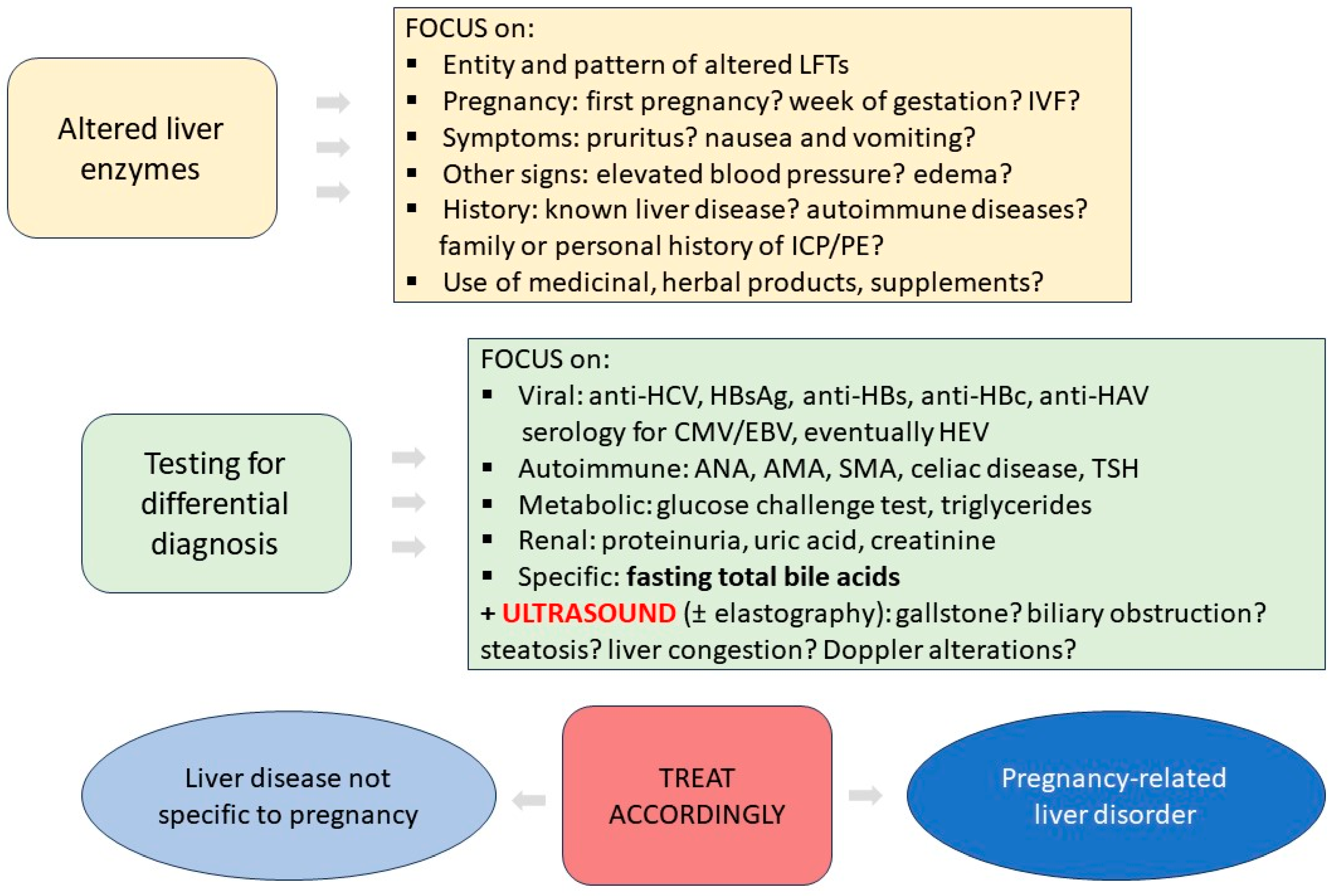
:1. Introduction
2. Acute and Chronic Liver Diseases Not Specific to Pregnancy
2.1. Viral Liver Disease
2.2. Autoimmune Liver Disease
3. Pregnancy-Related Liver Disorders
3.1. Hyperemesis Gravidarum
3.2. Intrahepatic Cholestasis of Pregnancy
3.3. Hypertension-Related Liver Disease during Pregnancy
3.4. Acute Fatty Liver of Pregnancy
4. Other Causes of Elevated Liver Enzymes: Hypotheses and Speculations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terrault, N.A.; Williamson, C. Pregnancy-Associated Liver Diseases. Gastroenterology 2022, 163, 97–117.e1. [Google Scholar] [CrossRef]
- Joshi, D.; James, A.; Quaglia, A.; Westbrook, R.H.; Heneghan, M.A. Liver disease in pregnancy. Lancet 2010, 375, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Ushida, T.; Kotani, T.; Kinoshita, F.; Imai, K.; Nakano-Kobayashi, T.; Nakamura, N.; Moriyama, Y.; Yoshida, S.; Yamashita, M.; Kajiyama, H. Liver transaminase levels during pregnancy: A Japanese multicenter study. J. Matern.-Fetal Neonatal Med. 2022, 35, 5761–5767. [Google Scholar] [CrossRef]
- Girling, J.C.; Dow, E.; Smith, J.H. Liver function tests in pre-eclampsia: Importance of comparison with a reference range derived for normal pregnancy. BJOG Int. J. Obstet. Gynaecol. 1997, 104, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kushner, T.M.; Park, C.B.; Masand, D.B.; Rosenbluth, E.; Carroll, C.B.; Grace, M.; Rodriguez-Rivas, C.; De La Cruz, H.; Overbey, J.; Sperling, R. Prevalence of elevated alanine aminotransferase (ALT) in pregnancy: A cross-sectional labor and delivery-based assessment. Medicine 2022, 101, e30408. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Levy, M.T.; Cheung, K.W.; Jourdain, G. Viral hepatitis and pregnancy. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 117–130. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Brady, C.W.; Fleckenstein, J.; Forde, K.A.; Khungar, V.; Molleston, J.P.; Afshar, Y.; Terrault, N.A. Reproductive Health and Liver Disease: Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 318–365. [Google Scholar] [CrossRef]
- Giles, M.; Visvanathan, K.; Lewin, S.; Bowden, S.; Locarnini, S.; Spelman, T.; Sasadeusz, J. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut 2015, 64, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Bzowej, N.H.; Tran, T.T.; Li, R.; Belle, S.H.; Smith, C.I.; Khalili, M.; Chung, R.; Tsai, N.; Terrault, N.; For the Hepatitis B Research Network (HBRN) Total Alanine Aminotransferase (ALT). Flares in Pregnant North American Women with Chronic Hepatitis B Infection: Results From a Prospective Observational Study. Am. J. Gastroenterol. 