Chest Tube Placement of Secondary Tracheoesophageal Voice Prosthesis: Overcoming Challenging Anatomy in the Laryngectomy Patient
Abstract
1. Background
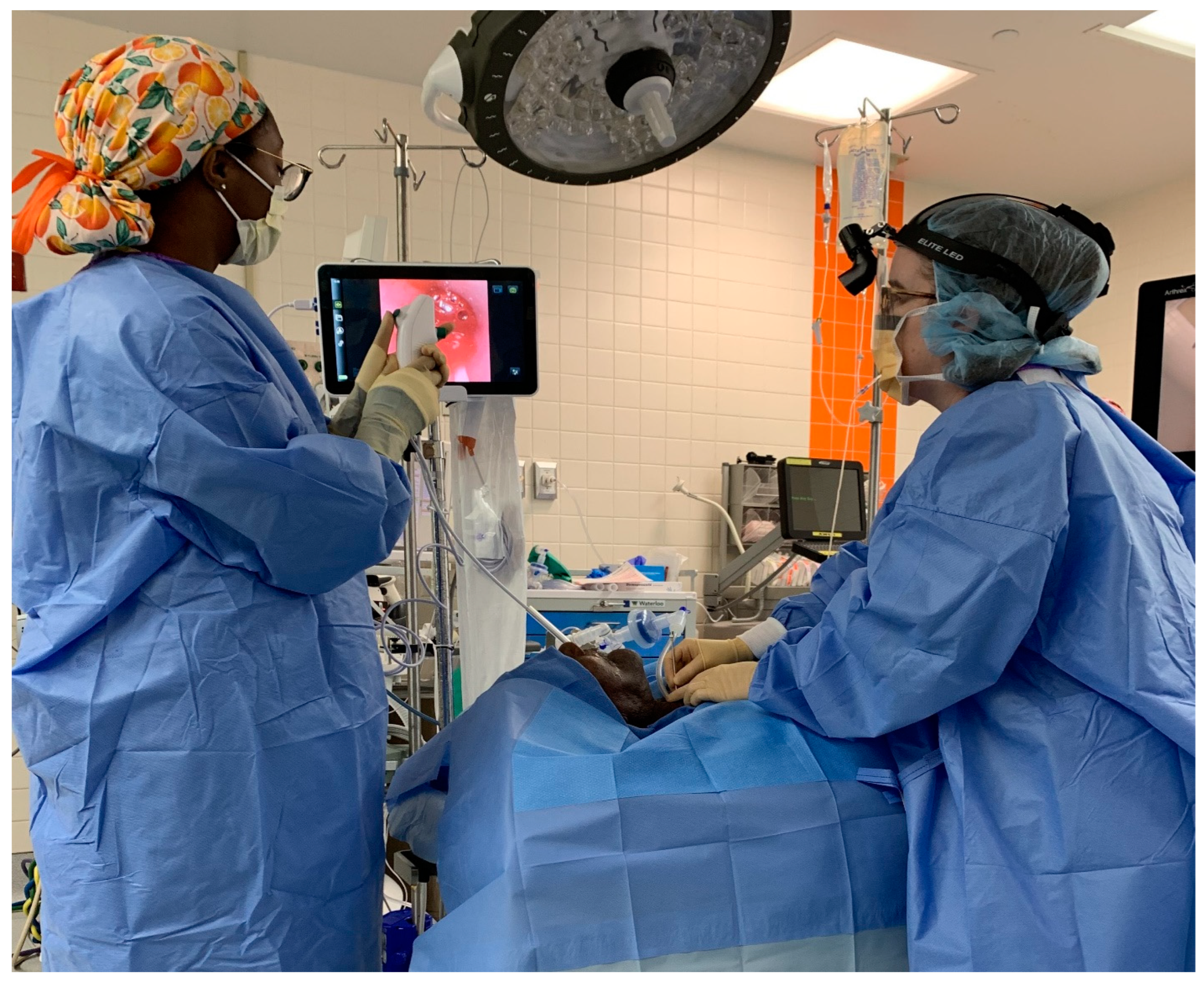
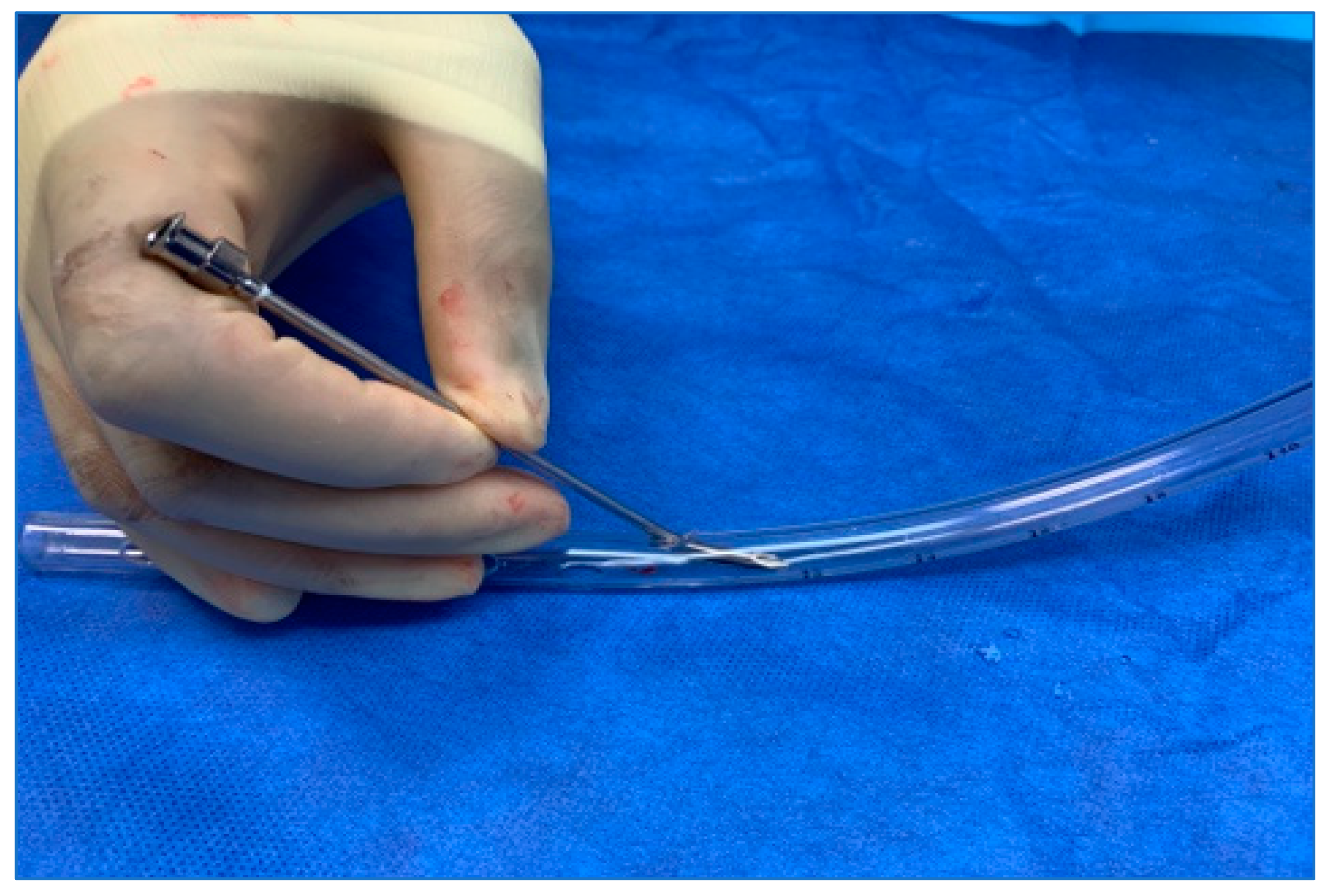
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Attieh, A.Y.; Searl, J.; Shahaltough, N.H.; Wreikat, M.M.; Lundy, D.S. Voice restoration following total laryngectomy by tracheoesophageal prosthesis: Effect on patients’ QOL and voice handicap in Jordan. Health Qual Life Outcomes 2008, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Clements, K.S.; Rassekh, C.H.; Seikaly, H.; Hokanson, J.A.; Calhoun, K.H. Communication After Laryngectomy: An Assessment of Patient Satisfaction. Arch. Otolaryngol. Neck Surg. 1997, 123, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.I.; Blom, E.D. An endoscopic technique for restoration of voice after laryngectomy. Ann. Otol. Rhinol. Laryngol. 1980, 89, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, P.D.; McMurran, A.E.L.; Banigo, A.; Shakeel, M.; Ah-See, K.W. Primary versus secondary tracheoesophageal puncture: Systematic review and meta-analysis. J. Laryngol. Otol. 2018, 132, 14–21. [Google Scholar] [CrossRef]
- Neto, J.C.B.; Dedivitis, R.A.; Aires, F.T.; Pfann, R.Z.; Matos, L.L.; Cernea, C.R. Comparison between Primary and Secondary Tracheoesophageal Puncture Prosthesis: A Systematic Review. ORL J. Otorhinolaryngol. Relat. Spec. 2017, 79, 222–229. [Google Scholar] [CrossRef]
- Chone, C.T.; Gripp, F.M.; Spina, A.L.; Crespo, A.N. Primary versus secondary tracheoesophageal puncture for speech rehabilitation in total laryngectomy: Long-term results with indwelling voice prosthesis. Otolaryngol. Head Neck Surg. 2005, 133, 89–93. [Google Scholar] [CrossRef]
- Gitomer, S.A.; Hutcheson, K.A.; Christianson, B.L.; Samuelson, M.B.; Barringer, D.A.; Roberts, D.B.; Hessel, A.C.; Weber, R.S.; Lewin, J.S.; Zafereo, M.E. Influence of timing, radiation, and reconstruction on complications and speech outcomes with tracheoesophageal puncture. Head Neck 2016, 38, 1765–1771. [Google Scholar] [CrossRef]
