Complement System Proteins in the Human Aqueous Humor and Their Association with Primary Open-Angle Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Liquid Chromatography–Tandem Mass Spectrometry Analysis
2.3. Protein Identification and Quantification
2.4. Optical Coherence Tomography (OCT) and Heidelberg Retinal Tomography (HRT) Measurements
2.5. Statistical Analyses
3. Results
3.1. Complement Proteins Detected in the Human Aqueous Humor
3.2. Alterations in Aqueous Humor Complement Proteins Associated with POAG
3.3. Race-Specific Alterations in Aqueous Humor Complement Proteins Associated with POAG
3.4. Sex-Specific Alterations in the Aqueous Humor Complement Proteins Associated with POAG
3.5. Alterations in Complement Proteins Correlated with Pattern Standard Deviation (PSD) and Visual Field Index (VFI)
3.6. Complement Proteins Correlated with Ocular Stereometric Heidelberg Retinal Tomography (HRT) Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benhar, I.; London, A.; Schwartz, M. The privileged immunity of immune privileged organs: The case of the eye. Front. Immunol. 2012, 3, 296. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003, 3, 879–889. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, C.S.; Leger, A.J.S.; Caspi, R.R. Mucosal immunology of the ocular surface. Mucosal Immunol. 2022, 15, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Crane, I.J.; Liversidge, J. Mechanisms of leukocyte migration across the blood–retina barrier. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 165–177. [Google Scholar]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Freddo, T.F. A contemporary concept of the blood–aqueous barrier. Prog. Retin. Eye Res. 2013, 32, 181–195. [Google Scholar] [CrossRef]
- Katamay, R.; Nussenblatt, R.B. Blood–retinal barrier, immune privilege, and autoimmunity. Retina 2013, 579–589. [Google Scholar]
- Keino, H.; Horie, S.; Sugita, S. Immune privilege and eye-derived T-regulatory cells. J. Immunol. Res. 2018, 2018, 1679197. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular immunosuppressive microenvironment. Immune Response Eye 2007, 92, 71–85. [Google Scholar]
- Sonoda, K.H.; Sakamoto, T.; Qiao, H.; Hisatomi, T.; Oshima, T.; Tsutsumi-Miyahara, C.; Exley, M.; Balk, S.P.; Taniguchi, M.; Ishibashi, T. The analysis of systemic tolerance elicited by antigen inoculation into the vitreous cavity: Vitreous cavity-associated immune deviation. Immunology 2005, 116, 390–399. [Google Scholar] [CrossRef]
- Williams, P.A.; Braine, C.E.; Kizhatil, K.; Foxworth, N.E.; Tolman, N.G.; Harder, J.M.; Scott, R.A.; Sousa, G.L.; Panitch, A.; Howell, G.R. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol. Neurodegener. 2019, 14, 6. [Google Scholar] [CrossRef]