2019, 114, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Q.; Duan, Z.; Dai, E.; Zhang, S.; Han, G.; Wang, Y.; Zhang, H.; Zou, H.; Zhu, B.; Zhao, W.; et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N. Engl. J. Med. 2016, 374, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-T.; Sun, C.; Liu, C.-X.; Xie, S.-S.; Xiao, D.; Liu, L.; Yu, J.-H.; Li, W.-W.; Li, Q. Clinical features and outcome of acute hepatitis B in pregnancy. BMC Infect. Dis. 2014, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Abbas, Z.; Azami, M.; Belopolskaya, M.; Dokmeci, A.K.; Ghazinyan, H.; Jia, J.; Jindal, A.; Lee, H.C.; Lei, W.; et al. Asian Pacific association for the study of liver (APASL) guidelines: Hepatitis B virus in pregnancy. Hepatol. Int. 2022, 16, 211–253. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.N.; Jiles, R.B.; Teshale, E.H.; Foster, M.A.; Pesano, R.L.; Holmberg, S.D. Hepatitis C Virus Infection Among Reproductive-Aged Women and Children in the United States, 2006 to 2014. Ann. Intern. Med. 2017, 166, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Conte, D.; Fraquelli, M.; Prati, D.; Colucci, A.; Minola, E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology 2000, 31, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-T.; Zhong, M.; Wei, S.-S.; Luo, W.; Li, F.; Yu, Y.-H. Chronic hepatitis C virus infection is associated with increased risk of preterm birth: A meta-analysis of observational studies. J. Viral Hepat. 2015, 22, 1033–1042. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Thongprayoon, C.; Sanguankeo, A.; Upala, S.; Ungprasert, P.; Cheungpasitporn, W. Hepatitis C infection and intrahepatic cholestasis of pregnancy: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 39–45. [Google Scholar] [CrossRef]
- Benova, L.; Mohamoud, Y.A.; Calvert, C.; Abu-Raddad, L.J. Vertical Transmission of Hepatitis C Virus: Systematic Review and Meta-analysis. Clin. Infect. Dis. 2014, 59, 765–773. [Google Scholar] [CrossRef]
- Mok, J.; Pembrey, L.; Tovo, P.A.; Newell, M.L. When does mother to child transmission of hepatitis C virus occur? Arch. Dis. Child.-Fetal Neonatal Ed. 2005, 90, F156–F160. [Google Scholar] [CrossRef] [PubMed]
- Hillemanns, P.; Dannecker, C.; Kimming, R.; Hasbargen, U. Obstetric risks and vertical transmission of hepatitis C virus infection in pregnancy. Acta Obstet. Gynecol. Scand. 2000, 79, 543–547. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/10929952/ (accessed on 12 May 2023).