- Sinclair, C.F.; Rosenthal, E.L.; McColloch, N.L.; Magnuson, J.S.; Desmond, R.A.; Peters, G.E.; Carroll, W.R. Primary versus delayed tracheoe sophageal puncture for laryngopharyngectomy with free flap reconstruction. Laryngoscope 2011, 121, 1436–1440. [Google Scholar] [CrossRef]
- Emerick, K.S.; Tomycz, L.; Bradford, C.R.; Lyden, T.H.; Chepeha, D.B.; Wolf, G.T.; Teknos, T.N. Primary versus secondary tracheoesophageal puncture in salvage total laryngectomy following chemoradiation. Otolaryngol. Head Neck Surg. 2009, 140, 386–390. [Google Scholar] [CrossRef]
- Dowthwaite, S.A.; Penhearow, J.; Szeto, C.; Nichols, A.; Franklin, J.; Fung, K.; Yoo, J. Postlaryngectomy pharyngocutaneous fistula: Determining the risk of preoperative tracheostomy and primary tracheoesophageal puncture. J. Otolaryngol. Head Neck Surg. 2012, 41, 169–175. [Google Scholar]
- Parikh, S.R.; Irish, J.C.; Curran, A.J.; Gullane, P.J.; Brown, D.H.; Rotstein, L.E. Pharyngocutaneous fistulae in laryngectomy patients: The Toronto Hospital experience. J. Otolaryngol. 1998, 27, 136–140. [Google Scholar] [PubMed]
- Sayles, M.; Grant, D.G. Preventing pharyngo-cutaneous fistula in total laryngectomy: A systematic review and meta-analysis. Laryngoscope 2014, 124, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.; Lujan, J.S.; Xia, T.; Zender, C.; Maronian, N. Outcomes of tracheoesophageal puncture in twice-radiated patients. Am. J. Otolaryngol. 2019, 40, 102272. [Google Scholar] [CrossRef] [PubMed]
- Shires, C.B.; Latour, M.; Sebelik, M.; Dewan, K. The use of Montgomery salivary bypass tubes and pharyngocutaneous fistula following salvage laryngectomy. World J. Otorhinolaryngol.—Head Neck Surg. 2024, 10, 43–48. [Google Scholar] [CrossRef]
- Azar, S.S.; Shires, C.B.; Dewan, K.; Chhetri, D.K. Total tracheoesophageal puncture failure: A scoping review of patient characteristics and etiologies. Head Neck 2024. [Google Scholar] [CrossRef]
- Bianco, M.R.; Saita, V.; Occhiuzzi, F.; Modica, D.M.; Latella, D.; Azzolina, A.; Galfano, M.; Allegra, E. Long-Term Complications of Tracheoesophageal Voice Prosthesis. J. Clin. Med. 2024, 13, 1912. [Google Scholar] [CrossRef]