- Mangan, B.G.; Al-Yahya, K.; Chen, C.T.; Gionfriddo, J.R.; Powell, C.C.; Dubielzig, R.R.; Ehrhart, E.J.; Madl, J.E. Retinal pigment epithelial damage, breakdown of the blood–retinal barrier, and retinal inflammation in dogs with primary glaucoma. Vet. Ophthalmol. 2007, 10, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Kodeboyina, S.K.; Lee, T.J.; Churchwell, L.; Ulrich, L.; Bollinger, K.; Bogorad, D.; Estes, A.; Zhi, W.; Sharma, S.; Sharma, A. The Constitutive Proteome of Human Aqueous Humor and Race Specific Alterations. Proteomes 2020, 8, 34. [Google Scholar] [CrossRef]
- Altay, L.; Sitnilska, V.; Schick, T.; Widmer, G.; Duchateau-Nguyen, G.; Piraino, P.; Jayagopal, A.; Drawnel, F.; Fauser, S. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye 2019, 33, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. The complement system and innate immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Bora, N.S.; Jha, P.; Bora, P.S. The role of complement in ocular pathology. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 85–95. [Google Scholar]
- Keir, L.S.; Firth, R.; Aponik, L.; Feitelberg, D.; Sakimoto, S.; Aguilar, E.; Welsh, G.I.; Richards, A.; Usui, Y.; Satchell, S.C.; et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J. Clin. Investig. 2017, 127, 199–214. [Google Scholar] [CrossRef]
- Botling Taube, A.; Konzer, A.; Alm, A.; Bergquist, J. Proteomic analysis of the aqueous humour in eyes with pseudoexfoliation syndrome. Br. J. Ophthalmol. 2019, 103, 1190–1194. [Google Scholar] [CrossRef]
- Yu, M.; Xie, F.; Liu, X.; Sun, H.; Guo, Z.; Liu, X.; Li, W.; Sun, W.; Wang, Y.; He, C. Proteomic Study of Aqueous Humor and Its Application in the Treatment of Neovascular Glaucoma. Front. Mol. Biosci. 2020, 7, 587677. [Google Scholar] [CrossRef]
- Schick, T.; Steinhauer, M.; Aslanidis, A.; Altay, L.; Karlstetter, M.; Langmann, T.; Kirschfink, M.; Fauser, S. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye 2017, 31, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Investig. 2011, 121, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Gassel, C.J.; Reinehr, S.; Gomes, S.C.; Dick, H.B.; Joachim, S.C. Preservation of optic nerve structure by complement inhibition in experimental glaucoma. Cell Tissue Res. 2020, 382, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Salgarello, T.; Colotto, A.; Falsini, B.; Buzzonetti, L.; Cesari, L.; Iarossi, G.; Scullica, L. Correlation of pattern electroretinogram with optic disc cup shape in ocular hypertension. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1989–1997. [Google Scholar]
- Eyre, T.A.; Ducluzeau, F.; Sneddon, T.P.; Povey, S.; Bruford, E.A.; Lush, M.J. The HUGO gene nomenclature database, 2006 updates. Nucleic Acids Res. 2006, 34, D319–D321. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Chowdhury, U.R.; Madden, B.J.; Charlesworth, M.C.; Fautsch, M.P. Proteome analysis of human aqueous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4921–4931. [Google Scholar] [CrossRef]