- Ahmad, T.; Hui, J.; Musa, T.H.; Behzadifar, M.; Baig, M. Seroprevalence of hepatitis E virus infection in pregnant women: A systematic review and meta-analysis. Ann. Saudi Med. 2020, 40, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Bigna, J.J.; Modiyinji, A.F.; Nansseu, J.R.; Amougou, M.A.; Nola, M.; Kenmoe, S.; Temfack, E.; Njouom, R. Burden of hepatitis E virus infection in pregnancy and maternofoetal outcomes: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Dagnew, M.; Belachew, A.; Tiruneh, M.; Moges, F. Hepatitis E virus infection among pregnant women in Africa: Systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 519. [Google Scholar] [CrossRef]
- Bergløv, A.; Hallager, S.; Weis, N. Hepatitis E during pregnancy: Maternal and foetal case-fatality rates and adverse outcomes—A systematic review. J. Viral Hepat. 2019, 26, 1240–1248. [Google Scholar] [CrossRef]
- Li, M.; Bu, Q.; Gong, W.; Li, H.; Li, S.; Sridhar, S.; Woo, P.C.; Wang, L. Hepatitis E virus infection and its associated adverse feto-maternal outcomes among pregnant women in Qinhuangdao, China. J. Matern.-Fetal Neonatal Med. 2019, 33, 3467–3651. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy*. J. Viral Hepat. 2003, 10, 61–69. [Google Scholar] [CrossRef]
- Norvell, J.P.; Blei, A.T.; Jovanovic, B.D.; Levitsky, J. Herpes simplex virus hepatitis: An analysis of the published literature and institutional cases. Liver Transplant. 2007, 13, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Lohse, A.W.; Chazouillères, O.; Dalekos, G. EASL clinical practice guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar]
- Grønbaek, L.; Vilstrup, H.; Jepsen, P. Pregnancy and birth outcomes in a Danish nationwide cohort of women with autoimmune hepatitis and matched population controls. Aliment. Pharmacol. Ther. 2018, 48, 655–663. [Google Scholar] [CrossRef]
- Schramm, C.; Herkel, J.; Beuers, U.; Kanzler, S.; Galle, P.R.; Lohse, A.W. Pregnancy in Autoimmune Hepatitis: Outcome and Risk Factors. Am. J. Gastroenterol. 2006, 101, 556–560. [Google Scholar] [CrossRef]
- Terrabuio, D.R.B.; Abrantes-Lemos, C.P.; Carrilho, F.J.; Cançado, E.L.R. Follow-up of pregnant women with autoimmune hepatitis: The disease behavior along with maternal and fetal outcomes. J. Clin. Gastroenterol. 2009, 43, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Huang, Z.; Hegarty, R.; Ma, Y.; Heneghan, M.A. Systematic review with meta-analysis: Outcomes of pregnancy in patients with autoimmune hepatitis. Aliment. Pharmacol. Ther. 2022, 55, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Buchel, E.; Van Steenbergen, W.; Nevens, F.; Fevery, J. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. Am. J. Gastroenterol. 2002, 97, 3160–3165. [Google Scholar] [CrossRef] [PubMed]
- Muratori, P.; Loffreda, S.; Muratori, L.; Ferrari, R.; Afandi, K.; Cassani, F.; Pappas, G.; Lenzi, M.; Bianchi, F. Spontaneous remission of autoimmune hepatitis during pregnancy. Dig. Liver Dis. 2002, 34, 608–609. [Google Scholar] [CrossRef]