- Noordiana, S.H.; Fang, F.T.H.; Kamarudin, N.A.; Azman, M. Sealing the Breach: A Surgical Solution for Tracheoesophageal Fistula with a Simple Two-Layer Closure. Cureus 2024, 16, e61934. [Google Scholar] [CrossRef]
- Suehiro, A.; Honda, K.; Kishimoto, Y.; Iwanaga, K.; Fujimura, S.; Kawai, Y.; Kojima, T.; Hamaguchi, K.; Omori, K. Modified method for tracheoesophageal fistula closure in intractable cases. Auris Nasus Larynx 2024, 51, 774–778. [Google Scholar] [CrossRef]
- Escandón, J.M.; Mohammad, A.; Mathews, S.; Bustos, V.P.; Santamaría, E.; Chen, H.C.; Langstein, H.N.; Manrique, O.J. Definitive Closure of the Tracheoesophageal Puncture Site after Oncologic Laryngectomy: A Systematic Review and Meta-Analysis. Arch. Plast. Surg. 2022, 49, 617–632. [Google Scholar] [CrossRef]
- Yavuz, H.; Vural, O. Tracheoesophageal puncture closure with annular mucosal flap. Head Neck 2021, 43, 1705–1710. [Google Scholar] [CrossRef]
- Patel, R.S.; Mohr, T.; Hartman, C.; Stach, C.; Sikora, A.G.; Zevallos, J.P.; Sandulache, V.C. Tracheoesophageal Prosthesis Use Is Associated with Improved Overall Quality of Life in Veterans with Laryngeal Cancer. Ann. Otol. Rhinol. Laryngol. 2018, 127, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ringash, J.; Bernstein, L.J.; Devins, G.; Dunphy, C.; Giuliani, M.; Martino, R.; McEwen, S. Head and Neck Cancer Survivorship: Learning the Needs, Meeting the Needs. In Seminars in Radiation Oncology; W.B. Saunders: Philadelphia, PA, USA, 2018; Volume 28, pp. 64–74. [Google Scholar]
- Galli, A.; Giordano, L.; Biafora, M.; Tulli, M.; Di Santo, D.; Bussi, M. Voice prosthesis rehabilitation after total laryngectomy: Are satisfaction and quality of life maintained over time? Acta Otorhinolaryngol. Ital. 2019, 39, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Kearney, A.; Damrose, E.J. Tracheoesophageal fistula length decreases over time. Eur. Arch. Otorhinolaryngol. 2016, 273, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Goodman, J.F. Tracheoesophageal Voice Prosthesis Use and Maintenance in Laryngectomees. Int. Arch. Otorhinolaryngol. 2020, 24, e535–e538. [Google Scholar] [CrossRef]