- Adav, S.S.; Wei, J.; Terence, Y.; Ang, B.C.; Yip, L.W.; Sze, S.K. Proteomic analysis of aqueous humor from primary open angle glaucoma patients on drug treatment revealed altered complement activation cascade. J. Proteome Res. 2018, 17, 2499–2510. [Google Scholar] [CrossRef]
- Aleshin, A.E.; Schraufstatter, I.U.; Stec, B.; Bankston, L.A.; Liddington, R.C.; DiScipio, R.G. Structure of Complement C6 Suggests a Mechanism for Initiation and Unidirectional, Sequential Assembly of Membrane Attack Complex (MAC)*♦. J. Biol. Chem. 2012, 287, 10210–10222. [Google Scholar] [CrossRef]
- Bubeck, D. The making of a macromolecular machine: Assembly of the membrane attack complex. Biochemistry 2014, 53, 1908–1915. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Kaplan, H.J. Oxidative stress and the regulation of complement activation in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Tegla, C.A.; Cudrici, C.; Patel, S.; Trippe, R.; Rus, V.; Niculescu, F.; Rus, H. Membrane attack by complement: The assembly and biology of terminal complement complexes. Immunol. Res. 2011, 51, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhao, J.; Madden, A.; Chen, M.; Xu, H. Complement expression in retinal pigment epithelial cells is modulated by activated macrophages. Exp. Eye Res. 2013, 112, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Agarwal, D.; Dana, N.; Schäfer, N.; Biber, J.; Wunderlich, K.A.; Jabri, Y.; Straub, T.; Zhang, N.R.; Gautam, A.K. Cell-type-specific complement expression in the healthy and diseased retina. Cell Rep. 2019, 29, 2835–2848.e2834. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef]
- Serna, M.; Giles, J.L.; Morgan, B.P.; Bubeck, D. Structural basis of complement membrane attack complex formation. Nat. Commun. 2016, 7, 10587. [Google Scholar] [CrossRef]
- Kojima, T.; Horiuchi, T.; Nishizaka, H.; Fukumori, Y.; Amano, T.; Nagasawa, K.; Niho, Y.; Hayashi, K. Genetic basis of human complement C8α-γ deficiency. J. Immunol. 1998, 161, 3762–3766. [Google Scholar] [CrossRef]
- Kim, J.-H.; Afridi, R.; Han, J.; Jung, H.-G.; Kim, S.-C.; Hwang, E.M.; Shim, H.S.; Ryu, H.; Choe, Y.; Hoe, H.-S. Gamma subunit of complement component 8 is a neuroinflammation inhibitor. Brain 2021, 144, 528–552. [Google Scholar] [CrossRef]
- Kim, J.-H.; Han, J.; Suk, K. Protective effects of complement component 8 gamma against blood-brain barrier breakdown. Front. Physiol. 2021, 12, 671250. [Google Scholar] [CrossRef]
- Suk, K. Gamma subunit of complement component 8 is an innate immune suppressor in brain. J. Immunol. 2020, 204, 144-14. [Google Scholar] [CrossRef]