- Fischer, S.E.; de Vries, E.S.; Tushuizen, M.E.; de Boer, Y.S.; van der Meer, A.J.P.; de Man, R.A.; Brouwer, J.T.; Kuyvenhoven, J.P.; Klemt-Kropp, M.; Gevers, T.J.G.; et al. Importance of complete response for outcomes of pregnancy in patients with autoimmune hepatitis. Liver Int. 2023, 43, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Llovet, L.-P.; Horta, D.; Eliz, M.G.; Berenguer, M.; Fábrega, E.; Sáez-Royuela, F.; García-Retortillo, M.; Torrijos, Y.S.; Romero-Gómez, M.; Fernández, C.; et al. Presentation and Outcomes of Pregnancy in Patients With Autoimmune Hepatitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2819–2821. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Riordan, S.; Strasser, S.; Kurtovic, J.; Singh-Grewel, I.; Koorey, D. Severe autoimmune hepatitis first presenting in the early post partum period. Clin. Gastroenterol. Hepatol. 2004, 2, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Efe, C.; Kahramanoğlu-Aksoy, E.; Yılmaz, B.; Ozseker, B.; Takcı, S.; Roach, E.C.; Purnak, T.; Kav, T.; Ozaslan, E.; Wahlin, S. Pregnancy in women with primary biliary cirrhosis. Autoimmun. Rev. 2014, 13, 931–935. [Google Scholar] [CrossRef]
- Poupon, R.; Chrétien, Y.; Chazouillères, O.; Poupon, R.E. Pregnancy in women with ursodeoxycholic acid-treated primary biliary cirrhosis. J. Hepatol. 2005, 42, 418–419. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Kumagi, T.; Al-Harthy, N.; Coltescu, C.; Ward, S.; Cheung, A.; Hirschfield, G.M. Good Maternal and Fetal Outcomes for Pregnant Women With Primary Biliary Cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1179–1185.e1. [Google Scholar] [CrossRef] [PubMed]
- Wellge, B.E.; Sterneck, M.; Teufel, A.; Rust, C.; Franke, A.; Schreiber, S.; Berg, T.; Günther, R.; Kreisel, W.; zu Eulenburg, C.; et al. Pregnancy in primary sclerosing cholangitis. Gut 2011, 60, 1117–1121. [Google Scholar] [CrossRef]
- Wronka, K.M.; Bik, E.; Milkiewicz, P. Outcome of pregnancy in patients with primary sclerosing cholangitis. Dig. Liver Dis. 2022, 54, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Cauldwell, M.; Mackie, F.L.; Steer, P.J.; Henehghan, M.; Baalman, J.H.; Brennand, J.; Johnston, T.; Dockree, S.; Hedley, C.; Jarvis, S.; et al. Pregnancy outcomes in women with primary biliary cholangitis and primary sclerosing cholangitis: A retrospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.H.; Dusheiko, G.; Williamson, C. Pregnancy and liver disease. J. Hepatol. 2016, 64, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Morali, G.A.; Braverman, D.Z. Abnormal Liver Enzymes and Ketonuria in Hyperemesis Gravidarum A Retrospective Review of 80 Patients. J. Clin. Gastroenterol. 1990, 12, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Gaba, N.; Gaba, S. Study of Liver Dysfunction in Hyperemesis Gravidarum. Cureus 2020, 12, e8709. [Google Scholar] [CrossRef]
- Chraïbi, Z.; Ouldamer, L.; Body, G.; Bacq, Y. Hyperemesis gravidarum: A ten-year French retrospective study of 109 patients. Presse Med. 2015, 44, e13–e22. [Google Scholar] [CrossRef]
- Abu-Hayyeh, S.; Papacleovoulou, G.; Williamson, C. Nuclear receptors, bile acids and cholesterol homeostasis series-bile acids and pregnancy. Mol. Cell. Endocrinol. 2013, 368, 120–128. [Google Scholar] [CrossRef]
- Turro, E.; Astle, W.J.; Megy, K.; Gräf, S.; Greene, D.; Shamardina, O.; Allen, H.L.; Sanchis-Juan, A.; Frontini, M.; Thys, C.; et al. Whole-genome sequencing of patients with rare diseases in a national health system. Nature 2020, 583, 96–102. [Google Scholar] [CrossRef]
