Analysis of Degenerative and Isthmic Lumbar Spondylolisthesis from the Difference of Pelvic Parameters and the Degree of Degeneration through Imaging Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Imaging Procedures
2.4. Image Analysis
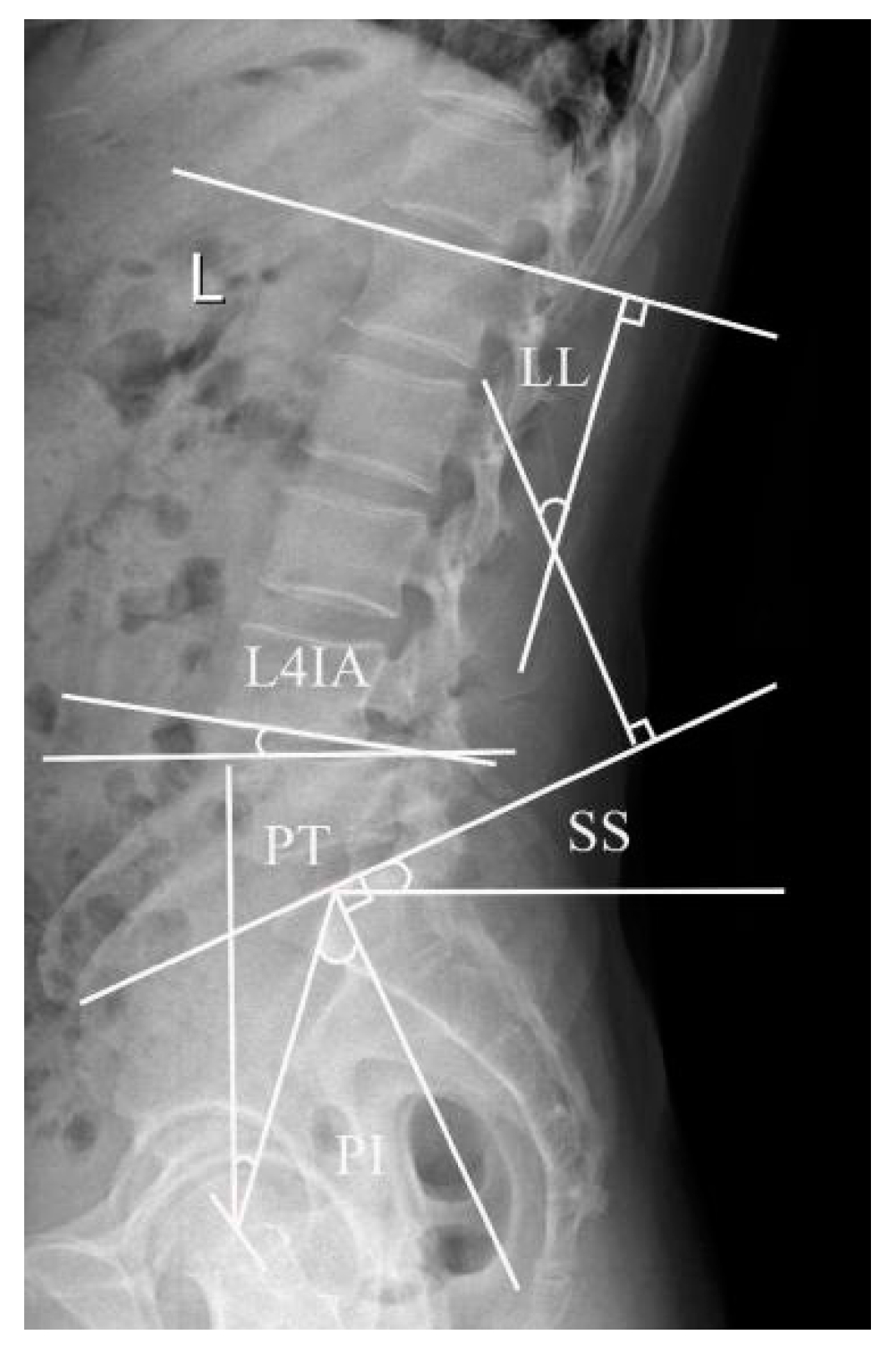
2.4.1. Lumbar Lordosis Angle, L4 Inclination Angle, and Pelvis-Related Parameters
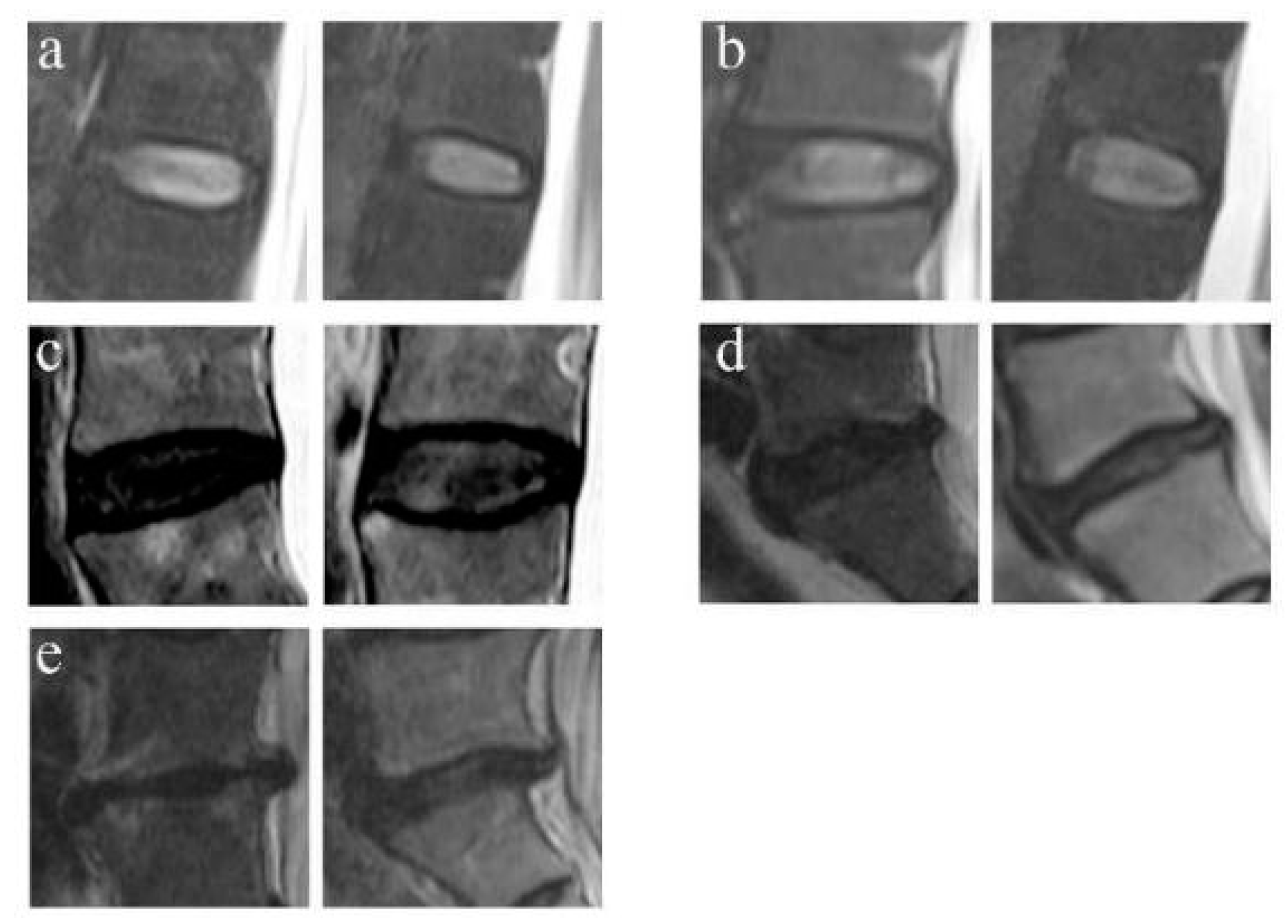
2.4.2. Disc Degeneration Grading
2.4.3. FJOA Grading
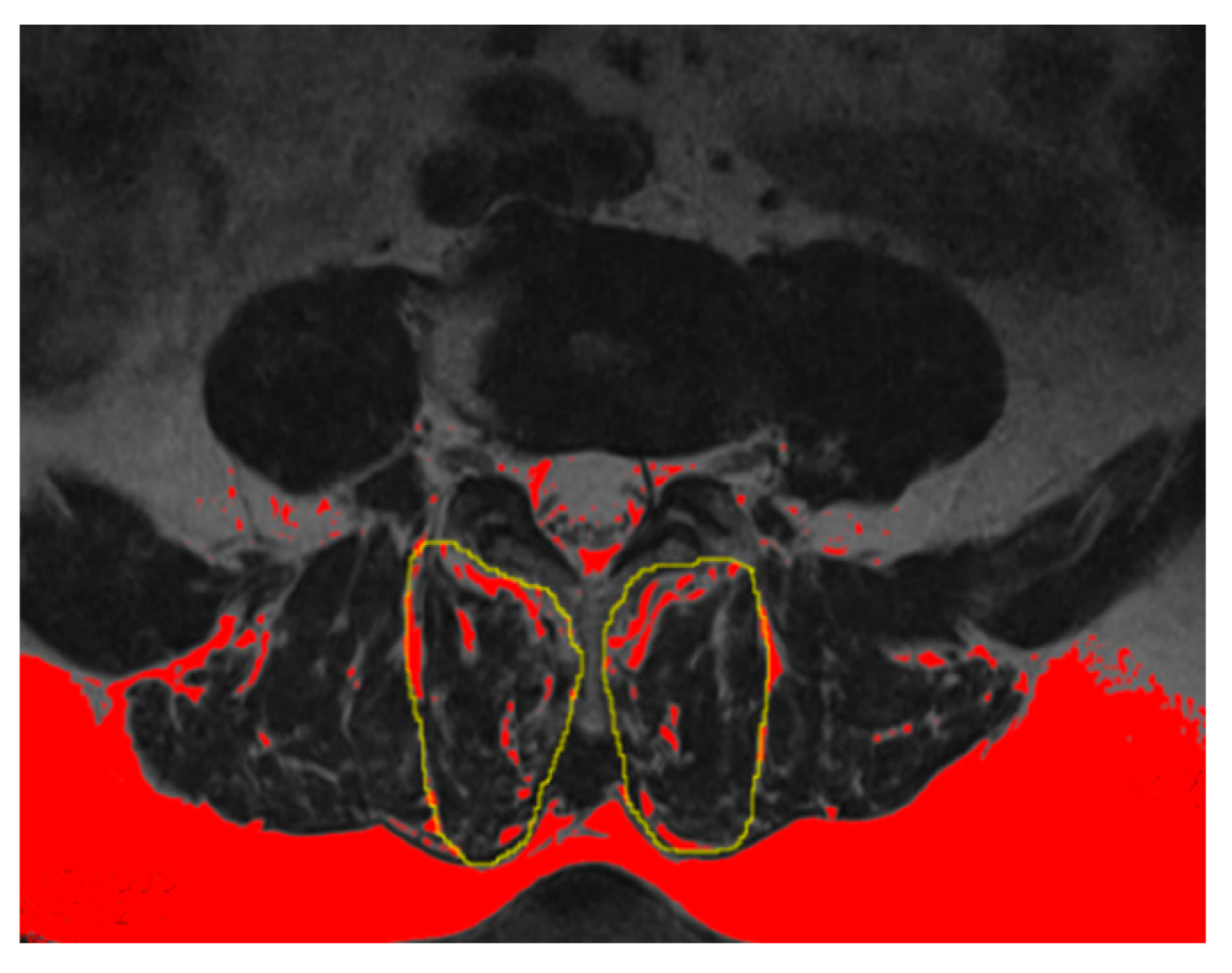
2.4.4. Evaluation of PVM Change
2.5. Statistical Analysis
3. Results
3.1. Lumbar Lordosis Angle, L4 Inclination Angle, and Pelvis-Related Parameters
3.2. Disc Degeneration Grading
3.2.1. FJOA Grading
3.2.2. Evaluation of PVM Change
3.2.3. Logistic Regression Analysis of DLS and ILS Predictors
4. Discussion
4.1. Degenerative Lumbar Spondylolisthesis and Isthmic Spondylolisthesis
4.2. Spinopelvic Parameters
4.3. Disc Degeneration
4.4. Facet Joint Osteoarthritis
4.5. Percentage of the Fat Infiltration Area (%FIA) of the Multifidus Muscle (MM)
4.6. Correlation between Different Imaging Parameters in the Two Groups
4.7. Possible Risk Factors for DLS and ILS
4.8. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, Z.; Yan, J.; Yang, Y.; Xu, Q.; Jin, Q. A Review of the Main Classifications of Lumbar Spondylolisthesis. World Neurosurg. 2023, 171, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Sharma, V.; Robinson, L.C.; Mummaneni, P.V. Summary of Guidelines for the Treatment of Lumbar Spondylolisthesis. Neurosurg. Clin. N. Am. 2019, 30, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Guigui, P.; Ferrero, E. Surgical treatment of degenerative spondylolisthesis. Orthop. Traumatol. Surg. Res. 2017, 103, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Bydon, M.; Alvi, M.A.; Goyal, A. Degenerative Lumbar Spondylolisthesis: Definition, Natural History, Conservative Management, and Surgical Treatment. Neurosurg. Clin. N. Am. 2019, 30, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, D.S.; Baisden, J.; Mazanec, D.J.; Patel, R.D.; Bess, R.S.; Burton, D.; Chutkan, N.B.; Cohen, B.A.; Crawford, C.H., 3rd. Guideline summary review: An evidence-based clinical guideline for the diagnosis and treatment of adult isthmic spondylolisthesis. Spine J. 2016, 16, 1478–1485. [Google Scholar] [CrossRef]
- Chen, I.R.; Wei, T.S. Disc height and lumbar index as independent predictors of degenerative spondylolisthesis in middle-aged women with low back pain. Spine 2009, 34, 1402–1409. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lyu, B.; Li, H.; Wang, S.Y. Effect of spinopelvic sagittal parameters and facet joint angle on degenerative lumbar spondylolisthesis. Zhongguo Gu Shang 2021, 34, 1016–1019. (In Chinese) [Google Scholar]
- Labelle, H.; Mac-Thiong, J.M.; Roussouly, P. Spino-pelvic sagittal balance of spondylolisthesis: A review and classification. Eur. Spine J. 2011, 20 (Suppl. S5), 641–646. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Pathria, M.; Sartoris, D.J.; Resnick, D. Osteoarthritis of the facet joints: Accuracy of oblique radiographic assessment. Radiology 1987, 164, 227–230. [Google Scholar] [CrossRef]
- Han, G.; Zou, D.; Li, X.; Zhang, S.; Li, Z.; Zhou, S.; Li, W.; Sun, Z.; Li, W. Can fat infiltration in the multifidus muscle be a predictor of postoperative symptoms and complications in patients undergoing lumbar fusion for degenerative lumbar spinal stenosis? A case-control study. J. Orthop. Surg. Res. 2022, 17, 289. [Google Scholar] [CrossRef] [PubMed]
- Deckers, K.; De Smedt, K.; Mitchell, B.; Vivian, D.; Russo, M.; Georgius, P.; Green, M.; Vieceli, J.; Eldabe, S.; Gulve, A.; et al. New Therapy for Refractory Chronic Mechanical Low Back Pain-Restorative Neurostimulation to Activate the Lumbar Multifidus: One Year Results of a Prospective Multicenter Clinical Trial. Neuromodulation 2018, 21, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhang, J.; Ding, W.; Yang, S.; Yang, D.; Ma, L.; Zhang, J. Abnormal change of