- Heinen, S.; Hartmann, A.; Lauer, N.; Wiehl, U.; Dahse, H.M.; Schirmer, S.; Gropp, K.; Enghardt, T.; Wallich, R.; Hälbich, S.; et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 2009, 114, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- De Córdoba, S.R.; De Jorge, E.G. Translational mini-review series on complement factor H: Genetics and disease associations of human complement factor H. Clin. Exp. Immunol. 2008, 151, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Borras, C.; Canonica, J.; Jorieux, S.; Abache, T.; El Sanharawi, M.; Klein, C.; Delaunay, K.; Jonet, L.; Salvodelli, M.; Naud, M.-C. CFH exerts anti-oxidant effects on retinal pigment epithelial cells independently from protecting against membrane attack complex. Sci. Rep. 2019, 9, 13873. [Google Scholar] [CrossRef] [PubMed]
- Weismann, D.; Hartvigsen, K.; Lauer, N.; Bennett, K.L.; Scholl, H.P.; Issa, P.C.; Cano, M.; Brandstätter, H.; Tsimikas, S.; Skerka, C. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 2011, 478, 76–81. [Google Scholar] [CrossRef]
- Kopp, A.; Hebecker, M.; Svobodová, E.; Józsi, M. Factor h: A complement regulator in health and disease, and a mediator of cellular interactions. Biomolecules 2012, 2, 46–75. [Google Scholar] [CrossRef]
- Chinnaraj, M.; Planer, W.; Pozzi, N. Structure of coagulation factor II: Molecular mechanism of thrombin generation and development of next-generation anticoagulants. Front. Med. 2018, 5, 281. [Google Scholar] [CrossRef]
- Grieshaber, M.C.; Mozaffarieh, M.; Flammer, J. What is the link between vascular dysregulation and glaucoma? Surv. Ophthalmol. 2007, 52, S144–S154. [Google Scholar] [CrossRef]
- O’Brien, C.; Butt, Z.; Ludlam, C.; Detkova, P. Activation of the coagulation cascade in untreated primary open-angle glaucoma. Ophthalmology 1997, 104, 725–730. [Google Scholar] [CrossRef]
- Siegfried, C.J.; Shui, Y.B. Racial Disparities in Glaucoma: From Epidemiology to Pathophysiology. Mo Med. 2022, 119, 49–54. [Google Scholar]
- Harboe, M.; Mollnes, T.E. The alternative complement pathway revisited. J. Cell. Mol. Med. 2008, 12, 1074–1084. [Google Scholar] [CrossRef]
- Harder, J.M.; Williams, P.A.; Braine, C.E.; Yang, H.S.; Thomas, J.M.; Foxworth, N.E.; John, S.W.; Howell, G.R. Complement peptide C3a receptor 1 promotes optic nerve degeneration in DBA/2J mice. J. Neuroinflammation 2020, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Soto, I.; Ryan, M.; Graham, L.C.; Smith, R.S.; John, S.W. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J. Neuroinflammation 2013, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; George, A.; Nimmagadda, M.; Ortolan, D.; Karla, B.-S.; Qureshy, Z.; Bose, D.; Dejene, R.; Liang, G.; Wan, Q. Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model. Nat. Commun. 2021, 12, 7293. [Google Scholar] [CrossRef]
