Increased Cadmium Load, Vitamin D Deficiency, and Elevated FGF23 Levels as Pathophysiological Factors Potentially Linked to the Onset of Acute Lymphoblastic Leukemia: A Review
Abstract
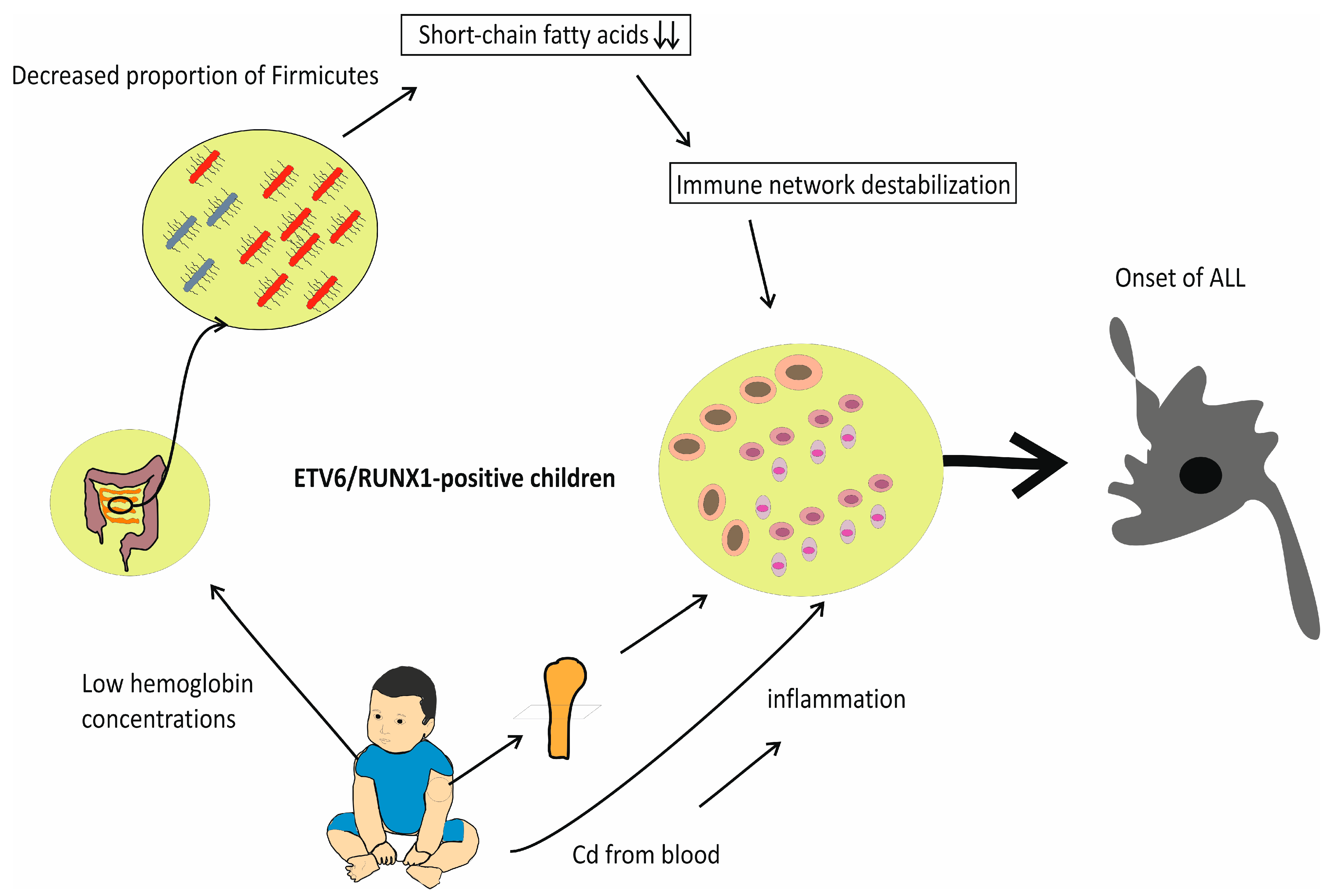
1. Introduction
2. Adverse Effects of IDA and Vitamin D Deficiency on Gut Microflora: Reduced Percentage of Firmicutes in the Stool of Individuals with ALL
3. Cd-Induced Toxicity in Bone Marrow
4. Maternal IDA during Pregnancy and Increased Risk of ALL in Children
5. Fibroblast Growth Factor 23 (FGF23) in Acute Leukemia: The IDA-FGF23-Vitamin D Deficiency Axis
6. Discussion with Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salah, R.W.; Hasab, A.A.H.; El-Nimr, N.A.; Tayel, D.I. The Prevalence and Predictors of Iron Deficiency Anemia among Rural Infants in Nablus Governorate. J. Res. Health Sci. 2018, 18, e00417. [Google Scholar] [PubMed]
- Chung, W.-S.; Lin, C.-L.; Lin, C.-L.; Kao, C.-H. Thalassaemia and risk of cancer: A population-based cohort study. J. Epidemiol. Community Health 2015, 69, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The role of gut microbiome in cancer genesis and cancer prevention. Health Sci. Rev. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Gordon, C.M.; Feldman, H.A.; Sinclair, L.; Williams, A.L.; Kleinman, P.K.; Perez-Rossello, J.; Cox, J.E. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch. Pediatr. Adolesc. Med. 2008, 162, 505–512. [Google Scholar] [CrossRef]
- Surve, S.; Chauhan, S.; Amdekar, Y.; Joshi, B. Vitamin D deficiency in Children: An update on its Prevalence, Therapeutics and Knowledge gaps. Indian J. Nutr. 2017, 4, 167. [Google Scholar]
- Mogire, R.M.; Muriuki, J.M.; Morovat, A.; Mentzer, A.J.; Webb, E.L.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Cutland, C.L.; Sirima, S.B.; et al. Vitamin D Deficiency and Its Association with Iron Deficiency in African Children. Nutrients 2022, 14, 1372. [Google Scholar] [CrossRef]
- Al-Zuhairy, S.H.; Darweesh, M.A.; Othman, M.A.; Al-Zuhairy, N.A.S. Vitamin D deficiency in young children with iron deficiency in Misan province, Iraq. J. Med. Life 2022, 15, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Kim, S.W.; Yoo, E.G.; Kim, M.K. Prevalence and risk factors for vitamin D deficiency in children with iron deficiency anemia. Korean J. Pediatr. 2012, 55, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef]
- Greaves, M.; Cazzaniga, V.; Ford, A. Can we prevent childhood Leukaemia? Leukemia 2021, 35, 1258–1264. [Google Scholar] [CrossRef]
- Peppas, I.; Ford, A.M.; Furness, C.L.; Greaves, M.F. Gut microbiome immaturity and childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2023, 23, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Garniasih, D.; Susanah, S.; Sribudiani, Y.; Hilmanto, D. The incidence and mortality of childhood acute lymphoblastic leukemia in Indonesia: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0269706. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, M.; Jam, N.; Karimi, M.; Shahriari, M.; Zareifar, S.; Zekavat, O.R.; Haghpanah, S.; Mottaghipisheh, H. The survival of childhood leukemia: An 8-year single-center experience. Cancer Rep. 2023, 6, e1784. [Google Scholar] [CrossRef] [PubMed]