paravertebral muscle in adult degenerative scoliosis and its association with bony structural parameters. Eur. Spine J. 2019, 28, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Mohile, N.V.; Kuczmarski, A.S.; Lee, D.; Warburton, C.; Rakoczy, K.; Butler, A.J. Spondylolysis and Isthmic Spondylolisthesis: A Guide to Diagnosis and Management. J. Am. Board. Fam. Med. 2022, 35, 1204–1216. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Li, H.; Wei, D.; Miao, J.; Xia, Q. Vertebral three-dimensional motion characteristics of adjacent segments in patients with isthmic spondylolisthesis in vivo. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 1560–1566. (In Chinese) [Google Scholar]
- Le Huec, J.C.; Thompson, W.; Mohsinaly, Y.; Barrey, C.; Faundez, A. Sagittal balance of the spine. Eur. Spine J. 2019, 28, 1889–1905. [Google Scholar] [CrossRef]
- Bao, H.; Liabaud, B.; Varghese, J.; Lafage, R.; Diebo, B.G.; Jalai, C.; Ramchandran, S.; Poorman, G.; Errico, T.; Zhu, F.; et al. Lumbosacral stress and age may contribute to increased pelvic incidence: An analysis of 1625 adults. Eur. Spine J. 2018, 27, 482–488. [Google Scholar] [CrossRef]
- Cecchinato, R.; Redaelli, A.; Martini, C.; Morselli, C.; Villafañe, J.H.; Lamartina, C.; Berjano, P. Long fusions to S1 with or without pelvic fixation can induce relevant acute variations in pelvic incidence: A retrospective cohort study of adult spine deformity surgery. Eur. Spine J. 2017, 26 (Suppl. S4), 436–441. [Google Scholar] [CrossRef]
- Beyer, G.; Khalifé, M.; Lafage, R.; Yang, J.; Elysee, J.; Frangella, N.; Steinmetz, L.; Ge, D.; Varlotta, C.; Stekas, N.; et al. Pelvic Compensation in Sagittal Malalignment: How Much Retroversion Can the Pelvis Accommodate? Spine 2020, 45, E203–E209. [Google Scholar] [CrossRef]
- Li, Y.; Hresko, M.T. Radiographic analysis of spondylolisthesis and sagittal spinopelvic deformity. J. Am. Acad. Orthop. Surg. 2012, 20, 194–205. [Google Scholar] [CrossRef]
