Robotic Rectosigmoid Resection with Totally Intracorporeal Colorectal Anastomosis (TICA) for Recurrent Ovarian Cancer: A Case Series and Description of the Technique
Abstract
1. Introduction
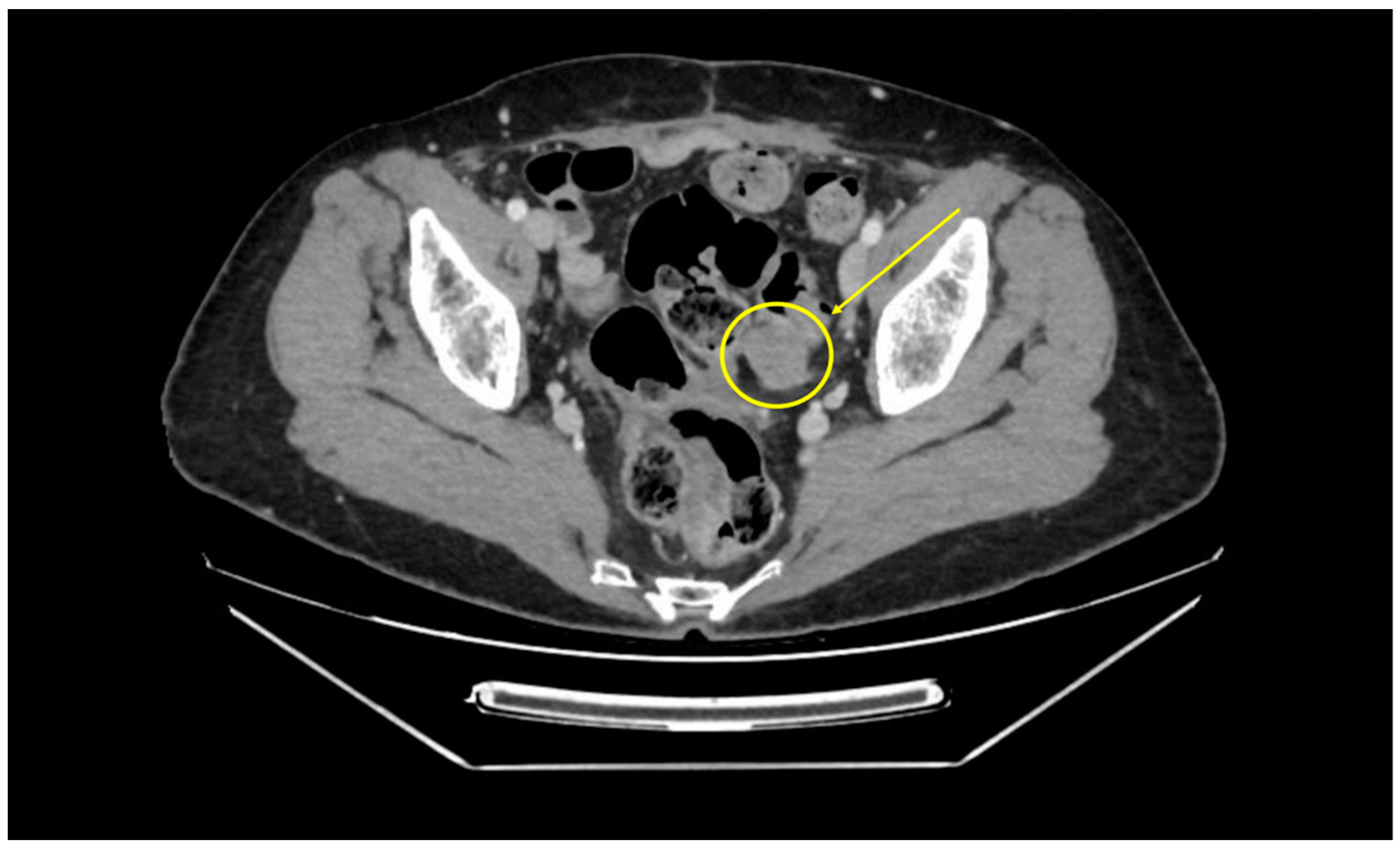
2. Material and Methods
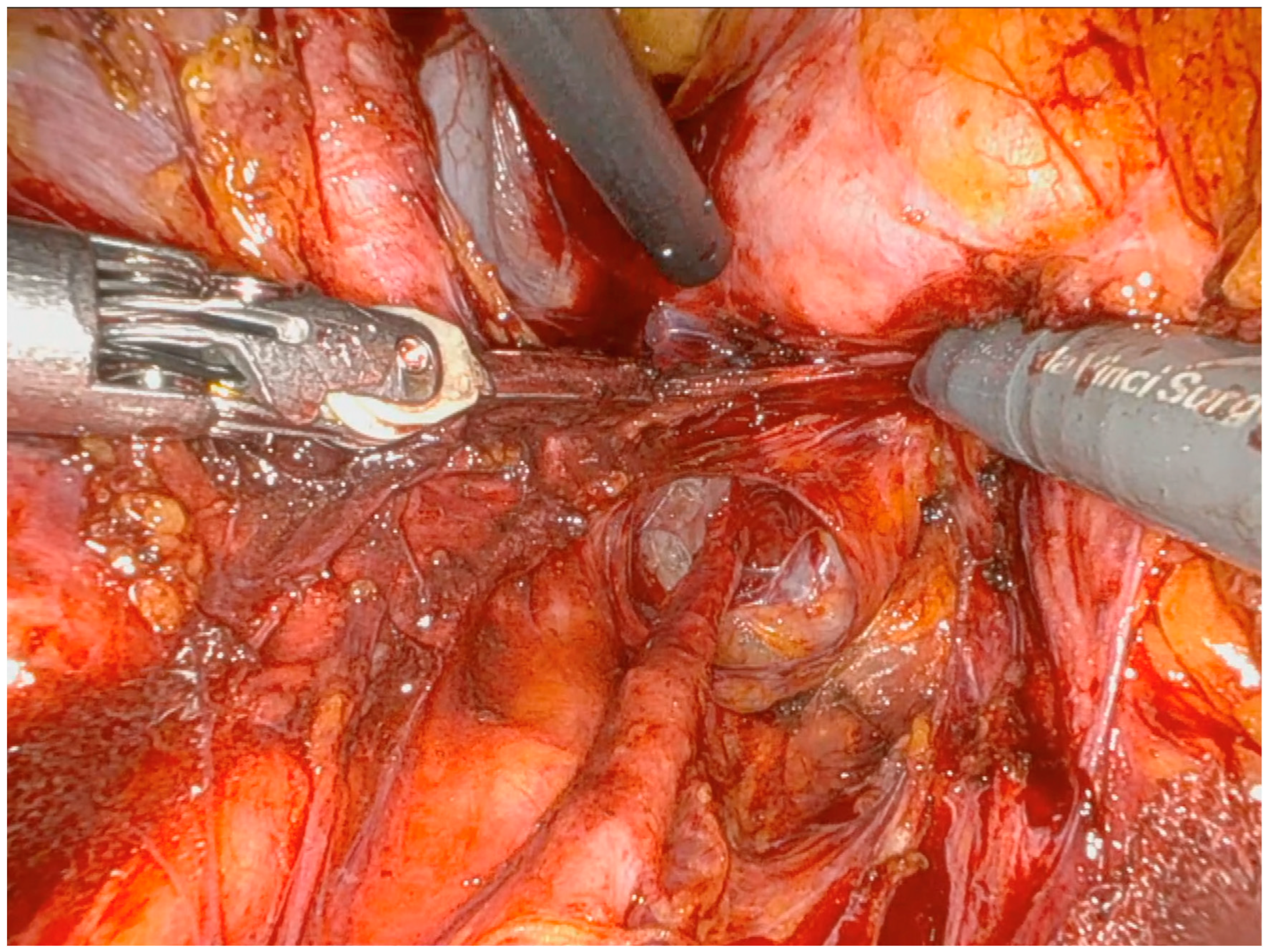
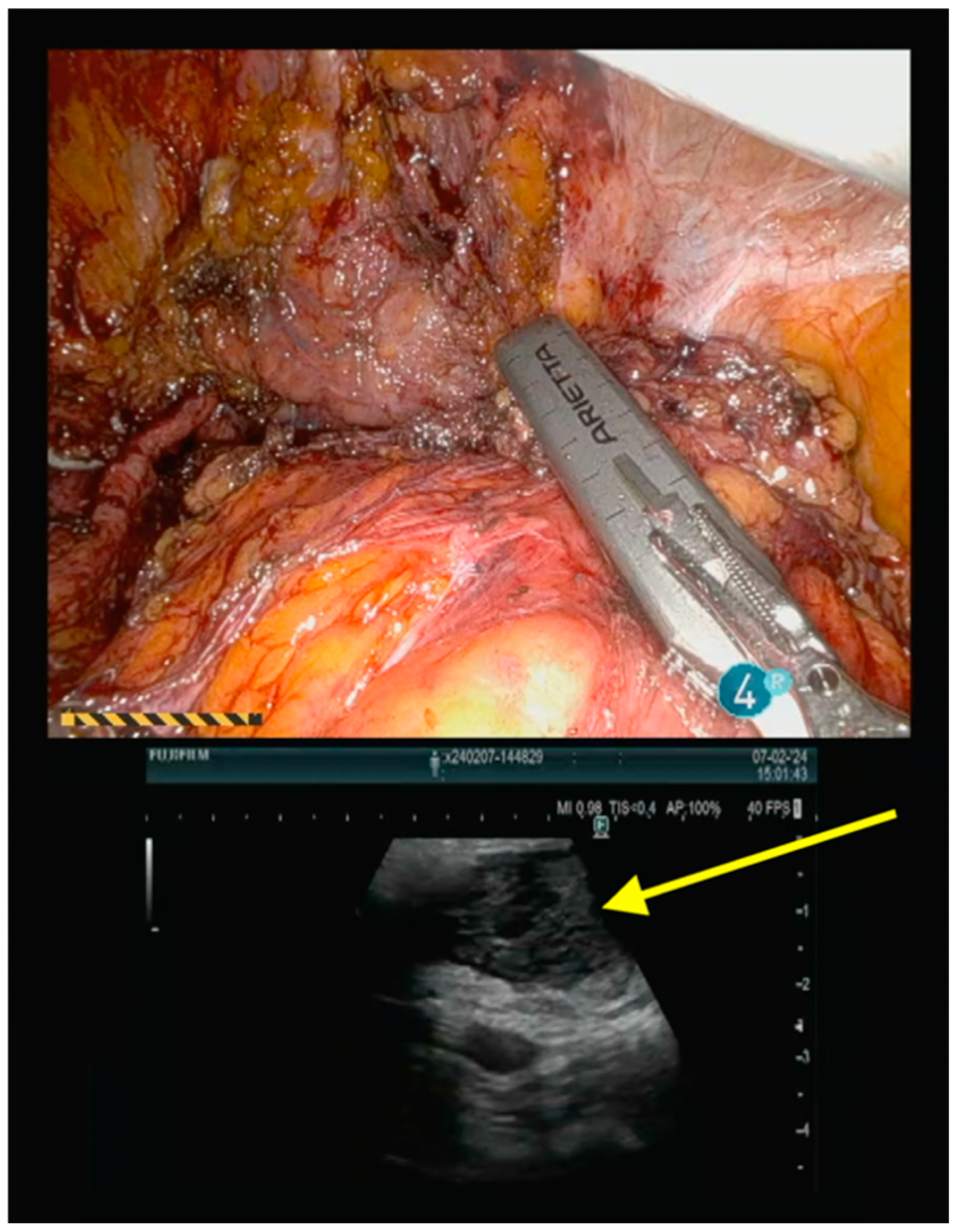
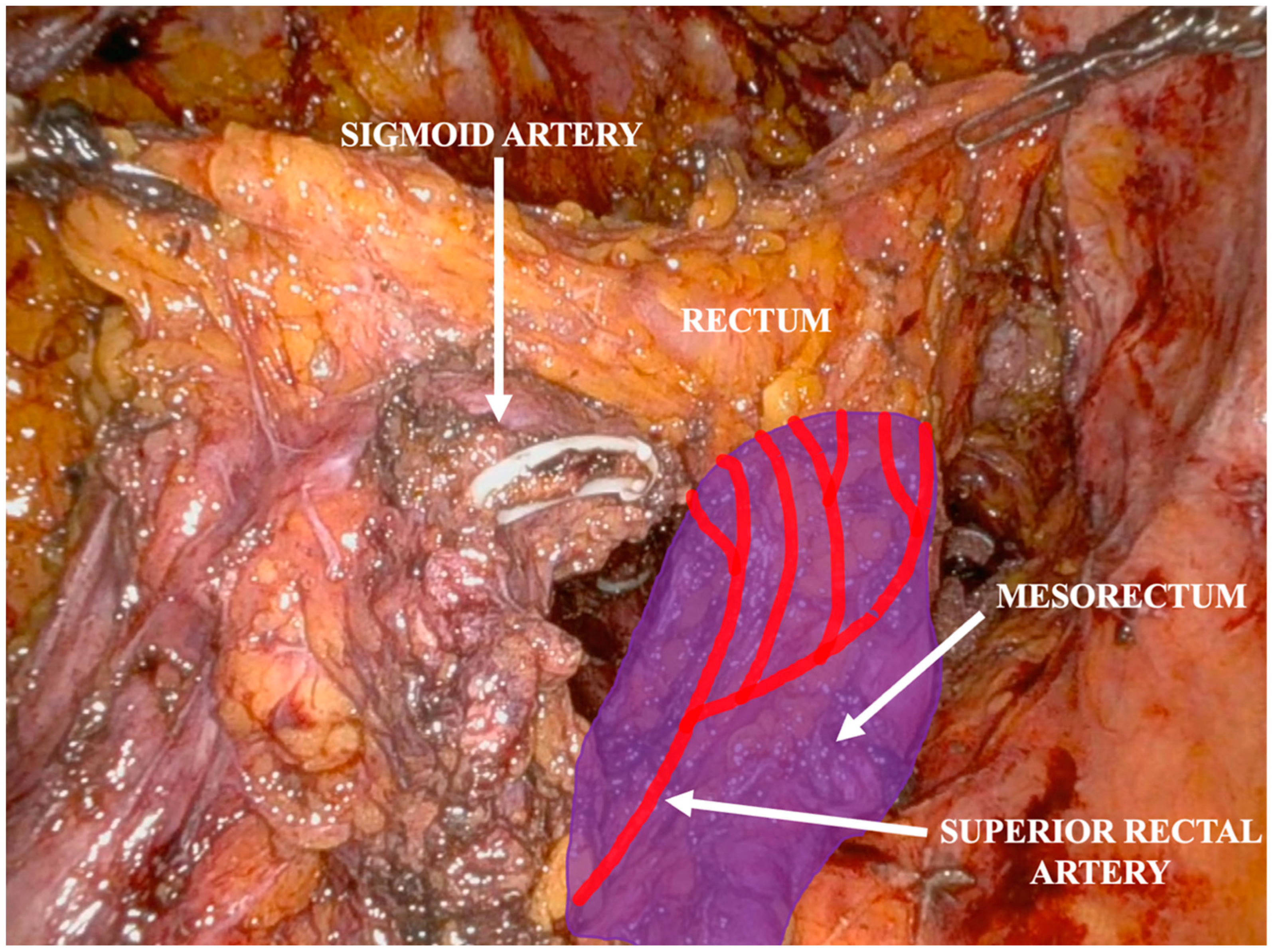
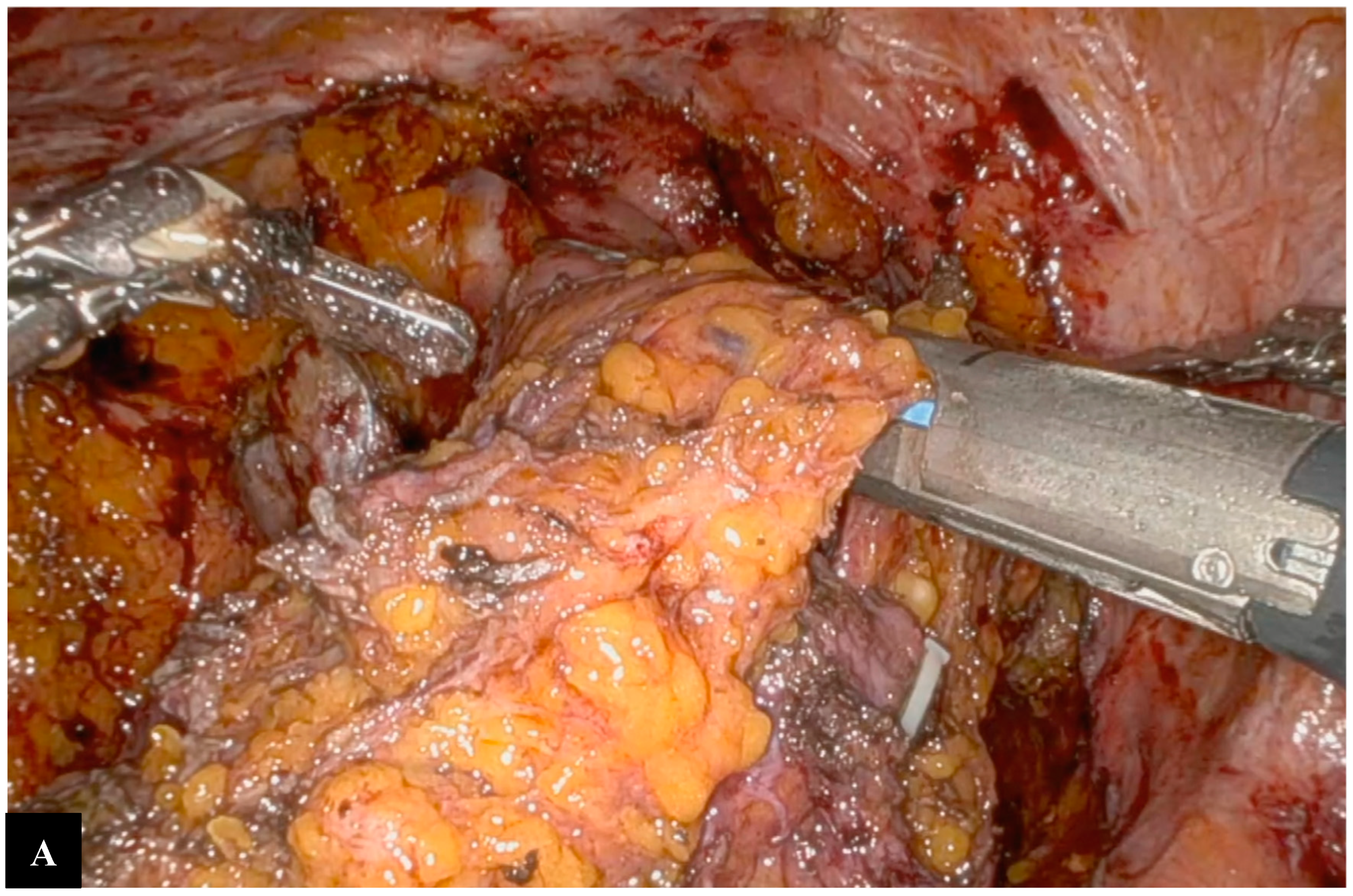
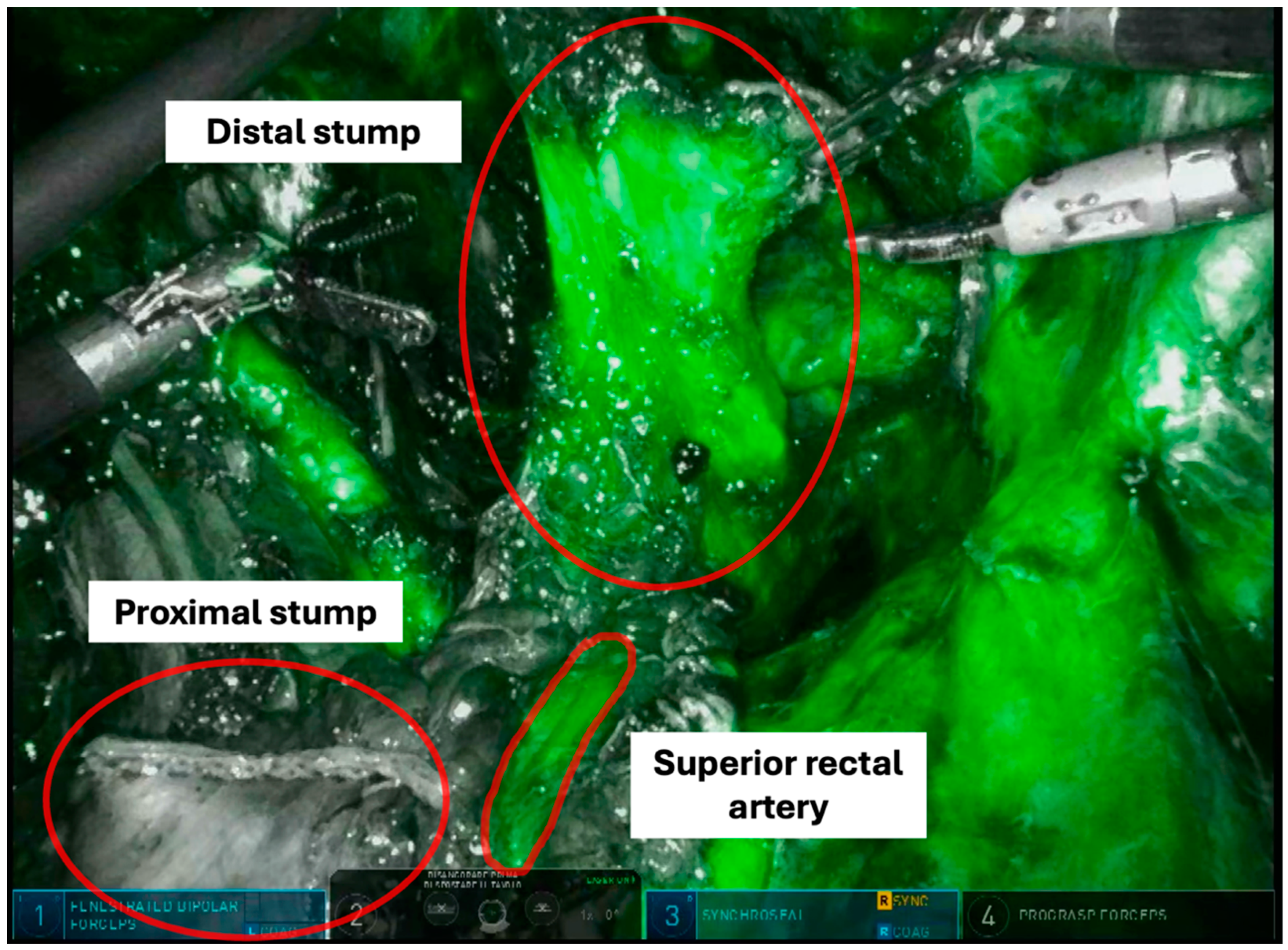
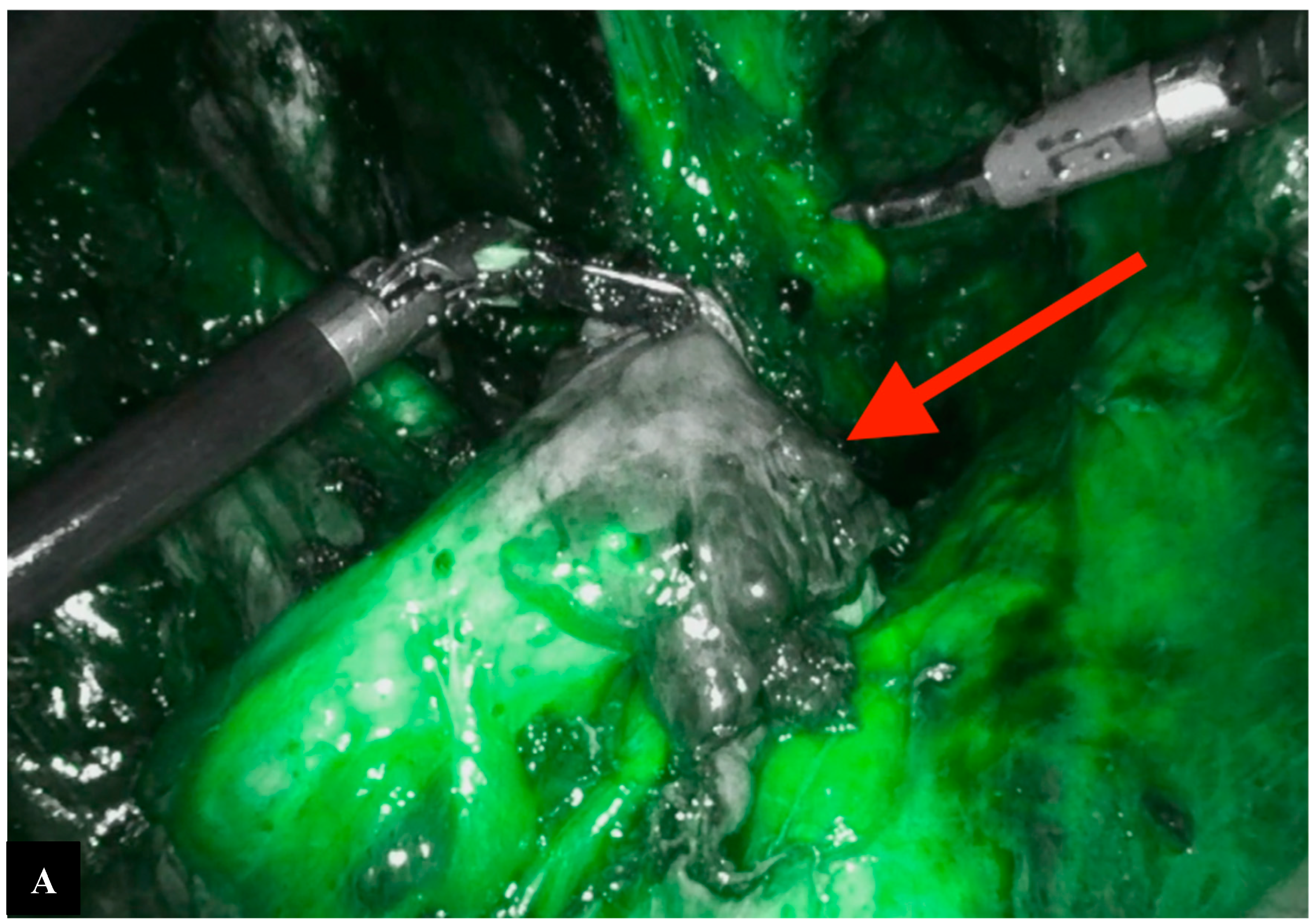
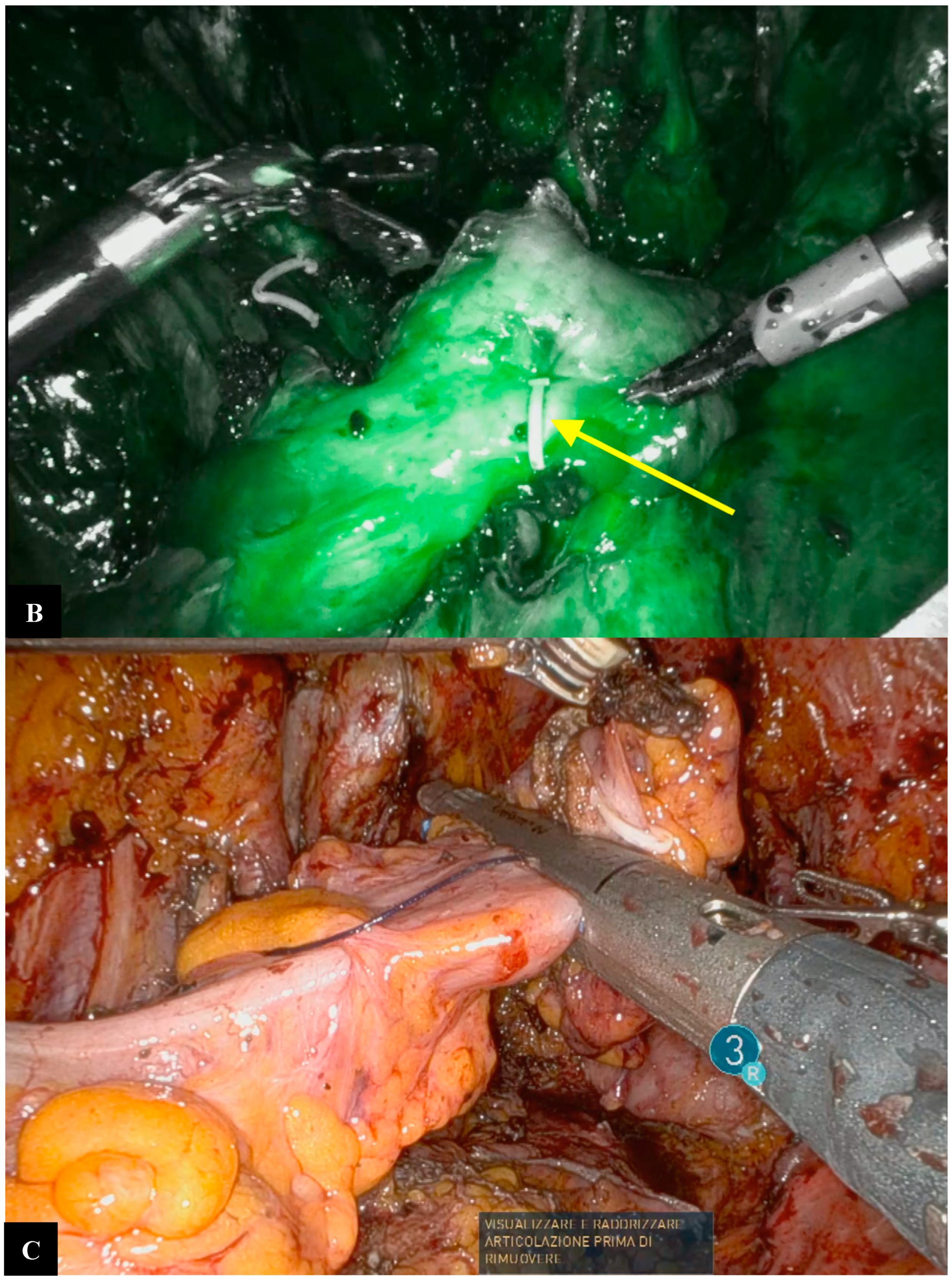
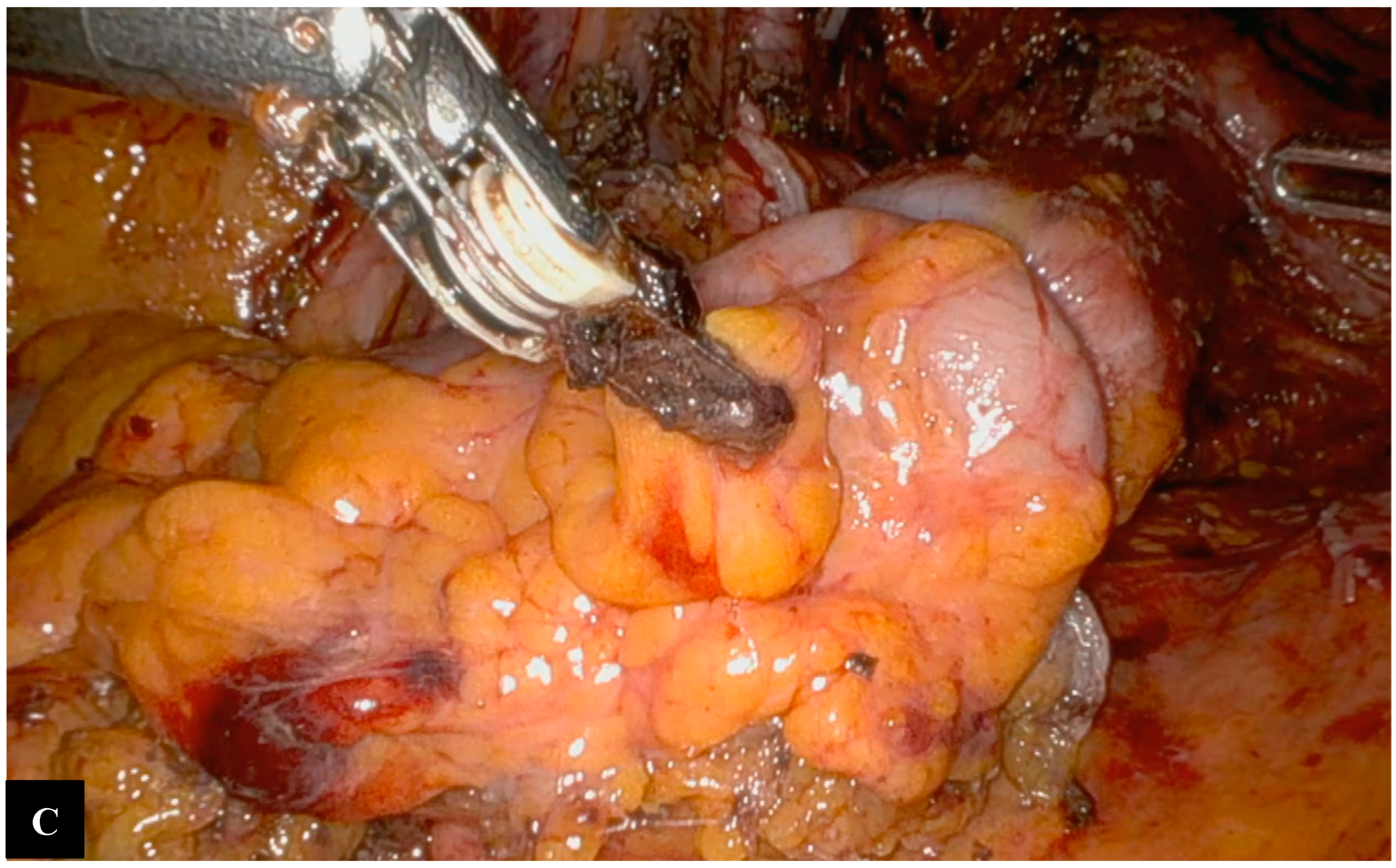
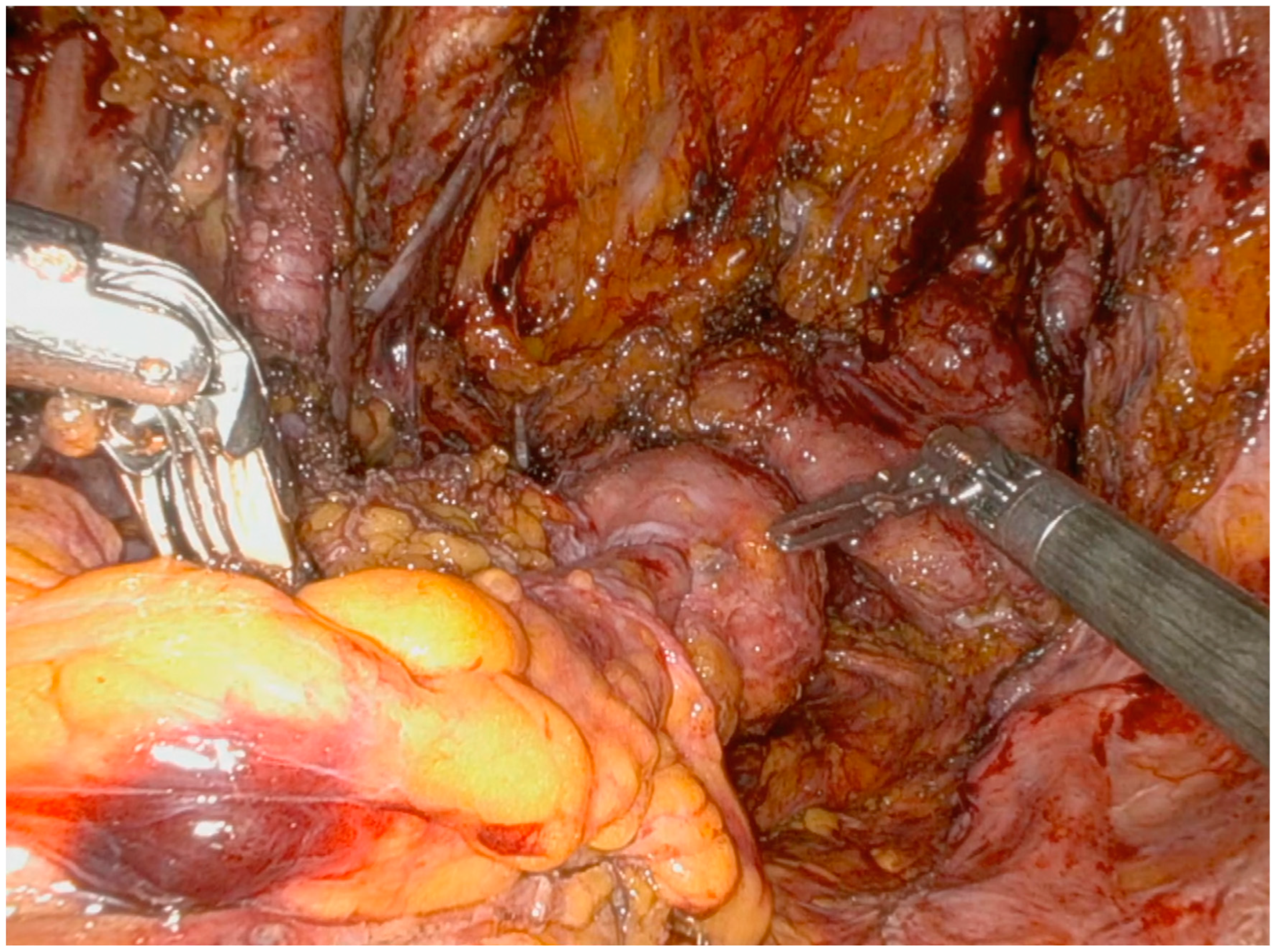
Operative Technique
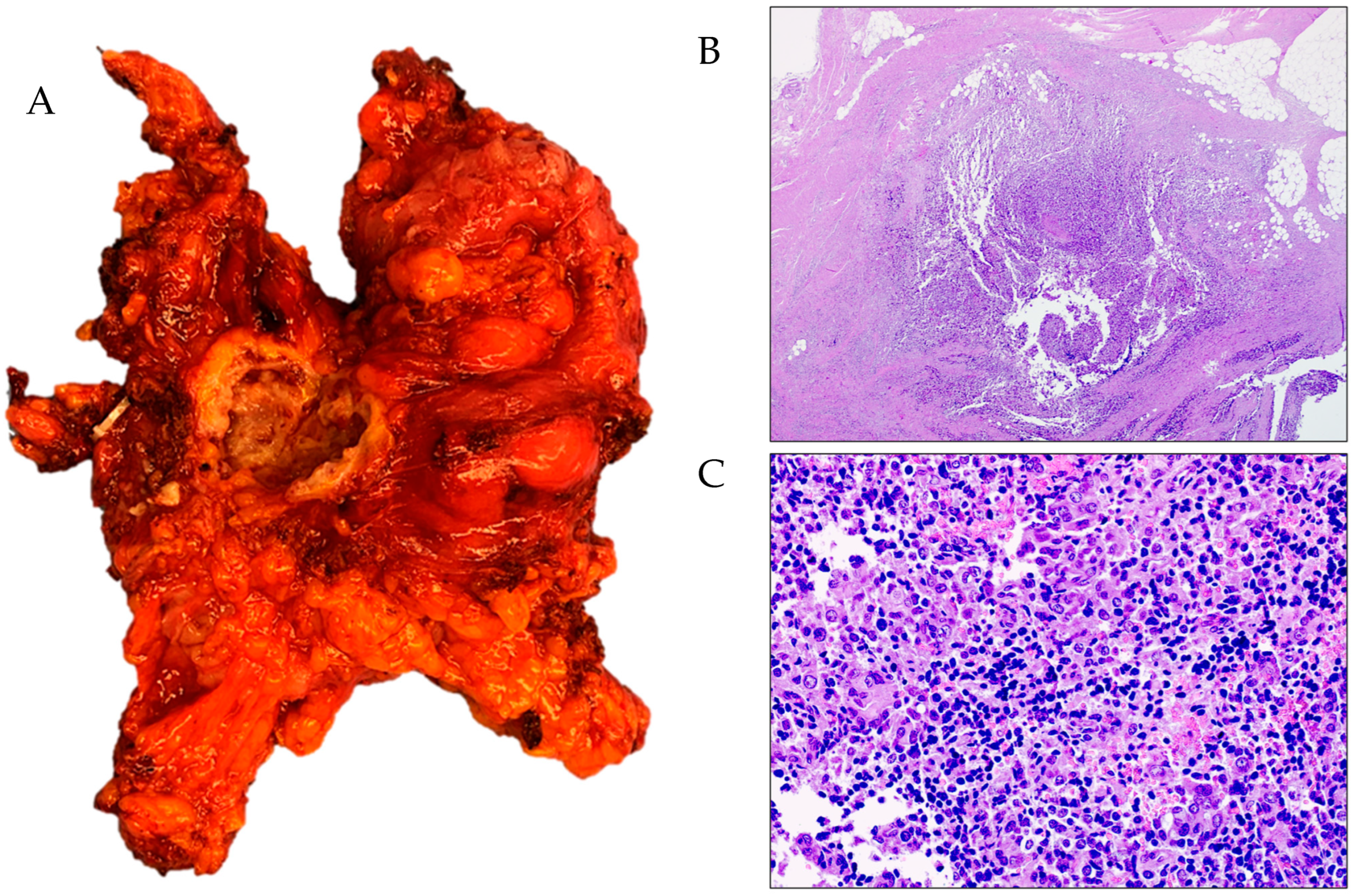
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.; Marth, C.; Largillier, R.; Kaern, J.; Brown, C.; Heywood, M.; Bonaventura, T.; Vergote, I.; Piccirillo, M.C.; Fossati, R.; et al. Final Overall Survival Results of Phase III GCIG CALYPSO Trial of Pegylated Liposomal Doxorubicin and Carboplatin vs Paclitaxel and Carboplatin in Platinum-Sensitive Ovarian Cancer Patients. Br. J. Cancer 2012, 107, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, J.; Plante, M.; Vergote, I.; du Bois, A.; Hirte, H.; Lacave, A.J.; Wagner, U.; Stähle, A.; Stuart, G.; Kimmig, R.; et al. Gemcitabine plus Carboplatin Compared with Carboplatin in Patients with Platinum-Sensitive Recurrent Ovarian Cancer: An Intergroup Trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J. Clin. Oncol. 2006, 24, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.C.; Cushing-Haugen, K.L.; Anglesio, M.; Wicklund, K.; Bentley, R.; Berchuck, A.; Kelemen, L.E.; Nazeran, T.M.; Gilks, C.B.; Harris, H.R.; et al. Histotype Classification of Ovarian Carcinoma: A Comparison of Approaches. Gynecol. Oncol. 2018, 151, 53–60. [Google Scholar] [CrossRef]
- Hollis, R.L.; Churchman, M.; Gourley, C. Distinct Implications of Different BRCA Mutations: Efficacy of Cytotoxic Chemotherapy, PARP Inhibition and Clinical Outcome in Ovarian Cancer. Onco Targets Ther. 2017, 10, 2539–2551. [Google Scholar] [CrossRef]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Dias, M.P.; Moser, S.C.; Ganesan, S.; Jonkers, J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 773–791. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O’Malley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/ GOG-3020/ENGOT-ov45). J. Clin. Oncol. 2022, 40, 3952–3964. [Google Scholar] [CrossRef]