- Kızılocak, H.; Okcu, F. Late Effects of Therapy in Childhood Acute Lymphoblastic Leukemia Survivors. Turk. J. Haematol. 2019, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021, 71, 101733. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef]
- Tun, H.M.; Konya, T.; Takaro, T.K.; Brook, J.R.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; et al. Exposure to household furry pets influences the gut microbiota of infants at 3–4 months following various birth scenarios. Microbiome 2017, 5, 40. [Google Scholar] [CrossRef]
- Tuğcu, D.; Karakaş, Z.; Gökçe, M.; Ağaoğlu, L.; Unüvar, A.; Sarıbeyoğlu, E.; Akçay, A.; Devecioğlu, O. Thalassemia Intermedia and Acute Lymphoblastic Leukemia: Is it a Coincidental Double Diagnosis? Turk. J. Haematol. 2014, 31, 311–312. [Google Scholar] [CrossRef]
- Karimi, M.; Giti, R.; Haghpanah, S.; Azarkeivan, A.; Hoofar, H.; Eslami, M. Malignancies in patients with beta-thalassemia major and beta-thalassemia intermedia: A multicenter study in Iran. Pediatr. Blood Cancer 2009, 53, 1064–1067. [Google Scholar] [CrossRef]
- Benetatos, L.; Alymara, V.; Vassou, A.; Bourantas, K.L. Malignancies in beta-thalassemia patients: A single-center experience and a concise review of the literature. Int. J. Lab. Hematol. 2008, 30, 167–172. [Google Scholar] [CrossRef]
- Hodroj, M.H.; Bou-Fakhredin, R.; Nour-Eldine, W.; Noureldine, H.A.; Noureldine, M.H.A.; Taher, A.T. Thalassemia and malignancy: An emerging concern? Blood Rev. 2019, 37, 100585. [Google Scholar] [CrossRef] [PubMed]
- Hodroj, M.H.; Taher, A. Thalassemia and malignancies: Updates from the literature. Ann. N. Y. Acad. Sci. 2023, 1529, 14–20. [Google Scholar] [CrossRef]
- Ho, T.T.B.; Kumar, A.; Louis-Jacques, A.F.; Dishaw, L.J.; Yee, A.L.; Groer, M.W. The development of intestinal dysbiosis in anemic preterm infants. J. Perinatol. 2020, 40, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, K.; Pacheco-Aranibar, J.; Manrique-Sam, C.; Ita-Balta, Y.; Carpio-Toia, A.M.D.; López-Casaperalta, P.; Chocano-Rosas, T.; Fernandez, F.F.; Villanueva-Salas, J.; Bernabe-Ortiz, J.C. Intestinal Microbiota in Children with Anemia in Southern Peru through Next-Generation Sequencing Technology. Children 2022, 9, 1615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yung, K.K.L.; KongYeung, C. Effects of common prebiotics on iron status and production of colonic short-chain fatty acids in anemic rats. Food Sci. Hum. Wellness 2021, 10, 327–334. [Google Scholar] [CrossRef]
- Sun, B.; Tan, B.; Zhang, P.; Huang, T.; Wei, H.; Li, C.; Yang, W. Effects of hemoglobin extracted from Tegillarca granosa on the gut microbiota in iron deficiency anemia mice. Food Funct. 2023, 14, 7040–7052. [Google Scholar] [CrossRef]
- Xiao, P.; Cai, X.; Zhang, Z.; Guo, K.; Ke, Y.; Hu, Z.; Song, Z.; Zhao, Y.; Yao, L.; Shen, M. Butyrate Prevents the Pathogenic Anemia-Inflammation Circuit by Facilitating Macrophage Iron Export. Adv. Sci. 2024, 11, 2306571. [Google Scholar] [CrossRef]