- Bederman, S.S.; Farhan, S.; Hu, X.; Lieberman, I.H.; Belanger, T.A.; Musa, A.; Eichler, M.C. Sagittal Spinal and Pelvic Parameters in Patients With Scheuermann’s Disease: A Preliminary Study. Int. J. Spine Surg. 2019, 13, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Kong, C.; Sun, S.; Sun, X.; Li, X.; Lu, S. Predictors of L4–L5 Degenerative Lumbar Spondylolisthesis: L4 Inclination Angle and Facet Joint Angle. World Neurosurg. 2019, 130, e680–e686. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Kim, S.M. Difference of Sagittal Spinopelvic Alignments between Degenerative Spondylolisthesis and Isthmic Spondylolisthesis. J. Korean Neurosurg. Soc. 2013, 53, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.S.; Bridwell, K.H.; Rhee, J.M.; Lenke, L.G. Correlation of pelvic incidence with low- and high-grade isthmic spondylolisthesis. Spine 2002, 27, 2026–2029. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Raja, S.N. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology 2007, 106, 591–614. [Google Scholar] [CrossRef]
- Inoue, N.; Orías, A.A.E.; Segami, K. Biomechanics of the Lumbar Facet Joint. Spine Surg. Relat. Res. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Faur, C.; Patrascu, J.M.; Haragus, H.; Anglitoiu, B. Correlation between multifidus fatty atrophy and lumbar disc degeneration in low back pain. BMC Musculoskelet. Disord. 2019, 20, 414. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Urquhart, D.M.; Wang, Y.; Wluka, A.E.; Wijethilake, P.; O’Sullivan, R.; Cicuttini, F.M. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J. 2015, 15, 1593–1601. [Google Scholar] [CrossRef]
- Hofste, A.; Soer, R.; Hermens, H.J.; Wagner, H.; Oosterveld, F.G.J.; Wolff, A.P.; Groen, G.J. Inconsistent descriptions of lumbar multifidus morphology: A scoping review. BMC Musculoskelet. Disord. 2020, 21, 312. [Google Scholar] [CrossRef]