- Frenel, J.S.; Kim, J.W.; Aryal, N.; Asher, R.; Berton, D.; Vidal, L.; Pautier, P.; Ledermann, J.A.; Penson, R.T.; Oza, A.M.; et al. Efficacy of subsequent chemotherapy for patients with BRCA1/2-mutated recurrent epithelial ovarian cancer progressing on olaparib versus placebo maintenance: Post-hoc analyses of the SOLO2/ENGOT Ov-21 trial. Ann. Oncol. 2022, 33, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.I.; Jeong, S.Y.; Kim, Y.; Bookman, M.A.; Kim, J.W.; Kim, B.G.; Lee, J.Y. Second-line olaparib maintenance therapy is associated with poor response to subsequent chemotherapy in BRCA1/2-mutated epithelial ovarian cancer: A multicentre retrospective study. Gynecol. Oncol. 2022, 165, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Mouret-Reynier, M.A.; Pignata, S.; Cropet, C.; González-Martín, A.; Bogner, G.; Fujiwara, K.; Vergote, I.; Colombo, N.; Nøttrup, T.J. Efficacy of maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed, advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Gynecol. Oncol. 2022, 164, 254–264. [Google Scholar] [CrossRef]
- Rose, P.G.; Yao, M.; Chambers, L.M.; Mahdi, H.; DeBernardo, R.; Michener, C.M.; AlHilli, M.; Ricci, S.; Vargas, R. PARP inhibitors decrease response to subsequent platinum-based chemotherapy in patients with BRCA mutated ovarian cancer. Anticancer Drugs 2021, 32, 1086–1092. [Google Scholar] [CrossRef]
- Gauduchon, T.; Kfoury, M.; Lorusso, D.; Floquet, A.; Ventriglia, J.; Salaun, H.; Moubarak, M.; Rivoirard, R.; Polastro, L.; Favier, L.; et al. PARP inhibitors (PARPi) prolongation after local therapy for oligo-metastatic progression in relapsed ovarian cancer patients. Gynecol. Oncol. 2023, 173, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.A.; Eriksson, A.G.Z.; Jaber, S.M.; Zhou, Q.; Iasonos, A.; Zivanovic, O.; Leitao, M.M.; Abu-Rustum, N.R.; Chi, D.S.; Gardner, G.J. A Comparative Analysis of Prediction Models for Complete Gross Resection in Secondary Cytoreductive Surgery for Ovarian Cancer. Gynecol. Oncol. 2017, 145, 230–235. [Google Scholar] [CrossRef]
- Lee, C.K.; Lord, S.; Grunewald, T.; Gebski, V.; Hardy-Bessard, A.-C.; Sehouli, J.; Woie, K.; Heywood, M.; Schauer, C.; Vergote, I.; et al. Impact of Secondary Cytoreductive Surgery on Survival in Patients with Platinum Sensitive Recurrent Ovarian Cancer: Analysis of the CALYPSO Trial. Gynecol. Oncol. 2015, 136, 18–24. [Google Scholar] [CrossRef]
- Al Rawahi, T.; Lopes, A.D.; Bristow, R.E.; Bryant, A.; Elattar, A.; Chattopadhyay, S.; Galaal, K. Surgical Cytoreduction for Recurrent Epithelial Ovarian Cancer. Cochrane Database Syst. Rev. 2013, 2013, CD008765. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary Cytoreduction Followed by Chemotherapy versus Chemotherapy Alone in Platinum-Sensitive Relapsed Ovarian Cancer (SOC-1): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef]