- Soriano-Lerma, A.; García-Burgos, M.; Barton, W.; Alférez, M.J.M.; Crespo-Pérez, J.V.; Soriano, M.; López-Aliaga, I.; Cotter, P.D.; García-Salcedo, J.A. Comprehensive insight into the alterations in the gut microbiome and the intestinal barrier as a consequence of iron deficiency anaemia. Biomedical J. 2024, 100701. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Singh, H.; Yu, Y.; Zabokrtsky, K.B.; Torralba, M.G.; Moncera, K.J.; Frank, B.; Pieper, R.; Sender, L.; Nelson, K.E. Persistent Gut Microbial Dysbiosis in Children with Acute Lymphoblastic Leukemia (ALL) During Chemotherapy. Microb. Ecol. 2020, 79, 1034–1043. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, P.; Li, D.; Ju, X. Changes in the gastrointestinal microbiota of children with acute lymphoblastic leukaemia and its association with antibiotics in the short term. J. Med. Microbiol. 2017, 66, 1297–1307. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Ran, Z.; Wang, J.; Ma, W.; Sheng, Q. Effect of Different Doses of Vitamin D on the Intestinal Flora of Babies with Eczema: An Experimental Study. Life 2022, 12, 1409. [Google Scholar] [CrossRef]
- Tabassum, A.; Ali, A.; Zahedi, F.D.; Ismail, N.A.S. Immunomodulatory Role of Vitamin D on Gut Microbiome in Children. Biomedicines 2023, 11, 1441. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Saadaoui, M.; Elhag, D.; Tomei, S.; Elanbari, M.; Akobeng, A.K.; Mustafa, A.; Abdelgadir, I.; Udassi, S.; et al. Tipping the Balance: Vitamin D Inadequacy in Children Impacts the Major Gut Bacterial Phyla. Biomedicines 2022, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Drall, K.M.; Field, C.J.; Haqq, A.M.; de Souza, R.J.; Tun, H.M.; Morales-Lizcano, N.P.; Konya, T.B.; Guttman, D.S.; Azad, M.B.; Becker, A.B.; et al. Vitamin D Supplementation in Pregnancy and Early Infancy in Relation to Gut Microbiota Composition and C. Difficile Colonization: Implications for Viral Respiratory Infections. Gut Microbes. 2020, 12, 1799734. [Google Scholar] [CrossRef] [PubMed]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Liu, X.-F.; Shao, J.-H.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-Z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Cresci, G.A. The immunomodulatory functions of butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Lee, B.-K.; Kim, Y. Iron deficiency is associated with increased levels of blood cadmium in the Korean general population: Analysis of 2008–2009 Korean National Health and Nutrition Examination Survey data. Environ. Res. 2012, 112, 155–163. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Azizi, G.; Eslami, S.; Alhassan Mohammed, H.; Fayyaz, F.; Hosseinzadeh, R.; Usman, A.B.; Kamali, A.N.; Mohammadi, H.; Jadidi-Niaragh, F. The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Cirovic, A.; Cirovic, A. Iron deficiency as promoter of heavy metals-induced acute myeloid leukemia. Leuk. Res. 2022, 112, 106755. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, X.; Wu, Q.; Jiang, L.; Chen, L.; Yu, Y.; Zhang, P.; Huang, X.; Wang, J.; Ju, Z. Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 2020, 105, 2071. [Google Scholar] [CrossRef] [PubMed]