- Ohyama, S.; Aoki, Y.; Inoue, M.; Nakajima, T.; Sato, Y.; Fukuchi, H.; Sakai, T.; Ochi, S.; Yanagawa, N.; Ohtori, S. The Quantity and Quality of Lumbar Muscles and Lumbopelvic Parameters in Patients With Degenerative Spondylolisthesis. Cureus 2021, 13, e18428. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Wang, J.D.; Lin, R.M.; Lin, C.J.; Huang, K.Y. Adjacent disc and facet joint degeneration in young adults with low-grade spondylolytic spondylolisthesis: A magnetic resonance imaging study. J. Formos. Med. Assoc. 2015, 114, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.W.; Simpson, A.K. High-Grade Lumbar Spondylolisthesis. Neurosurg. Clin. N. Am. 2019, 30, 291–298. [Google Scholar] [CrossRef] [PubMed]

| Variable | Control Group | DLS Group | ILS Group | Statistics | p-Value |
|---|---|---|---|---|---|
| Cases | 164 | 164 | 161 | ||
| Male/female | 59/105 | 49/115 | 53/108 | χ2 = 1.381 | 0.501 |
| Age (years) | 61.0 ± 8.7 | 62.3 ± 6.3 | 60.5 ± 6.9 | t = 2.588 | 0.076 |
| BMI (kg/m2) | 24.75 ± 2.38 | 24.02 ± 2.72 | 24.51 ± 4.13 | t = 2.012 | 0.1348 |
| Spinopelvic Parameters | Control Group | DLS Group | ILS Group | p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| PI (°) | 40.13 ± 8.72 | 51.91 ± 12.23 | 53.28 ± 11.12 | <0.001 * | <0.001 * | 0.29 |
| SS (°) | 26.33 ± 6.96 | 22.28 ± 7.25 | 30.20 ± 7.95 | <0.001 * | <0.001 * | <0.001 * |
| PT (°) | 13.81 ± 8.87 | 29.64 ± 11.37 | 23.07 ± 8.60 | <0.001 * | <0.001 * | <0.001 * |
| LL (°) | 44.40 ± 11.79 | 39.34 ± 8.57 | 55.16 ± 12.31 | <0.001 * | <0.001 * | <0.001 * |
| L4IA (°) | 6.27 ± 2.15 | 14.87 ± 4.02 | 11.89 ± 8.59 | <0.001 * | <0.001 * | <0.001 * |
| DD | Control Group | DLS Group | ILS Group | Statistics | p-Value |
|---|---|---|---|---|---|
| Grade 1 | 8 | 0 | 0 | Z1 = 5.90 Z2 = 11.18 Z3 = 7.79 | p1 < 0.001 * p2 < 0.001 * p3 < 0.001 * |
| Grade 2 | 51 | 11 | 3 | ||
| Grade 3 | 65 | 82 | 24 | ||
| Grade 4 | 36 | 59 | 87 | ||
| Grade 5 | 4 | 12 | 47 |
| FJOA | Control Group | DLS Group | ILS Group | Statistics | p-Value |
|---|---|---|---|---|---|
| Grade 0 | 64 | 10 | 54 | Z1 = 15.86 Z2 = 10.17 Z3 = 4.06 | p1 < 0.001 * p2 < 0.001 * p3 < 0.001 * |