- Klos, A.; Tenner, A.J.; Johswich, K.-O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Dong, N.; Yang, M.; Wang, J.; Feng, X.; Wang, Y. Complement inhibitors in age-related macular degeneration: A potential therapeutic option. J. Immunol. Res. 2021, 2021, 9945725. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential role of complement in pregnancy: From implantation to parturition and beyond. Front. Immunol. 2020, 11, 1681. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M. Complement C4, infections, and autoimmune diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef]
- Nilsson, S.C.; Sim, R.B.; Lea, S.M.; Fremeaux-Bacchi, V.; Blom, A.M. Complement factor I in health and disease. Mol. Immunol. 2011, 48, 1611–1620. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Kövari, E.; French, L.; Viard, I.; Hof, P.; Bouras, C. Possible neuroprotective role of clusterin in Alzheimer’s disease: A quantitative immunocytochemical study. Acta Neuropathol. 1998, 95, 387–394. [Google Scholar] [CrossRef]
- Wilson, M.R.; Easterbrook-Smith, S.B. Clusterin is a secreted mammalian chaperone. Trends Biochem. Sci. 2000, 25, 95–98. [Google Scholar] [CrossRef]
- Menny, A.; Lukassen, M.V.; Couves, E.C.; Franc, V.; Heck, A.J.; Bubeck, D. Structural basis of soluble membrane attack complex packaging for clearance. Nat. Commun. 2021, 12, 6086. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, Y.; Shao, M.; Tang, B.; Cao, W.; Sun, X. Association between coagulation function and patients with primary angle closure glaucoma: A 5-year retrospective case-control study. BMJ Open 2017, 7, e016719. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Tribble, J.R.; Pepper, K.W.; Cross, S.D.; Morgan, B.P.; Morgan, J.E.; John, S.W.; Howell, G.R. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol. Neurodegener. 2016, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Dikopf, M.S.; Vajaranant, T.S.; Edward, D.P. Topical treatment of glaucoma: Established and emerging pharmacology. Expert Opin. Pharmacother. 2017, 18, 885–898. [Google Scholar] [CrossRef] [PubMed]

| Demographic Characteristic | Cataract (n = 196) | POAG (n = 62) | p-Value |
|---|---|---|---|
| Age (years, mean ± SD) | 67.38 ± 9.48 | 67.87 ± 11.23 | 0.76 a |
| Sex (F/M) | 122/74 | 38/24 | 1 b |
| Race (African Americans/Caucasians) | 107/89 | 38/24 | 0.44 b |
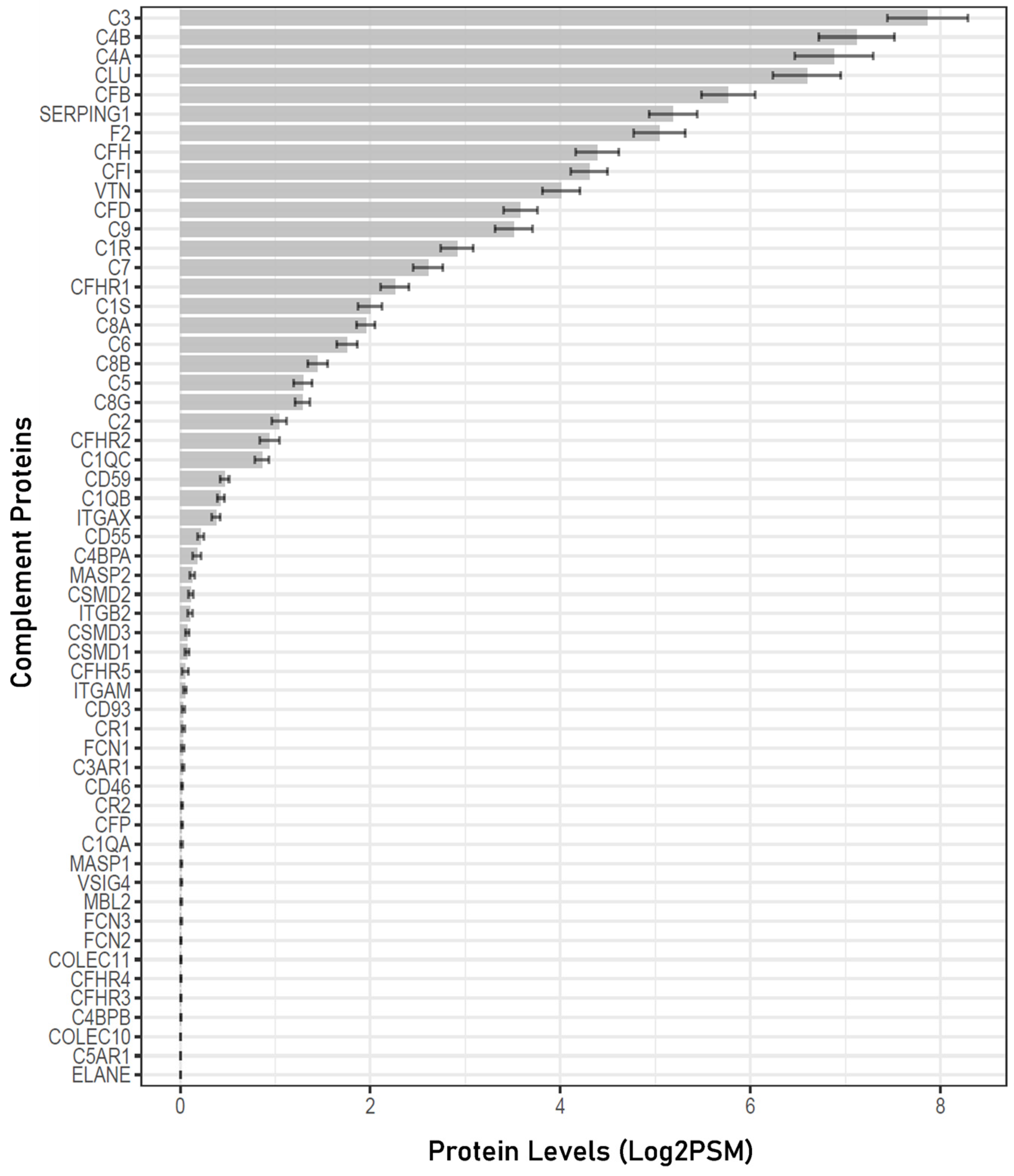
| UniProt ID | Gene Symbol | Description | Mean PSM Value (±SD) | Proportion of Samples Detected (%) |
|---|---|---|---|---|
| Activation Components | ||||
| P01024 | C3 | Complement C3 | 227.59 ± 68.1 | 100.0 |
| P0C0L5 | C4B | Complement C4B | 134.07 ± 64.43 | 96.5 |
| P0C0L4 | C4A | Complement C4-A | 112.39 ± 77.95 | 79.8 |
| P00751 | CFB | Complement factor B | 52.12 ± 13.43 | 100 |
| P00746 | CFD | Complement factor D | 11.32 ± 6.3 | 99.2 |
| P02748 | C9 | Complement component C9 | 11.03 ± 8.76 | 93.8 |
| P00736 | C1R | Complement C1r subcomponent | 6.92 ± 5.76 | 85.7 |
| P10643 | C7 | Complement component C7 | 5.14 ± 4.49 | 91.5 |
| P09871 | C1S | Complement C1s subcomponent | 3.26 ± 3.33 | 76.4 |
| P07357 | C8A | Complement component C8 alpha chain | 3.13 ± 2.59 | 90.3 |