- Demir, C.; Demir, H.; Esen, R.; Sehitogullari, A.; Atmaca, M.; Alay, M. Altered serum levels of elements in acute leukemia cases in Turkey. Asian Pac. J. Cancer Prev. 2011, 12, 3471–3474. [Google Scholar]
- Chrysochou, E.; Koukoulakis, K.; Kanellopoulos, P.G.; Sakellari, A.; Karavoltsos, S.; Dassenakis, M.; Minaidis, M.; Maropoulos, G.; Bakeas, E. Human serum elements’ levels and leukemia: A first pilot study from an adult Greek cohort. J. Trace Elem. Med. Biol. 2021, 68, 126833. [Google Scholar] [CrossRef]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; Zheng, Y.; Liu, Q.; Shi, G.; Zhang, Z. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef] [PubMed]
- Suljevic, D.; Corbic, A.; Islamagic, E.; Focak, M.; Filipic, F.; Alijagic, A. Impairments of bone marrow hematopoietic cells followed by the sever erythrocyte damage and necrotic liver as the outcome of chronic in vivo exposure to cadmium: Novel insights from quails. Environ. Toxicol. Pharmacol. 2019, 72, 103250. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, Z.; Xu, T.; Peng, C.; Hu, A.; Jiang, W.; Xiong, Z.; Wu, Y.; Yang, F.; Cao, H. Compound probiotics alleviate cadmium-induced intestinal dysfunction and microbiota disorders in broilers. Ecotoxicol. Environ. Saf. 2022, 234, 113374. [Google Scholar] [CrossRef]
- Odewumi, C.; Latinwo, L.M.; Sinclair, A.; Badisa, V.L.; Abdullah, A.; Badisa, R.B. Effect of cadmium on the expression levels of interleukin-1α and interleukin-10 cytokines in human lung cells. Mol. Med. Rep. 2015, 12, 6422–6426. [Google Scholar] [CrossRef]
- Schwerdtle, T.; Ebert, F.; Thuy, C.; Richter, C.; Mullenders, L.H.; Hartwig, A. Genotoxicity of soluble and particulate cadmium compounds: Impact on oxidative DNA damage and nucleotide excision repair. Chem. Res. Toxicol. 2010, 23, 432–442. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Yao, W.; Ba, Q.; Wang, H. Effects of Cadmium Exposure on the Immune System and Immunoregulation. Front. Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.-L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chi, H.; Zhu, W.; Yang, G.; Song, J.; Mo, L.; Zhang, Y.; Deng, Y.; Xu, F.; Yang, J.; et al. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 2021, 95, 3497–3513. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Gritsenko, V.A.; Skalnaya, M.G.; Cherkasov, S.V.; Aaseth, J.; Skalny, A.V. Gut as a target for cadmium toxicity. Environ. Pollut. 2018, 235, 429–434. [Google Scholar] [CrossRef]
- Djulejic, V.; Petrovic, B.; Jevtic, J.; Vujacic, M.; Clarke, B.L.; Cirovic, A.; Cirovic, A. The role of cadmium in the pathogenesis of myeloid leukemia in individuals with anemia, deficiencies in vitamin D, zinc, and low calcium dietary intake. J. Trace Elem. Med. Biol. 2023, 79, 127263. [Google Scholar] [CrossRef]
- Orimoloye, H.T.; Qureshi, N.; Lee, P.C.; Wu, C.K.; Saechao, C.; Federman, N.; Li, C.Y.; Ritz, B.; Arah, O.A.; Heck, J.E. Maternal anemia and the risk of childhood cancer: A population-based cohort study in Taiwan. Pediatr. Blood Cancer 2023, 70, e30188. [Google Scholar] [CrossRef]
- Qureshi, N.; Orimoloye, H.; Hansen, J.; Saechao, C.; Olsen, J.; Federman, N.; Huang, X.; He, D.; Ritz, B.; Heck, J.E. Maternal anemia and childhood cancer: A population-based case-control study in Denmark. Cancer Epidemiol. 2023, 82, 102308. [Google Scholar] [CrossRef] [PubMed]