- Jiang, R.; Feng, Y.; Chen, Y.; Cheng, X.; Shi, T.; Gao, W.; Jia, H.; Jiang, S.; Guo, Y.; Huang, X.; et al. Surgery versus no surgery in platinum-sensitive relapsed ovarian cancer: Final overall survival analysis of the SOC-1 randomized phase 3 trial. Nat. Med. 2024, 30, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Pappa, C.; Galaal, K.; Smyth, S.; Bristow, R.E.; Khashan, A.S.; Alazzam, M. Cytoreductive surgery plus chemotherapy versus chemotherapy alone for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev. 2024, 2024, CD015297. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, H.; Chen, Y.; Yao, H.; Li, N.; Wu, L.; Yuan, G. Outcomes of secondary cytoreductive surgery in patients with platinum-sensitive recurrent ovarian cancer progressed after prior poly (adenosine diphosphate-ribose) polymerase inhibitors: A retrospective cohort study. Eur. J. Surg. Oncol. 2024, 50, 108383. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, S.; Lam, C.; Zhou, Q.; Iasonos, A.; Grisham, R.N.; Tew, W.P.; O’Cearbhaill, R.E.; Long Roche, K.; Zivanovic, O.; Sonoda, Y.; et al. Secondary cytoreductive surgery and oncologic outcomes in the era of targeted maintenance therapy for recurrent, platinum-sensitive ovarian cancer. Gynecol. Oncol. 2024, 186, 104–109. [Google Scholar] [CrossRef]
- Chen, T.; Xu, J.; Xia, B.; Wang, H.; Shen, Y. Secondary cytoreduction surgery for recurrent epithelial ovarian cancer patients after PARPi maintenance: A multicenter, randomized, controlled clinical trial. Int. J. Gynecol. Cancer 2023, 34, 320–331. [Google Scholar] [CrossRef]
- Petrillo, M.; Pedone Anchora, L.; Tortorella, L.; Fanfani, F.; Gallotta, V.; Pacciani, M.; Scambia, G.; Fagotti, A. Secondary Cytoreductive Surgery in Patients with Isolated Platinum-Resistant Recurrent Ovarian Cancer: A Retrospective Analysis. Gynecol. Oncol. 2014, 134, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; du Bois, A.; Hahmann, M.; Hasenburg, A.; Burges, A.; Loibl, S.; Gropp, M.; Huober, J.; Fink, D.; Schröder, W.; et al. Surgery in recurrent ovarian cancer: The Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol. 2006, 13, 1702–1710. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Reuss, A.; Hasenburg, A.; Scambia, G.; Cibula, D.; Mahner, S.; Vergote, I.; Reinthaller, A.; Burges, A.; et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: The Multi- center Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int. J. Gynecol. Cancer 2011, 21, 289–295. [Google Scholar] [CrossRef]
- Sehouli, J.; Richter, R.; Braicu, E.I.; Bühling, K.J.; Bahra, M.; Neuhaus, P.; Lichtenegger, W.; Fotopoulou, C. Role of secondary cytoreductive surgery in ovarian cancer relapse: Who will benefit? A systematic analysis of 240 consecutive patients. J. Surg. Oncol. 2010, 102, 656–662. [Google Scholar] [CrossRef]