| P13671 | C6 | Complement component C6 | 2.57 ± 2.72 | 74.8 |
| P07358 | C8B | Complement component C8 beta chain | 1.91 ± 2.62 | 54.7 |
| P07360 | C8G | Complement component C8 gamma chain | 1.58 ± 1.63 | 65.9 |
| P01031 | C5 | Complement C5 | 1.55 ± 2.16 | 58.9 |
| P06681 | C2 | Complement C2 | 1.11 ± 1.43 | 54.7 |
| P02747 | C1QC | Complement C1q subcomponent subunit C | 0.81 ± 1.2 | 43.0 |
| P02746 | C1QB | Complement C1q subcomponent subunit B | 0.36 ± 0.6 | 29.8 |
| O00187 | MASP2 | Mannan-binding lectin serine protease 2 | 0.09 ± 0.31 | 8.1 |
| O00602 | FCN1 | Ficolin-1 | ND | ND |
| P48740 | MASP1 | Mannan-binding lectin serine protease 1 | ND | ND |
| P02745 | C1QA | Complement C1q subcomponent subunit A | ND | ND |
| P27918 | CFP | Properdin | ND | ND |
| O75636 | FCN3 | Ficolin-3 | ND | ND |
| P11226 | MBL2 | Mannose-binding protein C | ND | ND |
| Q9BWP8 | COLEC11 | Collectin subfamily member 11 | ND | ND |
| Q15485 | FCN2 | Ficolin-2 | ND | ND |
| Q9Y6Z7 | COLEC10 | Collectin subfamily member 10 | ND | ND |
| Regulators and Receptors | ||||
| P10909 | CLU | Clusterin | 95.22 ± 40.97 | 100.0 |
| P05155 | SERPING1 | Plasma protease C1 inhibitor | 35.57 ± 12.07 | 100.0 |
| P00734 | F2 | Prothrombin | 31.86 ± 15.52 | 100.0 |
| P08603 | CFH | Complement factor H | 21.11 ± 12.14 | 98.1 |
| P05156 | CFI | Complement factor I | 19.12 ± 7.18 | 100.0 |
| P04004 | VTN | Vitronectin | 14.92 ± 6.39 | 98.1 |
| Q03591 | CFHR1 | Complement factor H-related protein 1 | 3.85 ± 4.28 | 54.7 |
| P36980 | CFHR2 | Complement factor H-related protein 2 | 0.86 ± 1.98 | 20.9 |
| P13987 | CD59 | CD59 glycoprotein | 0.38 ± 0.7 | 29.1 |
| P20702 | ITGAX | Integrin alpha-X | 0.31 ± 0.68 | 20.5 |
| P08174 | CD55 | Complement decay-accelerating factor | 0.17 ± 0.48 | 13.6 |
| P04003 | C4BPA | C4b-binding protein alpha chain | 0.12 ± 0.64 | 6.6 |
| Q7Z408 | CSMD2 | CUB and sushi domain-containing protein 2 | 0.08 ± 0.3 | 6.6 |
| P05107 | ITGB2 | Integrin beta-2 | 0.07 ± 0.3 | 6.2 |
| Q7Z407 | CSMD3 | CUB and sushi domain-containing protein 3 | ND | ND |
| Q96PZ7 | CSMD1 | CUB and sushi domain-containing protein 1 | ND | ND |
| P11215 | ITGAM | Integrin alpha-M | ND | ND |
| Q9BXR6 | CFHR5 | Complement factor H-related protein 5 | ND | ND |
| P17927 | CR1 | Complement receptor type 1 | ND | ND |
| Q9NPY3 | CD93 | Complement component C1q receptor | ND | ND |
| Q16581 | C3AR1 | C3a anaphylatoxin chemotactic receptor | ND | ND |
| P20023 | CR2 | Complement receptor type 2 | ND | ND |
| P15529 | CD46 | Membrane cofactor protein | ND | ND |
| Q9Y279 | VSIG4 | V-set and immunoglobulin domain-containing protein 4 | ND | ND |
| P20851 | C4BPB | C4b-binding protein beta chain | ND | ND |
| Q92496 | CFHR4 | Complement factor H-related protein 4 | ND | ND |
| Q02985 | CFHR3 | Complement factor H-related protein 3 | ND | ND |