- de Smith, A.J.; Spector, L.G. In Utero Origins of Acute Leukemia in Children. Biomedicines 2024, 12, 236. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free. Radic. Biol. Med. 2019, 133, 254–261. [Google Scholar] [CrossRef]
- Ohta, H.; Ohba, K. Involvement of metal transporters in the intestinal uptake of cadmium. J. Toxicol. Sci. 2020, 45, 539–548. [Google Scholar] [CrossRef]
- Li, S.H.; Yin, H.B.; Ren, M.R.; Wu, M.J.; Huang, X.L.; Li, J.J.; Luan, Y.P.; Wu, Y.L. TRPV5 and TRPV6 are expressed in placenta and bone tissues during pregnancy in mice. Biotech. Histochem. 2019, 94, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Wang, C.Y.; Mackenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 2012, 25, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Bai, B.; Cao, X.X.; Zhang, Y.H.; Yan, H.; Zheng, Q.Q.; Zhuang, G.H. Divalent metal transporter 1 expression and regulation in human placenta. Biol. Trace Elem. Res. 2012, 146, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Courbebaisse, M.; Lanske, B. Biology of Fibroblast Growth Factor 23: From Physiology to Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a031260. [Google Scholar] [CrossRef]
- Hanudel, M.R.; Chua, K.; Rappaport, M.; Gabayan, V.; Valore, E.; Goltzman, D.; Ganz, T.; Nemeth, E.; Salusky, I.B. Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am. J. Physiol. Ren. Physiol. 2016, 311, F1369–F1377. [Google Scholar] [CrossRef]
- Clinkenbeard, E.L.; Farrow, E.G.; Summers, L.J.; Cass, T.A.; Roberts, J.L.; Bayt, C.A.; Lahm, T.; Albrecht, M.; Allen, M.R.; Peacock, M.; et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J. Bone Miner. Res. 2014, 29, 361–369. [Google Scholar] [CrossRef]
- Farrow, E.G.; Yu, X.; Summers, L.J.; Davis, S.I.; Fleet, J.C.; Allen, M.R.; Robling, A.G.; Stayrook, K.R.; Jideonwo, V.; Magers, M.J.; et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl. Acad. Sci. USA 2011, 108, E1146–E1155. [Google Scholar] [CrossRef]
- Imel, E.A.; Peacock, M.; Gray, A.K.; Padgett, L.R.; Hui, S.L.; Econs, M.J. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J. Clin. Endocrinol. Metab. 2011, 96, 3541–3549. [Google Scholar] [CrossRef]
- Babitt, J.L.; Sitara, D. Crosstalk between fibroblast growth factor 23, iron, erythropoietin, and inflammation in kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 304–310. [Google Scholar] [CrossRef]
- Kido, S.; Fujihara, M.; Nomura, K.; Sasaki, S.; Mukai, R.; Ohnishi, R.; Kaneko, I.; Segawa, H.; Tatsumi, S.; Izumi, H. Molecular mechanisms of cadmium-induced fibroblast growth factor 23 upregulation in osteoblast-like cells. Toxicol. Sci. 2014, 139, 301–316. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Reinert, R.B.; Bixby, D.; Koenig, R.J. Fibroblast Growth Factor 23-Induced Hypophosphatemia in Acute Leukemia. J. Endocr. Soc. 2018, 2, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Weidner, H.; Baschant, U.; Lademann, F.; Colunga, M.G.L.; Balaian, E.; Hofbauer, C.; Misof, B.M.; Roschger, P.; Blouin, S.; Richards, W.G. Increased FGF-23 levels are linked to ineffective erythropoiesis and impaired bone mineralization in myelodysplastic syndromes. JCI Insight 2020, 5, e137062. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Verma, N.; Kumar, A. Prevalence of vitamin D deficiency in childhood acute lymphoblastic leukemia and its association with adverse outcomes during induction phase of treatment. Nutr. Cancer 2020, 72, 1321–1325. [Google Scholar] [CrossRef]