- van de Laar, R.; Zusterzeel, P.L.; Van Gorp, T.; Buist, M.R.; van Driel, W.J.; Gaarenstroom, K.N.; Arts, H.J.; van Huisseling, J.C.; Hermans, R.H.; Pijnenborg, J.M.; et al. Cytoreductive surgery followed by chemotherapy versus chemotherapy alone for recurrent platinum-sensitive epithelial ovarian cancer (SOCceR trial): A multi- center randomised controlled study. BMC Cancer 2014, 14, 22. [Google Scholar] [CrossRef]
- van de Laar, R.; Kruitwagen, R.F.; Zusterzeel, P.L.; Van Gorp, T.; Massuger, L.F. Correspondence: Premature stop of the SOCceR trial, a multi- center randomized controlled trial on secondary cytoreductive surgery: Netherlands Trial Register number: NTR3337. Int. J. Gynecol. Cancer 2017, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Jensen, P.T.; Selle, F.; Guyon, F.; Pomel, C.; et al. Ran- domized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ ENGOT ov20. J. Clin. Oncol. 2017, 35, 5501. [Google Scholar] [CrossRef]
- Bristow, R.E.; Peiretti, M.; Gerardi, M.; Zanagnolo, V.; Ueda, S.; Diaz-Montes, T.; Giuntoli, R.L.; Maggioni, A. Secondary Cytoreductive Surgery Including Rectosigmoid Colectomy for Recurrent Ovarian Cancer: Operative Technique and Clinical Outcome. Gynecol. Oncol. 2009, 114, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Certelli, C.; Russo, S.A.; Palmieri, L.; Foresta, A.; Pedone Anchora, L.; Vargiu, V.; Santullo, F.; Fagotti, A.; Scambia, G.; Gallotta, V. Minimally-Invasive Secondary Cytoreduction in Recurrent Ovarian Cancer. Cancers 2023, 15, 4769. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Giudice, M.T.; Conte, C.; Sarandeses, A.V.; D’Indinosante, M.; Federico, A.; Tortorella, L.; Carbone, M.V.; Gueli Alletti, S.; Vizzielli, G.; et al. Minimally invasive salvage lymphadenectomy in gynecological cancer patients: A single institution series. Eur. J. Surg. Oncol. 2018, 44, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.G.Z.; Graul, A.; Yu, M.C.; Halko, A.; Chi, D.S.; Zivanovic, O.; Gardner, G.J.; Sonoda, Y.; Barakat, R.R.; Abu-Rustum, N.R.; et al. Minimal access surgery compared to laparotomy for secondary surgical cytoreduction in patients with recurrent ovarian carcinoma: Perioperative and oncologic outcomes. Gynecol. Oncol. 2017, 146, 263–267. [Google Scholar] [CrossRef]
- Magrina, J.F.; Cetta, R.L.; Chang, Y.H.; Guevara, G.; Magtibay, P.M. Analysis of secondary cytoreduction for recurrent ovarian cancer by robotics, laparoscopy and laparotomy. Gynecol. Oncol. 2013, 129, 336–340. [Google Scholar] [CrossRef]
- Gallotta, V.; Conte, C.; Giudice, M.T.; Nero, C.; Vizzielli, G.; Gueli Alletti, S.; Cianci, S.; Lodoli, C.; Di Giorgio, A.; De Rose, A.M.; et al. Secondary laparoscopic cytoreduction in recurrent ovarian cancer: A large, single-institution experience. J. Minim. Invasive Gynecol. 2018, 25, 644–650. [Google Scholar] [CrossRef]