| P08246 | ELANE | Neutrophil elastase | ND | ND |
| P21730 | C5AR1 | Complement C5a receptor 1 | ND | ND |
| Gene Symbol | Description | Overall | African American | Caucasian | |||
|---|---|---|---|---|---|---|---|
| FC | p-Value | FC | p-Value | FC | p-Value | ||
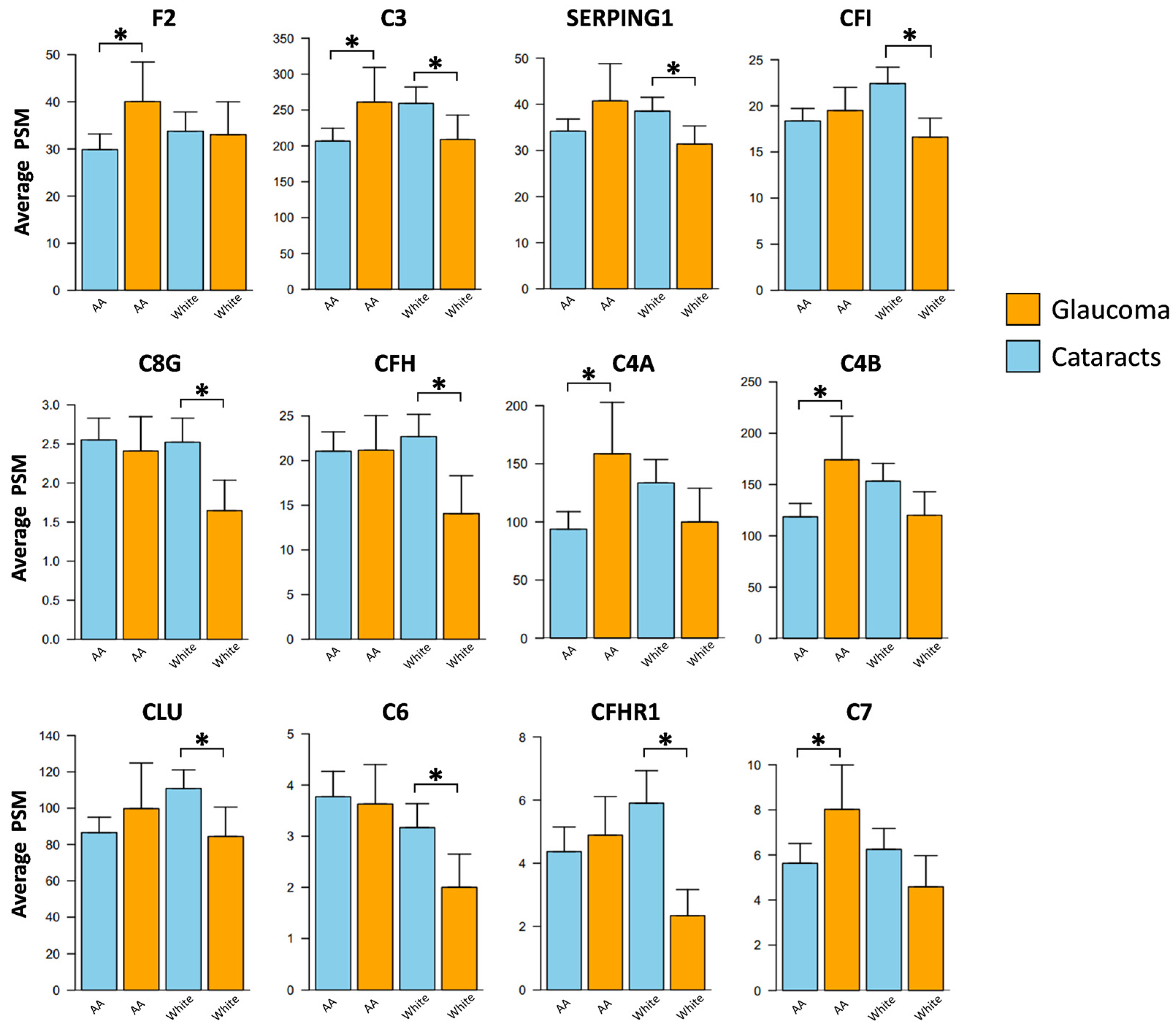
| C4A | Complement C4-A | 1.44 | 0.210 | 2.33 | 0.027 * | 0.72 | 0.452 |
| C4B | Complement C4B | 1.30 | 0.074 | 1.69 | 0.015 * | 0.89 | 0.517 |
| F2 | Prothrombin | 1.19 | 0.046 * | 1.31 | 0.023 * | 1.03 | 0.811 |
| C7 | Complement component C7 | 1.06 | 0.576 | 1.38 | 0.025 * | 0.72 | 0.057 |
| C3 | Complement C3 | 1.03 | 0.607 | 1.21 | 0.027 * | 0.82 | 0.038 * |
| SERPING1 | Plasma protease C1 inhibitor | 1.00 | 0.946 | 1.13 | 0.114 | 0.83 | 0.015 * |
| CFI | Complement factor I | 0.93 | 0.261 | 1.06 | 0.490 | 0.77 | 0.007 * |
| CLU | Clusterin | 0.93 | 0.325 | 1.05 | 0.626 | 0.77 | 0.021 * |
| CFHR1 | Complement factor H-related protein 1 | 0.85 | 0.273 | 1.25 | 0.257 | 0.48 | 0.002 * |
| C8G | Complement component C8 gamma chain | 0.83 | 0.026 * | 0.94 | 0.600 | 0.67 | 0.004 * |
| C6 | Complement component C6 | 0.81 | 0.032 * | 0.95 | 0.716 | 0.63 | 0.002 * |
| CFH | Complement factor H | 0.77 | 0.024 * | 0.95 | 0.702 | 0.57 | 0.003 * |
| Gene Symbol | Description | Overall | Male | Female | |||
|---|---|---|---|---|---|---|---|
| FC | p-Value | FC | p-Value | FC | p-Value | ||
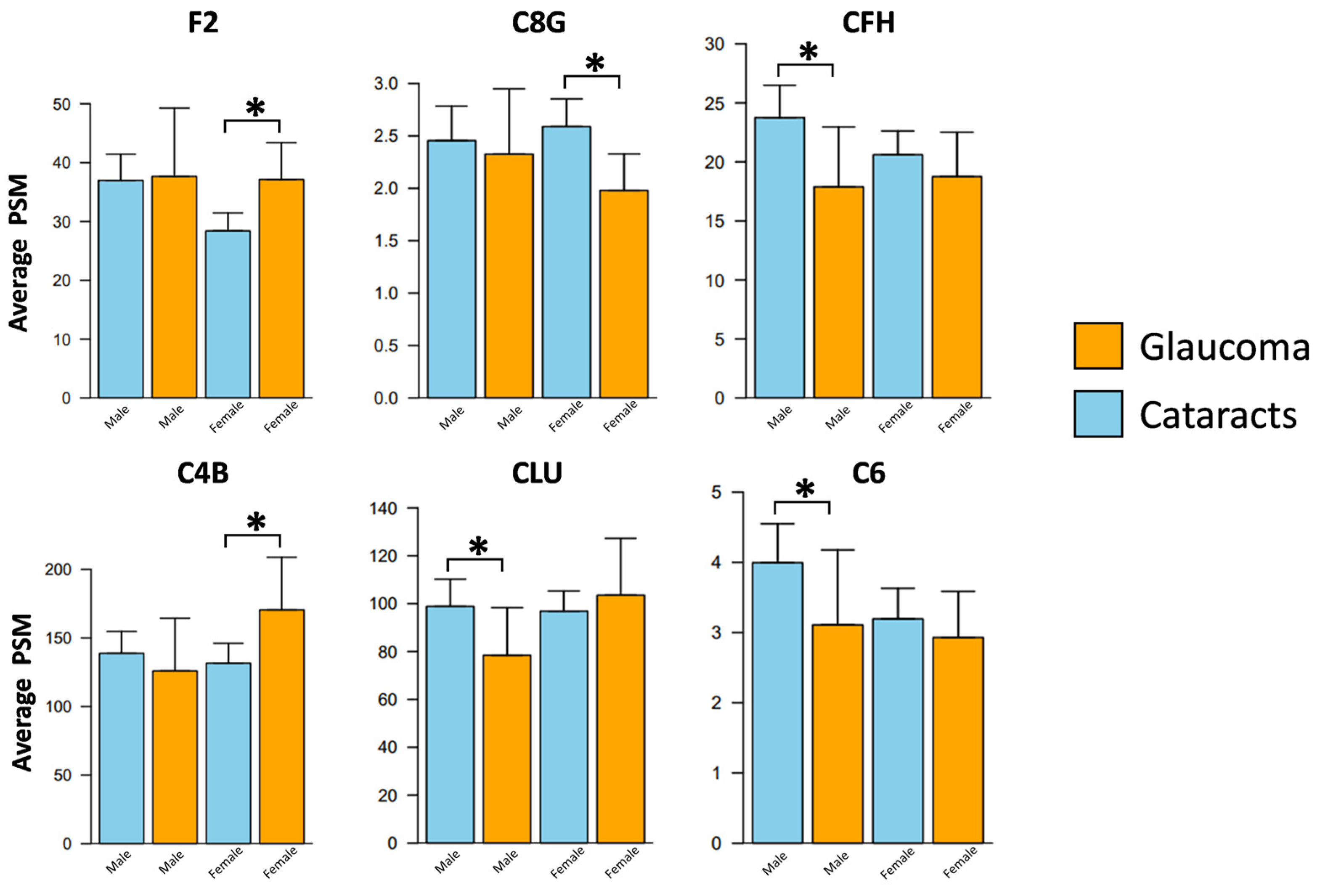
| C4B | Complement C4B | 1.30 | 0.074 | 0.96 | 0.853 | 1.56 | 0.030 * |
| F2 | Prothrombin | 1.19 | 0.046 * | 0.94 | 0.630 | 1.37 | 0.007 * |
| CLU | Clusterin | 0.93 | 0.325 | 0.78 | 0.046 * | 1.03 | 0.781 |
| C8G | Complement component C8 gamma chain | 0.83 | 0.026 * | 0.88 | 0.388 | 0.78 | 0.025 * |
| C6 | Complement component C6 | 0.81 | 0.032 * | 0.67 | 0.009 * | 0.92 | 0.519 |
| CFH | Complement factor H | 0.77 | 0.024 * | 0.63 | 0.015 * | 0.87 | 0.339 |
| Gene Symbol | Description | PSD Glaucoma | VFI Glaucoma | ||
|---|---|---|---|---|---|
| Correlation Coefficient (R) | p-Value | Correlation Coefficient (R) | p-Value | ||
| C5 | Complement C5 | 0.47 | 0.002 * | −0.31 | 0.125 |
| C7 | Complement component C7 | 0.43 | 0.006 * | −0.64 | 0.0006 * |
| C1R | Complement C1r subcomponent | 0.39 | 0.014 * | −0.52 | 0.008 * |
| C4B | Complement C4B | 0.38 | 0.019 * | −0.46 | 0.020 * |
| C8A | Complement component C8 alpha chain | 0.37 | 0.020 * | −0.29 | 0.162 |
| F2 | Prothrombin | 0.36 | 0.024 * | −0.49 | 0.014 * |
| C4A | Complement C4-A | 0.33 | 0.038 * | −0.44 | 0.027 * |
| C9 | Complement component C9 | 0.30 | 0.063 | −0.44 | 0.027 * |
| C8G | Complement component C8 gamma chain | 0.24 | 0.143 | −0.54 | 0.006 * |