- Lewin, J.S.; Baumgart, L.M.; Barrow, M.P.; Hutcheson, K.A. Device life of the trachesophageal voice prosthesis revisited. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 65–71. [Google Scholar] [CrossRef]
- Silver, F.M.; Gluckman, J.L.; Donegan, J.O. Operative complications of tracheoesophageal puncture. Laryngoscope 1985, 95, 1360–1362. [Google Scholar] [CrossRef]
- Andrews, J.C.; Mickel, R.A.; Monahan, G.P.; Hanson, D.G.; Ward, P.H. Major complications following tracheoesophageal puncture for voice rehabilitation. Laryngoscope 1987, 97, 562–567. [Google Scholar] [CrossRef]
- Padhya, T.A.; Athavale, S.M.; Morgan, J.M.; McCaffrey, T.V. An Alternative Approach for Secondary Tracheoesophageal Puncture in the Difficult Laryngectomy Neck. Laryngoscope 2008, 118, 266–269. [Google Scholar] [CrossRef]
- Mohr, R.M.; Paddock, B.H.; Boehler, J. An adaptation of tracheoesophageal puncture. Laryngoscope 1983, 93, 1086–1088. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, S.; Jabbour, J.; Lai, S.; Suruliraj, A.; Smith, M.; Riffat, F.; Devadas, M.; Liem, H.; Sritharan, N. A Multidisciplinary Approach to Secondary Tracheoesophageal Puncture for Voice Prosthesis Insertion Using Flexible Esophagoscopy. J. Voice 2022, 38, 1255.e1–1255.e8. [Google Scholar] [CrossRef]
- LeBert, B.; McWhorter, A.J.; Kunduk, M.; Walvekar, R.R.; Lewin, J.S.; Hutcheson, K.A.; Barringer, D.A.; Hessel, A.C.; Holsinger, F.C. Secondary Tracheoesophageal Puncture with In-Office Transnasal Esophagoscopy. Arch. Otolaryngol. Neck Surg. 2009, 135, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Spofford, B.; Jafek, B.; Barcz, D. An improved method for creating tracheoesophageal fistulas for Blom-Singer or Panje voice prostheses. Laryngoscope 1984, 94 Pt 1, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, A.J. Newer technique of tracheoesophageal fistula for vocal rehabilitation after total laryngectomy. Laryngoscope 1985, 95, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Thimmappa, V.; Jones, J.; Shires, C.; Sebelik, M. The Use of Ultrasound for Sizing Tracheoesophageal Speech Prostheses. Otolaryngol.–Head Neck Surg. 2016, 157, 1075–1078. [Google Scholar] [CrossRef]