- Gallotta, V.; Fagotti, A.; Fanfani, F.; Ferrandina, G.; Nero, C.; Costantini, B.; Gueli Alletti, S.; Chiantera, V.; Ercoli, A.; Scambia, G. Laparoscopic surgical management of localized recurrent ovarian cancer: A single-institution experience. Surg. Endosc. 2014, 28, 1808–1815. [Google Scholar] [CrossRef]
- Gallotta, V.; Fanfani, F.; Vizzielli, G.; Panico, G.; Rossitto, C.; Gagliardi, M.L.; Margariti, P.A.; Salerno, M.G.; Zannoni, G.F.; Pacelli, F.; et al. Douglas Peritonectomy Compared to Recto-Sigmoid Resection in Optimally Cytoreduced Advanced Ovarian Cancer Patients: Analysis of Morbidity and Oncological Outcome. Eur. J. Surg. Oncol. 2011, 37, 1085–1092. [Google Scholar] [CrossRef]
- Escobar, P.F.; Levinson, K.L.; Magrina, J.; Martino, M.A.; Barakat, R.R.; Fader, A.N.; Leitao, M.M., Jr. Feasibility and perioperative outcomes of robotic-assisted surgery in the management of recurrent ovarian cancer: A multi-institutional study. Gynecol. Oncol. 2014, 134, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Conte, C.; D’Indinosante, M.; Capoluongo, E.; Minucci, A.; De Rose, A.M.; Ardito, F.; Giuliante, F.; Di Giorgio, A.; Zannoni, G.F.; et al. Prognostic factors value of germline and somatic BRCA in patients undergoing surgery for recurrent ovarian cancer with liver metastases. Eur. J. Surg. Oncol. 2019, 45, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Bruno, M.; Conte, C.; Giudice, M.T.; Davià, F.; Moro, F.; Zannoni, G.F.; Fagotti, A.; De Bonis, M.; Capoluongo, E.; et al. Salvage lymphadenectomy in recurrent ovarian cancer patients: Analysis of clinical outcome and BRCA1/2 gene mutational status. Eur. J. Surg. Oncol. 2020, 46, 1327–1333. [Google Scholar] [CrossRef]
- Conte, C.; Marchetti, C.; Loverro, M.; Giudice, M.T.; Rosati, A.; Gallotta, V.; Scambia, G.; Fagotti, A. Role of Minimally Invasive Secondary Cytoreduction in Patients with Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer 2023, 33, 137–144. [Google Scholar] [CrossRef]
- Gallotta, V.; Certelli, C.; Oliva, R.; Rosati, A.; Federico, A.; Loverro, M.; Lodoli, C.; Foschi, N.; Lathouras, K.; Fagotti, A.; et al. Robotic surgery in ovarian cancer. Erratum Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 91, 102419. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Wong, S.W.; Crowe, P. Visualisation ergonomics and robotic surgery. J. Robot. Surg. 2023, 17, 1873–1878. [Google Scholar] [CrossRef]
- Kavoussi, L.R.; Moore, R.G.; Adams, J.B.; Partin, A.W. Comparison of robotic versus human laparoscopic camera control. J. Urol. 1995, 154, 2134–2136. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, T.; Jeekel, J.; Arends, J.W.; Brinkhorst, A.P.; Kluck, H.M.; Luyk, C.I.; Munting, J.D.; Povel, J.A.; Rutten, A.P.; Volovics, A. No-Touch Isolation Technique in Colon Cancer: A Controlled Prospective Trial. Br. J. Surg. 1988, 75, 409–415. [Google Scholar] [CrossRef]