- Dixon, P.H.; Sambrotta, M.; Chambers, J.; Taylor-Harris, P.; Syngelaki, A.; Nicolaides, K.; Knisely, A.S.; Thompson, R.J.; Williamson, C. An expanded role for heterozygous mutations of ABCB4, ABCB11, ATP8B1, ABCC2 and TJP2 in intrahepatic cholestasis of pregnancy. Sci. Rep. 2017, 7, 11823. [Google Scholar] [CrossRef] [PubMed]
- Girling, J.; Knight, C.L.; Chappell, L. Intrahepatic cholestasis of pregnancy: Green-top Guideline No. 43 June 2022. BJOG Int. J. Obstet. Gynaecol. 2022, 129, e95–e114. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- Ovadia, C.; Seed, P.T.; Sklavounos, A.; Geenes, V.; Di Ilio, C.; Chambers, J.; Kohari, K.; Bacq, Y.; Bozkurt, N.; Brun-Furrer, R.; et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: Results of aggregate and individual patient data meta-analyses. Lancet 2019, 393, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Geenes, V.; Chappell, L.C.; Seed, P.T.; Steer, P.J.; Knight, M.; Williamson, C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: A prospective population-based case-control study. Hepatology 2014, 59, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Parikh, L.I.; Ramsey, P.S.; Huang, C.-C.; Zeymo, A.; Fernandez, M.; Smith, S.; Iqbal, S.N. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am. J. Obstet. Gynecol. 2015, 213, 570.e1–570.e8. [Google Scholar] [CrossRef] [PubMed]
- Agaoglu, R.T.; Celik, O.Y.; Yakut, K.; Celen, S.; Caglar, A.T. Maternal serum calprotectin level in intrahepatic cholestasis of pregnancy. J. Obstet. Gynaecol. Res. 2021, 47, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Bacq, Y.; Sentilhes, L.; Reyes, H.B.; Glantz, A.; Kondrackiene, J.; Binder, T.; Nicastri, P.L.; Locatelli, A.; Floreani, A.; Hernandez, I.; et al. Efficacy of Ursodeoxycholic Acid in Treating Intrahepatic Cholestasis of Pregnancy: A Meta-analysis. Gastroenterology 2012, 143, 1492–1501. [Google Scholar] [CrossRef]
- Bacq, Y.; le Besco, M.; Lecuyer, A.-I.; Gendrot, C.; Potin, J.; Andres, C.R.; Aubourg, A. Ursodeoxycholic acid therapy in intrahepatic cholestasis of pregnancy: Results in real-world conditions and factors predictive of response to treatment. Dig. Liver Dis. 2017, 49, 63–69. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 145 (Suppl. S1), 1–33. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.; Menkhorst, E. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertens 2013, 62, 1046–1054. [Google Scholar] [CrossRef]
- Gerretsen, G.; Huisjes, H.J.; Elema, J.D. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br. J. Obstet. Gynaecol. 1981, 88, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, J.; Baker, P.N.; Tong, C. Revisiting preeclampsia: A metabolic disorder of the placenta. FEBS J. 2022, 289, 336–354. [Google Scholar] [CrossRef] [PubMed]
- Bahado-Singh, R.O.; Syngelaki, A.; Mandal, R.; Graham, S.F.; Akolekar, R.; Han, B.; Bjondahl, T.C.; Dong, E.; Bauer, S.; Alpay-Savasan, Z.; et al. Metabolomic determination of pathogenesis of late-onset preeclampsia. J. Matern.-Fetal Neonatal Med. 2017, 30, 658–664. [Google Scholar] [CrossRef]
- Fondjo, L.A.; Amoah, B.; Annan, J.J.; Adu-Gyamfi, E.A.; Asamaoh, E.A. Hematobiochemical variability and predictors of new-onset and persistent postpartum preeclampsia. Sci. Rep. 2022, 12, 3583. [Google Scholar] [CrossRef]
- Gelaw, Y.; Asrie, F.; Walle, M.; Getaneh, Z. The value of eosinophil count in the diagnosis of preeclampsia among pregnant women attending the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2021. BMC Pregnancy Childbirth 2022, 22, 557. [Google Scholar] [CrossRef]