- Shires, C.B.; Boughter, J.D., Jr.; Smith, A.; Sebelik, M.E. Head and Neck Ultrasound Utilization Rates: 2012 to 2019. OTO Open 2023, 7, e97. [Google Scholar] [CrossRef]
- Glazer, T.A.; Meraj, T.; Lyden, T.H.; Spector, M.E. In-Office Secondary Tracheoesophageal Puncture with Immediate Prosthesis Placement. Otolaryngol. Head Neck Surg. 2016, 155, 360–363. [Google Scholar] [CrossRef]
- Tkaczuk, A.T.; Taylor, R.J.; Wolf, J.S. A Novel Device for Placement of a Secondary Tracheoesophageal Voice Prosthesis: A Preliminary Feasibility Study. ORL J. Otorhinolaryngol. Relat. Spec. 2018, 80, 36–40. [Google Scholar] [CrossRef]
- Ricci, E.; Riva, G.; Dagna, F.; Seglie, E.; Cavalot, A. In-clinic secondary tracheoesophageal puncture and voice prosthesis placement in laryngectomees. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 349–352. [Google Scholar] [CrossRef]
- Wu, M.P.; Sullivan, C.; Deschler, D.G. Modified Technique for Retrograde Placement of the Tracheoesophageal Voice Prosthesis in the Office. Laryngoscope 2024, ahead of print. [Google Scholar] [CrossRef]
- Inhealth Technologies. Blom-Singer Indwelling Voice Prosthesis for Primary, Secondary, Replacement TEP Procedures: Medical Professional Instructoins for Use; Freudenberg Medical: Beverly, MA, USA, 2018. [Google Scholar]
- Atos Medical. Provox ActiValve: Clinician Instructions for Use; Atos Medical AB: Malmö, Sweden, 2020. [Google Scholar]
- Maniglia, A.J. Vocal rehabilitation after total laryngectomy: A flexible fiberoptic endoscopic technique for tracheoesophageal fistula. Laryngoscope 1982, 92, 1437–1439. [Google Scholar] [CrossRef]
- Abdul-Aziz, D.; Zenga, J.; Deschler, D.G. The Difficult Secondary Tracheoesophageal Puncture: A Technique for Safe Insertion. ORL J. Otorhinolaryngol. Relat. Spec. 2019, 81, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Scherl, C.; Kauffels, J.; Schützenberger, A.; Döllinger, M.; Bohr, C.; Dürr, S.; Fietkau, R.; Haderlein, M.; Koch, M.; Traxdorf, M.; et al. Secondary Tracheoesophageal Puncture After Laryngectomy Increases Complications with Shunt and Voice Prosthesis. Laryngoscope 2020, 130, E865–E873. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Schultz-Coulon, H.J. Management of complications after prosthetic voice rehabilitation. HNO 2000, 48, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Izdebski, K.; Reed, C.G.; Ross, J.C.; Hilsinger, R.L. Problems with tracheoesophageal fistula voice restoration in totally laryngectomized patients. A review of 95 cases. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 840–845. [Google Scholar] [CrossRef]





| Patient Characteristics (N = 76) | |
|---|---|
| Average age | 62 years |
| Average time from TL to TEP | 197 days |
| Sex (M:F) | 3.1:1.2 |
| Prior attempt with another technique | 74% (n = 56) |
| Prior XRT | 92% (n = 70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shires, C.B.; Schertzer, J.S.; Ottenstein, L.; Harris, T.; Sebelik, M.E. Chest Tube Placement of Secondary Tracheoesophageal Voice Prosthesis: Overcoming Challenging Anatomy in the Laryngectomy Patient. J. Pers. Med. 2024, 14, 1021. https://doi.org/10.3390/jpm14101021
Shires CB, Schertzer JS, Ottenstein L, Harris T, Sebelik ME. Chest Tube Placement of Secondary Tracheoesophageal Voice Prosthesis: Overcoming Challenging Anatomy in the Laryngectomy Patient. Journal of Personalized Medicine. 2024; 14(10):1021. https://doi.org/10.3390/jpm14101021
Chicago/Turabian StyleShires, Courtney B., Joseph S. Schertzer, Lauren Ottenstein, Tricia Harris, and Merry E. Sebelik. 2024. "Chest Tube Placement of Secondary Tracheoesophageal Voice Prosthesis: Overcoming Challenging Anatomy in the Laryngectomy Patient" Journal of Personalized Medicine 14, no. 10: 1021. https://doi.org/10.3390/jpm14101021
APA StyleShires, C. B., Schertzer, J. S., Ottenstein, L., Harris, T., & Sebelik, M. E. (2024). Chest Tube Placement of Secondary Tracheoesophageal Voice Prosthesis: Overcoming Challenging Anatomy in the Laryngectomy Patient. Journal of Personalized Medicine, 14(10), 1021. https://doi.org/10.3390/jpm14101021