- Takii, Y.; Shimada, Y.; Moriya, Y.; Nakamura, K.; Katayama, H.; Kimura, A.; Shibata, T.; Fukuda, H. Colorectal Cancer Study Group (CCSG) of Japan Clinical Oncology Group A Randomized Controlled Trial of the Conventional Technique versus the No-Touch Isolation Technique for Primary Tumor Resection in Patients with Colorectal Cancer: Japan Clinical Oncology Group Study JCOG1006. Jpn. J. Clin. Oncol. 2014, 44, 97–100. [Google Scholar] [CrossRef]
- Kuroki, T.; Eguchi, S. No-Touch Isolation Techniques for Pancreatic Cancer. Surg. Today 2017, 47, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Markauskas, A.; Blaakær, J.; Traen, K.J.; Neumann, G.A.; Chunsen, W.; Petersen, L.K. Morbidity following robot-assisted surgery in a gynecological oncology setting: A cohort study. Acta Obs. Gynecol. Scand. 2024, 103, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-H.; Kim, J.; Shim, J.; Kong, T.-W.; Paek, J.; Chang, S.-J.; Ryu, H.-S. Comparison of Posterior Rectal Dissection Techniques during Rectosigmoid Colon Resection as Part of Cytoreductive Surgery in Patients with Epithelial Ovarian Cancer: Close Rectal Dissection versus Total Mesorectal Excision. Gynecol. Oncol. 2019, 153, 362–367. [Google Scholar] [CrossRef]
- Praiss, A.M.; Hirani, R.; Zhou, Q.; Iasonos, A.; Sonoda, Y.; Abu-Rustum, N.R.; Leitao, M.M.; Long Roche, K.; Broach, V.; Gardner, G.J.; et al. Impact of Postoperative Morbidity on Outcomes in Patients with Advanced Epithelial Ovarian Cancer Undergoing Intestinal Surgery at the Time of Primary or Interval Cytoreductive Surgery: A Memorial Sloan Kettering Cancer Center Team Ovary Study. Gynecol. Oncol. 2023, 179, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Pazdírek, F.; Vjaclovský, M.; Kocian, P.; Bockova, M.; Hoch, J. Robotic resection of the rectum - what are the advantages? Rozhl. Chir. 2023, 102, 459–463. [Google Scholar] [CrossRef]
- Grass, J.K.; Chen, C.C.; Melling, N.; Lingala, B.; Kemper, M.; Scognamiglio, P.; Persiani, R.; Tirelli, F.; Caricato, M.; Capolupo, G.T.; et al. Robotic rectal resection preserves anorectal function: Systematic review and meta-analysis. Int. J. Med. Robot. 2021, 17, e2329. [Google Scholar] [CrossRef]
- Tjandra, J.J.; Chan, M.K. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Color. Dis. 2008, 8, 305–306. [Google Scholar] [CrossRef]
- Lorenzon, L.; Bini, F.; Balducci, G.; Ferri, M.; Salvi, P.F.; Marinozzi, F. Laparoscopic versus Robotic-Assisted Colectomy and Rectal Resection: A Systematic Review and Meta-Analysis. Int. J. Color. Dis. 2016, 31, 161–173. [Google Scholar] [CrossRef]