- Gedik, E.; Yücel, N.; Sahin, T.; Koca, E.; Colak, Y.Z.; Togal, T. Hemolysis, elevated liver enzymes, and low platelet syndrome: Outcomes for patients admitted to intensive care at a tertiary referral hospital. Hypertens. Pregnancy 2017, 36, 21–29. [Google Scholar] [CrossRef]
- Carpani, G.; Bozzo, M.; Ferrazzi, E.; D’Amato, B.; Pizzotti, D.; Radaelli, T.; Pardi, G. The evaluation of maternal parameters at diagnosis may predict HELLP syndrome severity. J. Matern.-Fetal Neonatal Med. 2003, 13, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.X.; Chen, C.P.; Sun, F.J.; Chen, C.Y. Comparison of maternal and neonatal outcomes between acute fatty liver of pregnancy and hemolysis, elevated liver enzymes and low platelets syndrome: A retrospective cohort study. BMC Pregnancy Childbirth 2021, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, D.N.; Das Neves, A.V.; Aphalo, V.M.; Vidal, L.; Moseinco, M.; Lapadula, J.; Santa-Maria, A.; Zakalik, G.; Gomez, R.A.; Capalbo, M.; et al. Predictability of adverse outcomes in hypertensive disorders of pregnancy: A multicenter prospective cohort study. Hypertens. Pregnancy 2021, 40, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Villalaín, C.; Herraiz, I.; Olmo, P.D.-D.; Angulo, P.; Ayala, J.L.; Galindo, A. Prediction of Delivery Within 7 Days After Diagnosis of Early Onset Preeclampsia Using Machine-Learning Models. Front. Cardiovasc. Med. 2022, 9, 910701. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Allotey, J.; Marlin, N.; Dodds, J.; Cheong-See, F.; von Dadelszen, P.; Ganzevoort, W.; Akkermans, J.; Kerry, S.; Mol, B.W.; et al. Prediction of complications in early-onset pre-eclampsia (PREP): Development and external multinational validation of prognostic models. BMC Med. 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Von Dadelszen, P.; Payne, B.; Li, J.; Ansermino, J.M.; Pipkin, F.B.; Côté, A.-M.; Douglas, M.J.; Gruslin, A.; Hutcheon, J.A.; Joseph, K.; et al. Prediction of adverse maternal outcomes in pre-eclampsia: Development and validation of the fullPIERS model. Lancet 2011, 377, 219–227. [Google Scholar] [CrossRef]
- Greiner, K.S.; Rincón, M.; Derrah, K.L.; Burwick, R.M. Elevated liver enzymes and adverse outcomes among patients with preeclampsia with severe features. J. Matern.-Fetal Neonatal Med. 2023, 36, 2160627. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, J.S.; Han, Y.J.; Kim, W.; Bang, S.H.; Kim, B.J.; Park, C.-W.; Kim, M.Y. Elevated Alanine Aminotransferase in Early Pregnancy and Subsequent Development of Gestational Diabetes and Preeclampsia. J. Korean Med. Sci. 2020, 35, e198. [Google Scholar] [CrossRef]
- Cho, G.J.; Kim, H.Y.; Park, J.H.; Ahn, K.-H.; Hong, S.-C.; Oh, M.-J.; Kim, H.-J. Prepregnancy liver enzyme levels and risk of preeclampsia in a subsequent pregnancy: A population-based cohort study. Liver Int. 2018, 38, 949–954. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, C.; Wang, D.; Chen, X.; Jiang, Y.; Dou, Y.; Wang, Y.; Li, M.; Chen, H.; He, W.; et al. High-normal liver enzyme levels in early pregnancy predispose the risk of gestational hypertension and preeclampsia: A prospective cohort study. Front. Cardiovasc. Med. 2022, 9, 963957. [Google Scholar] [CrossRef]