- Seyedalipour, F.; Mansouri, A.; Vaezi, M.; Gholami, K.; Heidari, K.; Hadjibabaie, M.; Ghavamzadeh, A. High Prevalence of Vitamin D Deficiency in Newly Diagnosed Acute Myeloid Leukemia Patients and Its Adverse Outcome. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 209–216. [Google Scholar] [PubMed]
- Ghazaey Zidanloo, S.; Jahantigh, D.; Amini, N. Vitamin D-Binding Protein and Acute Myeloid Leukemia: A Genetic Association Analysis in Combination with Vitamin D Levels. Nutr. Cancer 2023, 75, 470–481. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, J.; Wang, Y.; Xu, C.; Liu, Y.; Du, L.; Wang, Q.; Ji, K.; He, N.; Zhang, M.; et al. Low-dose ionizing radiation exposure and risk of leukemia: Results from 1950–1995 Chinese medical X-ray workers’ cohort study and meta-analysis. J. Natl. Cancer Cent. 2022, 2, 90–97. [Google Scholar] [CrossRef]
- Cirovic, A.; Cirovic, A. Factors moderating cadmium bioavailability: Key considerations for comparing blood cadmium levels between groups. Food Chem. Toxicol. 2024, 191, 114865. [Google Scholar] [CrossRef]
- Stajnko, A.; Lundh, T.; Assarson, E.; Krook, E.Å.; Broberg, K. Lead, cadmium, and mercury blood levels in schoolchildren in southern Sweden: Time trends over the last decades. Chemosphere 2024, 346, 140562. [Google Scholar] [CrossRef]
- García-Pérez, J.; López-Abente, G.; Gómez-Barroso, D.; Morales-Piga, A.; Pardo Romaguera, E.; Tamayo, I.; Fernández-Navarro, P.; Ramis, R. Childhood leukemia and residential proximity to industrial and urban sites. Environ. Res. 2015, 140, 542–553. [Google Scholar] [CrossRef]
- Boonhat, H.; Lin, R.-T. Association between leukemia incidence and mortality and residential petrochemical exposure: A systematic review and meta-analysis. Environ. Int. 2020, 145, 106090. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Lee, J.-Y.; Banno, H.; Imai, S.; Tokumoto, M.; Hasegawa, T.; Seko, Y.; Nagase, H.; Satoh, M. Cadmium induces iron deficiency anemia through the suppression of iron transport in the duodenum. Toxicol. Lett. 2020, 332, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, M.; Lee, J.-Y.; Fujiwara, Y.; Satoh, M. Long-Term Exposure to Cadmium Causes Hepatic Iron Deficiency through the Suppression of Iron-Transport-Related Gene Expression in the Proximal Duodenum. Toxics 2023, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Rabe, H. Prevention of iron deficiency anemia in infants and toddlers. Pediatr. Res. 2021, 89, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Meinzen-Derr, J.K.; Guerrero, M.L.; Altaye, M.; Ortega-Gallegos, H.; Ruiz-Palacios, G.M.; Morrow, A.L. Risk of Infant Anemia Is Associated with Exclusive Breast-Feeding and Maternal Anemia in a Mexican Cohort12. J. Nutr. 2006, 136, 452–458. [Google Scholar] [CrossRef]