- Vaghiri, S.; Prassas, D.; Krieg, S.; Knoefel, W.T.; Krieg, A. Intracorporeal Versus Extracorporeal Colo-colic Anastomosis in Minimally-invasive Left Colectomy: A Systematic Review and Meta-analysis. J. Gastrointest. Surg. 2023, 27, 3024–3037. [Google Scholar] [CrossRef]
- Brown, R.F.; Cleary, R.K. Intracorporeal anastomosis versus extracorporeal anastomosis for minimally invasive colectomy. J. Gastrointest. Oncol. 2020, 11, 500–507. [Google Scholar] [CrossRef]
- Al Natour, R.H.; Obias, V.; Albright, J.; Wu, J.; Ferraro, J.; Akram, W.M.; McClure, A.M.; Shanker, B.A.; Cleary, R.K. A propensity score-matched comparison of intracorporeal and extracorporeal techniques for robotic-assisted sigmoidectomy in an enhanced recovery pathway. J. Robot. Surg. 2019, 13, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Achilli, P.; Perry, W.; Grass, F.; Abd El Aziz, M.A.; Kelley, S.R.; Larson, D.W.; Behm, K.T. Completely intracorporeal anastomosis in robotic left colonic and rectal surgery: Technique and 30-day outcomes. Updates Surg. 2021, 73, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Santullo, F.; Attalla El Halabieh, M.; Lodoli, C.; Abatini, C.; Rosati, A.; Ianieri, M.; Scambia, G.; De Cicco Nardone, A. Totally Intracorporeal Colorectal Anastomosis after Segmental Sigmoid Resection with Inferior Mesenteric Artery Preservation for Deep Infiltrating Endometriosis. Tech. Coloproctol. 2021, 25, 745–746. [Google Scholar] [CrossRef]
- Hur, C.; Falcone, T. Robotic treatment of bowel endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Stabilini, C.; Barra, F.; Clarizia, R.; Roviglione, G.; Ceccaroni, M. Bowel resection for intestinal endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 114–128. [Google Scholar] [CrossRef]
- Reitz, A.C.W.; Lin, E.; Rosen, S.A. A single surgeon’s experience transitioning to robotic-assisted right colectomy with intracorporeal anastomosis. Surg. Endosc. 2018, 32, 3525–3532. [Google Scholar] [CrossRef]
- Larsen, P.O.; Nerup, N.; Andersen, J.; Dohrn, N.; Klein, M.F.; Brisling, S.; Salomon, S.; Andersen, P.V.; Möller, S.; Svendsen, M.B.S.; et al. Anastomotic perfusion assessment with indocyanine green in robot-assisted low-anterior resection, a multicenter study of interobserver variation. Surg. Endosc. 2023, 37, 3602–3609. [Google Scholar] [CrossRef]
- Jiang, Z.; Salcudean, S.E.; Navab, N. Robotic ultrasound imaging: State-of-the-art and future perspectives. Med. Image Anal. 2023, 89, 102878. [Google Scholar] [CrossRef]




| Age | BMI | Number of Pervious Surgeries | NACT | FIRST Surgery | Hystology | FIGO Stage | BRAC Status | Number of Recurrencec | PFI | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | 40 | 1 | no | LPT: TH + BSO + omentectomy + pelvic and aortic LND + appendectomy + WP | HGSOC | IIIA1(ii) | WT | I | 19 |