- Knight, M.; Nelson-Piercy, C.; Kurinczuk, J.J.; Spark, P.; Brocklehurst, P.; (Ukoss), O.B.O.U.O.S.S. A prospective national study of acute fatty liver of pregnancy in the UK. Gut 2008, 57, 951–956. [Google Scholar] [CrossRef]
- Ibdah, J.A.; Bennett, M.J.; Rinaldo, P.; Zhao, Y.; Gibson, B.; Sims, H.F.; Strauss, A.W. A Fetal Fatty-Acid Oxidation Disorder as a Cause of Liver Disease in Pregnant Women. N. Engl. J. Med. 1999, 340, 1723–1731. [Google Scholar] [CrossRef]
- Casey, L.C.; Fontana, R.J.; Aday, A.; Nelson, D.B.; Rule, J.A.; Gottfried, M.; Tran, M.; Lee, W.M. Acute Liver Failure (ALF) in Pregnancy: How Much Is Pregnancy Related? Hepatology 2020, 72, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Azzaroli, F.; Mazzella, G.; Marchesini, G.; Brodosi, L.; Petroni, M.L. Fatty liver in pregnancy: A narrative review of two distinct conditions. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ghaziani, T.T.; Wolf, J.L. Acute Fatty Liver Disease of Pregnancy: Updates in Pathogenesis, Diagnosis, and Management. Am. J. Gastroenterol. 2017, 112, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Yarrington, C.D.; Cantonwine, D.E.; Seely, E.W.; Mcelrath, T.F.; Zera, C.A. The association of early unexplained elevated alanine aminotransferase with large-for-gestational-age birthweight. Am. J. Obstet. Gynecol. 2016, 215, 474.e1–474.e5. [Google Scholar] [CrossRef]
- Ch’Ng, C.L.; Morgan, M.; Hainsworth, I.; Kingham, J.G.C. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut 2002, 51, 876–880. [Google Scholar] [CrossRef]
- Yarrington, C.D.; Cantonwine, D.E.; Seely, E.W.; McElrath, T.F.; Zera, C.A. The Association of Alanine Aminotransferase in Early Pregnancy with Gestational Diabetes. Metab. Syndr. Relat. Disord. 2016, 14, 254–258. [Google Scholar] [CrossRef]
- Leng, J.; Zhang, C.; Wang, P.; Li, N.; Li, W.; Liu, H.; Zhang, S.; Hu, G.; Yu, Z.; Ma, R.C.; et al. Plasma Levels of Alanine Aminotransferase in the First Trimester Identify High Risk Chinese Women for Gestational Diabetes. Sci. Rep. 2016, 6, 27291. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Duo, Y.; Zhang, Y.; Qiao, X.; Xu, J.; Zhang, J.; Peng, Z.; Chen, Y.; Nie, X.; Sun, Q.; et al. The Predictive Ability of Hepatic Steatosis Index for Gestational Diabetes Mellitus and Large for Gestational Age Infant Compared with Other Noninvasive Indices Among Chinese Pregnancies: A Preliminary Double-center Cohort Study. Diabetes Metab. Syndr. Obes. 2021, 14, 4791–4800. [Google Scholar] [CrossRef]
- Bulut, A.N.; Ceyhan, V.; Dolanbay, M. Can alanine aminotransferase measured in early pregnancy predict macrosomia? J. Obstet. Gynaecol. 2022, 42, 1799–1802. [Google Scholar] [CrossRef]
- Serra, C.; Dajti, E.; De Molo, C.; Montaguti, E.; Porro, A.; Seidenari, A.; Angilletta, E.; Bernardi, V.; Salsi, G.; Bakken, S.M.; et al. Utility of Doppler-Ultrasound and Liver Elastography in the Evaluation of Patients with Suspected Pregnancy-Related Liver Disease. J. Clin. Med. 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Grab, J.; Dodge, J.L.; Gunderson, E.P.; Rubin, J.; Irani, R.A.; Cedars, M.; Terrault, N. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J. Hepatol. 2020, 73, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Chung, Y.; Park, J.; Park, J.; Han, K.; Park, Y.; Park, I.Y.; Ko, H.S. Influences of pregravid liver enzyme levels on the development of gestational diabetes mellitus. Liver Int. 2021, 41, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Lao, T.T. Implications of abnormal liver function in pregnancy and non-alcoholic fatty liver disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 68, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S988–S1005. [Google Scholar] [CrossRef] [PubMed]