| Gene Symbol | Cup Area (mm2) | Cup Volume (mm3) | Maximum Cup Depth (mm) | Mean Cup Depth (mm) | Disc Area (mm2) | CD Area Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corr. Coeff. (R) | p-Value | Corr. Coeff. (R) | p-Value | Corr. Coeff. (R) | p-Value | Corr. Coeff. (R) | p-Value | Corr. Coeff. (R) | p-Value | Corr. Coeff. (R) | p-Value | |
| CFI | −0.33 | 6 × 10−5 * | −0.33 | 5 × 10−5 * | −0.27 | 0.001 * | −0.23 | 0.005 * | −0.19 | 0.020 * | −0.28 | 0.0004 * |
| VTN | −0.26 | 0.001 * | −0.29 | 0.0005 * | −0.22 | 0.009 * | −0.15 | 0.061 | −0.19 | 0.021 * | −0.19 | 0.022 * |
| CLU | −0.26 | 0.002 * | −0.25 | 0.002 * | −0.15 | 0.066 | −0.13 | 0.121 | −0.09 | 0.291 | −0.21 | 0.010 * |
| C3 | −0.22 | 0.007 * | −0.26 | 0.002 * | −0.22 | 0.007 * | −0.19 | 0.022 * | −0.17 | 0.045 * | −0.19 | 0.022 * |
| CFH | −0.20 | 0.015 * | −0.23 | 0.005 * | −0.22 | 0.008 * | −0.20 | 0.014 * | −0.14 | 0.105 | −0.21 | 0.009 * |
| CFHR1 | −0.17 | 0.036 * | −0.20 | 0.018 * | −0.18 | 0.030 * | −0.17 | 0.032 * | −0.18 | 0.034 * | −0.16 | 0.049 * |
| C4B | −0.15 | 0.074 | −0.19 | 0.023 * | −0.15 | 0.074 | −0.15 | 0.062 | −0.09 | 0.307 | −0.12 | 0.143 |
| C2 | −0.15 | 0.065 | −0.16 | 0.049 * | −0.13 | 0.116 | −0.13 | 0.116 | −0.11 | 0.188 | −0.12 | 0.146 |
| F2 | −0.14 | 0.093 | −0.18 | 0.034 * | −0.20 | 0.015 * | −0.16 | 0.054 | −0.08 | 0.358 | −0.10 | 0.213 |
| C5 | −0.08 | 0.358 | −0.11 | 0.194 | −0.17 | 0.038 * | −0.13 | 0.102 | −0.14 | 0.083 | −0.05 | 0.533 |
| C8B | −0.08 | 0.360 | −0.11 | 0.171 | −0.17 | 0.044 * | −0.13 | 0.111 | −0.07 | 0.395 | −0.05 | 0.504 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vashishtha, A.; Maina, S.W.; Altman, J.; Jones, G.; Lee, T.J.; Bollinger, K.E.; Ulrich, L.; Töteberg-Harms, M.; Estes, A.J.; Zhi, W.; et al. Complement System Proteins in the Human Aqueous Humor and Their Association with Primary Open-Angle Glaucoma. J. Pers. Med. 2023, 13, 1400. https://doi.org/10.3390/jpm13091400
Vashishtha A, Maina SW, Altman J, Jones G, Lee TJ, Bollinger KE, Ulrich L, Töteberg-Harms M, Estes AJ, Zhi W, et al. Complement System Proteins in the Human Aqueous Humor and Their Association with Primary Open-Angle Glaucoma. Journal of Personalized Medicine. 2023; 13(9):1400. https://doi.org/10.3390/jpm13091400
Chicago/Turabian StyleVashishtha, Ayushi, Sharon W. Maina, Jeremy Altman, Garrett Jones, Tae Jin Lee, Kathryn E. Bollinger, Lane Ulrich, Marc Töteberg-Harms, Amy J. Estes, Wenbo Zhi, and et al. 2023. "Complement System Proteins in the Human Aqueous Humor and Their Association with Primary Open-Angle Glaucoma" Journal of Personalized Medicine 13, no. 9: 1400. https://doi.org/10.3390/jpm13091400
APA StyleVashishtha, A., Maina, S. W., Altman, J., Jones, G., Lee, T. J., Bollinger, K. E., Ulrich, L., Töteberg-Harms, M., Estes, A. J., Zhi, W., Sharma, S., & Sharma, A. (2023). Complement System Proteins in the Human Aqueous Humor and Their Association with Primary Open-Angle Glaucoma. Journal of Personalized Medicine, 13(9), 1400. https://doi.org/10.3390/jpm13091400