| Grade 1 | 237 | 89 | 85 | ||
| Grade 2 | 25 | 154 | 126 | ||
| Grade 3 | 2 | 75 | 57 |
| L4-5 | %FIA | Statistics | p-Value | ||
|---|---|---|---|---|---|
| Control Group | DLS Group | ILS Group | |||
| Mean | 19.71 ± 9.05 | 21.89 ± 7.51 | 25.29 ± 8.12 | t1 = 2.37 t2 = 5.85 t3 = 3.92 | p1 < 0.05 * p2 < 0.001 * p3 < 0.001 * |
| Risk | β | 95%CI | OR | p-Value |
|---|---|---|---|---|
| L4IA | 0.850 | 1.832–2.988 | 2.340 | 0.000 * |
| FJOA | 1.420 | 1.713–9.986 | 4.136 | 0.002 * |
| PT | 0.153 | 1.058–1.283 | 1.166 | 0.002 * |
| Risk | β | 95%CI | OR | p-Value |
|---|---|---|---|---|
| L4IA | 0.172 | 1.113–1.267 | 1.188 | 0.000 * |
| FJOA | 0.686 | 1.218–3.237 | 1.986 | 0.006 * |
| DD | 1.672 | 3.083–9.182 | 5.321 | 0.000 * |
| PT | 0.110 | 1.050–1.188 | 1.117 | 0.000 * |
| LL | 0.057 | 1.023–1.096 | 1.059 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Dai, G.; Cao, Y.; Duan, C. Analysis of Degenerative and Isthmic Lumbar Spondylolisthesis from the Difference of Pelvic Parameters and the Degree of Degeneration through Imaging Data. J. Pers. Med. 2023, 13, 1420. https://doi.org/10.3390/jpm13091420
Liu Z, Dai G, Cao Y, Duan C. Analysis of Degenerative and Isthmic Lumbar Spondylolisthesis from the Difference of Pelvic Parameters and the Degree of Degeneration through Imaging Data. Journal of Personalized Medicine. 2023; 13(9):1420. https://doi.org/10.3390/jpm13091420
Chicago/Turabian StyleLiu, Zhide, Guoyu Dai, Yong Cao, and Chunyue Duan. 2023. "Analysis of Degenerative and Isthmic Lumbar Spondylolisthesis from the Difference of Pelvic Parameters and the Degree of Degeneration through Imaging Data" Journal of Personalized Medicine 13, no. 9: 1420. https://doi.org/10.3390/jpm13091420
APA StyleLiu, Z., Dai, G., Cao, Y., & Duan, C. (2023). Analysis of Degenerative and Isthmic Lumbar Spondylolisthesis from the Difference of Pelvic Parameters and the Degree of Degeneration through Imaging Data. Journal of Personalized Medicine, 13(9), 1420. https://doi.org/10.3390/jpm13091420