- Power, G.; Stratas, A.; Landry, C.; Morrison, L.; Kulkarni, K.; Campbell-Yeo, M.; Singh, B.; Higgins, M.; Ghotra, S. Formula Feeding Significantly Increases Risk of Iron Deficiency in Very Preterm Infants during the First 4–6 Months of Life. Blood 2022, 140, 8196–8197. [Google Scholar] [CrossRef]
- Miller, M.F.; Humphrey, J.H.; Iliff, P.J.; Malaba, L.C.; Mbuya, N.V.; Stoltzfus, R.J. Neonatal erythropoiesis and subsequent anemia in HIV-positive and HIV-negative Zimbabwean babies during the first year of life: A longitudinal study. BMC Infect. Dis. 2006, 6, 1. [Google Scholar] [CrossRef]
- Freeman, H.J. Iron deficiency anemia in celiac disease. World J. Gastroenterol. 2015, 21, 9233–9238. [Google Scholar] [CrossRef]
- Levy-Costa, R.B.; Monteiro, C.A. Cow’s milk consumption and childhood anemia in the city of São Paulo, southern Brazil. Rev. Saude Publica 2004, 38, 797–803. [Google Scholar] [CrossRef][Green Version]
- Graczykowska, K.; Kaczmarek, J.; Wilczyńska, D.; Łoś-Rycharska, E.; Krogulska, A. The Consequence of Excessive Consumption of Cow’s Milk: Protein-Losing Enteropathy with Anasarca in the Course of Iron Deficiency Anemia-Case Reports and a Literature Review. Nutrients 2021, 13, 828. [Google Scholar] [CrossRef]
- Gaitán, D.; Flores, S.; Saavedra, P.; Miranda, C.; Olivares, M.; Arredondo, M.; de Romana, D.L.; Lönnerdal, B.; Pizarro, F. Calcium does not inhibit the absorption of 5 milligrams of nonheme or heme iron at doses less than 800 milligrams in nonpregnant women. J. Nutr. 2011, 141, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.E. Consumption of cow’s milk as a cause of iron deficiency in infants and toddlers. Nutr. Rev. 2011, 69, S37–S42. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djulejic, V.; Ivanovski, A.; Cirovic, A.; Cirovic, A. Increased Cadmium Load, Vitamin D Deficiency, and Elevated FGF23 Levels as Pathophysiological Factors Potentially Linked to the Onset of Acute Lymphoblastic Leukemia: A Review. J. Pers. Med. 2024, 14, 1036. https://doi.org/10.3390/jpm14101036
Djulejic V, Ivanovski A, Cirovic A, Cirovic A. Increased Cadmium Load, Vitamin D Deficiency, and Elevated FGF23 Levels as Pathophysiological Factors Potentially Linked to the Onset of Acute Lymphoblastic Leukemia: A Review. Journal of Personalized Medicine. 2024; 14(10):1036. https://doi.org/10.3390/jpm14101036
Chicago/Turabian StyleDjulejic, Vuk, Ana Ivanovski, Ana Cirovic, and Aleksandar Cirovic. 2024. "Increased Cadmium Load, Vitamin D Deficiency, and Elevated FGF23 Levels as Pathophysiological Factors Potentially Linked to the Onset of Acute Lymphoblastic Leukemia: A Review" Journal of Personalized Medicine 14, no. 10: 1036. https://doi.org/10.3390/jpm14101036
APA StyleDjulejic, V., Ivanovski, A., Cirovic, A., & Cirovic, A. (2024). Increased Cadmium Load, Vitamin D Deficiency, and Elevated FGF23 Levels as Pathophysiological Factors Potentially Linked to the Onset of Acute Lymphoblastic Leukemia: A Review. Journal of Personalized Medicine, 14(10), 1036. https://doi.org/10.3390/jpm14101036