| 2 | 46 | 35 | 1 | no | LPT BSO Restaging LPT: LND + omentectomy | Granulosa cell tumor | IIIB | - | II | 10 |
| 3 | 56 | 24 | 2 | yes | IDS-LPT: TH + BSO + omentectomy + Douglas peritonectomy | HGSOC | IIIC | WT | I | 28 |
| 4 | 64 | 24 | 4 | yes | IDS-LPT: TH + BSO + omentectomy + Douglas peritonectomy + splenctomy + HIPEC | HGSOC | IVB | WT | I | 11 |
| 5 | 57 | 26 | 1 | no | LPT: TH + BSO + omentectomy + appendectomy + Douglas peritonectomy | Endometrioid G1 | IIA | - | I | 19 |
| Operative Time (min) | EBL (mL) | Intraoperative Complications | Hospital Stay (Days) | Adjuvant Therapy | FUP (Months) | Status | |
|---|---|---|---|---|---|---|---|
| 1 | 480 | 200 | no | 6 | VI cycles Carboplatin-Caelyx + Rucaparib | 28 | alive |
| 2 | 300 | 300 | No | 9 | Letrozole | 16 | Recurrence at 6 months; alive |
| 3 | 210 | 200 | no | 6 | VI cycles Carboplatin | 16 | alive |
| 4 | 270 | 100 | no | 7 | Niraparib continuation | 7 | alive |
| 5 | 210 | 100 | no | 13 | Letrozole | 5 | alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallotta, V.; Palmieri, L.; Santullo, F.; Certelli, C.; Lodoli, C.; Abatini, C.; El Halabieh, M.A.; D’Indinosante, M.; Federico, A.; Rosati, A.; et al. Robotic Rectosigmoid Resection with Totally Intracorporeal Colorectal Anastomosis (TICA) for Recurrent Ovarian Cancer: A Case Series and Description of the Technique. J. Pers. Med. 2024, 14, 1052. https://doi.org/10.3390/jpm14101052
Gallotta V, Palmieri L, Santullo F, Certelli C, Lodoli C, Abatini C, El Halabieh MA, D’Indinosante M, Federico A, Rosati A, et al. Robotic Rectosigmoid Resection with Totally Intracorporeal Colorectal Anastomosis (TICA) for Recurrent Ovarian Cancer: A Case Series and Description of the Technique. Journal of Personalized Medicine. 2024; 14(10):1052. https://doi.org/10.3390/jpm14101052
Chicago/Turabian StyleGallotta, Valerio, Luca Palmieri, Francesco Santullo, Camilla Certelli, Claudio Lodoli, Carlo Abatini, Miriam Attalla El Halabieh, Marco D’Indinosante, Alex Federico, Andrea Rosati, and et al. 2024. "Robotic Rectosigmoid Resection with Totally Intracorporeal Colorectal Anastomosis (TICA) for Recurrent Ovarian Cancer: A Case Series and Description of the Technique" Journal of Personalized Medicine 14, no. 10: 1052. https://doi.org/10.3390/jpm14101052
APA StyleGallotta, V., Palmieri, L., Santullo, F., Certelli, C., Lodoli, C., Abatini, C., El Halabieh, M. A., D’Indinosante, M., Federico, A., Rosati, A., Conte, C., Oliva, R., Fagotti, A., & Scambia, G. (2024). Robotic Rectosigmoid Resection with Totally Intracorporeal Colorectal Anastomosis (TICA) for Recurrent Ovarian Cancer: A Case Series and Description of the Technique. Journal of Personalized Medicine, 14(10), 1052. https://doi.org/10.3390/jpm14101052