- Trottmann, F.; Baumann, M.; Amylidi-Mohr, S.; Surbek, D.; Risch, L.; Mosimann, B.; Raio, L. Angiogenic profiling in HELLP syndrome cases with or without hypertension and proteinuria. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W.; Mullens, W.; Tomsin, K.; Mesens, T.; Peeters, L. Role of dysfunctional maternal venous hemodynamics in the pathophysiology of pre-eclampsia: A review. Ultrasound Obstet. Gynecol. 2011, 38, 123–129. [Google Scholar] [CrossRef]
- Ammon, F.J.; Kohlhaas, A.; Elshaarawy, O.; Mueller, J.; Bruckner, T.; Sohn, C.; Fluhr, G.; Fluhr, H.; Mueller, S. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. World J. Gastroenterol. 2018, 24, 4393–4402. [Google Scholar] [CrossRef]
- Carmiel Haggai, M.; Sgayer, I.; Bornstein, J.; Odeh, M.; Lowenstein, L.; Frank Wolf, M. Liver stiffness and steatosis in preeclampsia as shown by transient elastography-a prospective cohort study. Am. J. Obstet. Gynecol. 2022, 227, 515.e1–515.e9. [Google Scholar] [CrossRef]
| Hyperemesis Gravidarum | Intrahepatic Cholestasis of Pregnancy | PE-Related Liver Dysfunction and HELLP Syndrome | Acute Fatty Liver of Pregnancy | |
|---|---|---|---|---|
| Diagnostic Criteria | Persistent vomiting with weight loss > 5% | Pruritus and bile acid > 10 µmol/L | Hypertension and maternal or fetal complication | Swansea criteria |
| Altered liver enzymes | ||||
| Prevalence | 8–15% overall 40–50% if hospitalized | 20–80% | 20–50% | Almost 100% |
| Mean AST/ALT values (IU/L) | 50 IU/L | 100–150 IU/L | 40–100 IU/L 300–500 IU/L if HELLP | 300–1000 IU/L |
| Range (× ULN) | 2–5 × ULN | 1.5–8 × ULN | 2–5 × ULN Up to 30 × ULN if HELLP | Up to 100 × ULN |
| Jaundice | Rare | Rare | Unlikely Possible (15%) if HELLP | Almost 100% Mean 5–6 mg/dL |
| Inclusion in diagnostic criteria? | No | No | Yes | Yes |
| Correlation with disease severity and prognosis? | Yes, with starvation | No prognostic role | Yes, with worse maternal outcomes | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dajti, E.; Bruni, A.; Barbara, G.; Azzaroli, F. Diagnostic Approach to Elevated Liver Function Tests during Pregnancy: A Pragmatic Narrative Review. J. Pers. Med. 2023, 13, 1388. https://doi.org/10.3390/jpm13091388
Dajti E, Bruni A, Barbara G, Azzaroli F. Diagnostic Approach to Elevated Liver Function Tests during Pregnancy: A Pragmatic Narrative Review. Journal of Personalized Medicine. 2023; 13(9):1388. https://doi.org/10.3390/jpm13091388
Chicago/Turabian StyleDajti, Elton, Angelo Bruni, Giovanni Barbara, and Francesco Azzaroli. 2023. "Diagnostic Approach to Elevated Liver Function Tests during Pregnancy: A Pragmatic Narrative Review" Journal of Personalized Medicine 13, no. 9: 1388. https://doi.org/10.3390/jpm13091388
APA StyleDajti, E., Bruni, A., Barbara, G., & Azzaroli, F. (2023). Diagnostic Approach to Elevated Liver Function Tests during Pregnancy: A Pragmatic Narrative Review. Journal of Personalized Medicine, 13(9), 1388. https://doi.org/10.3390/jpm13091388







