Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Control Subjects
2.2. Clinical Evaluation
2.3. Determination of Neutrophil Lymphocyte Ratio (NLR)
2.4. Statistics
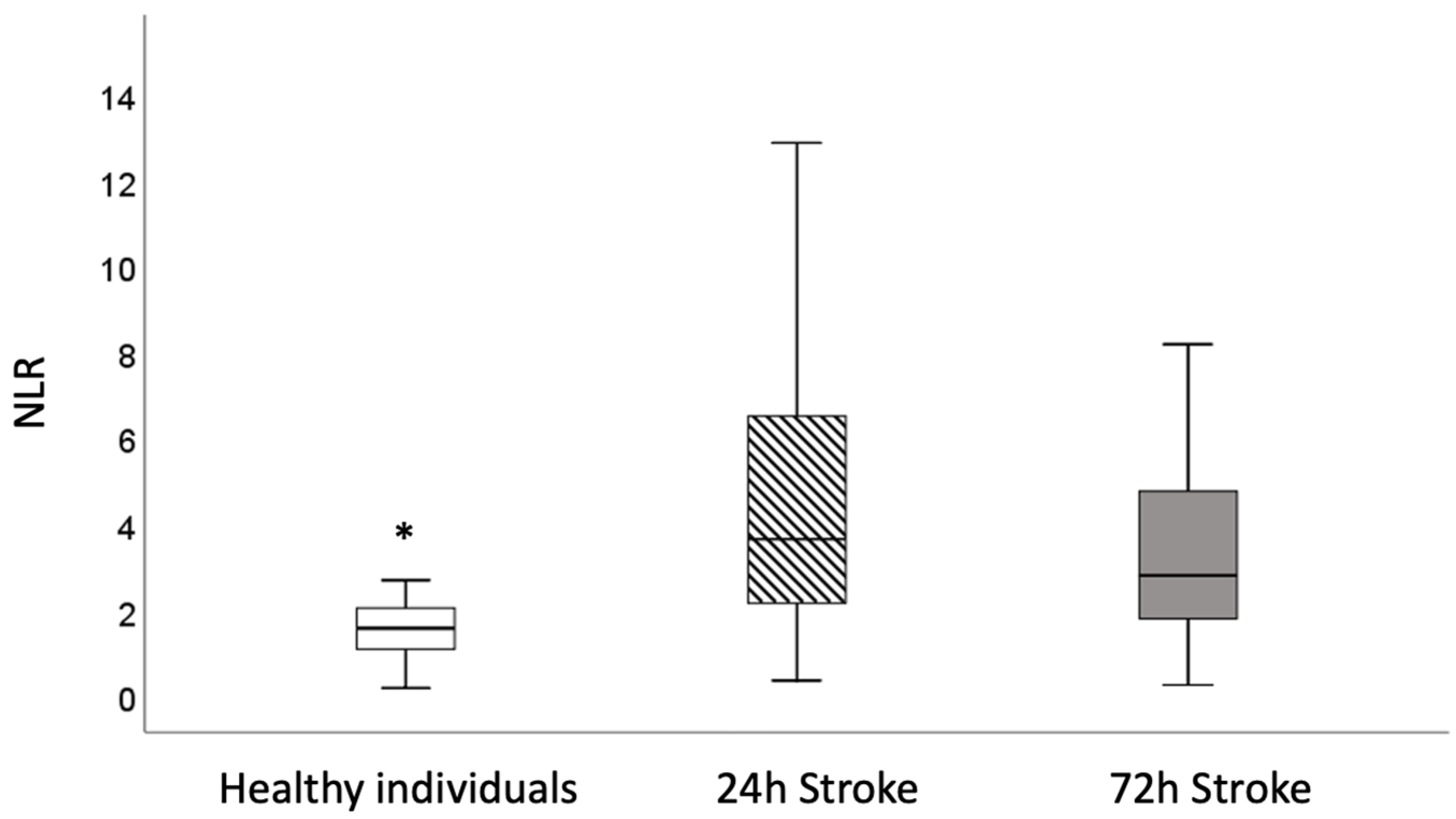
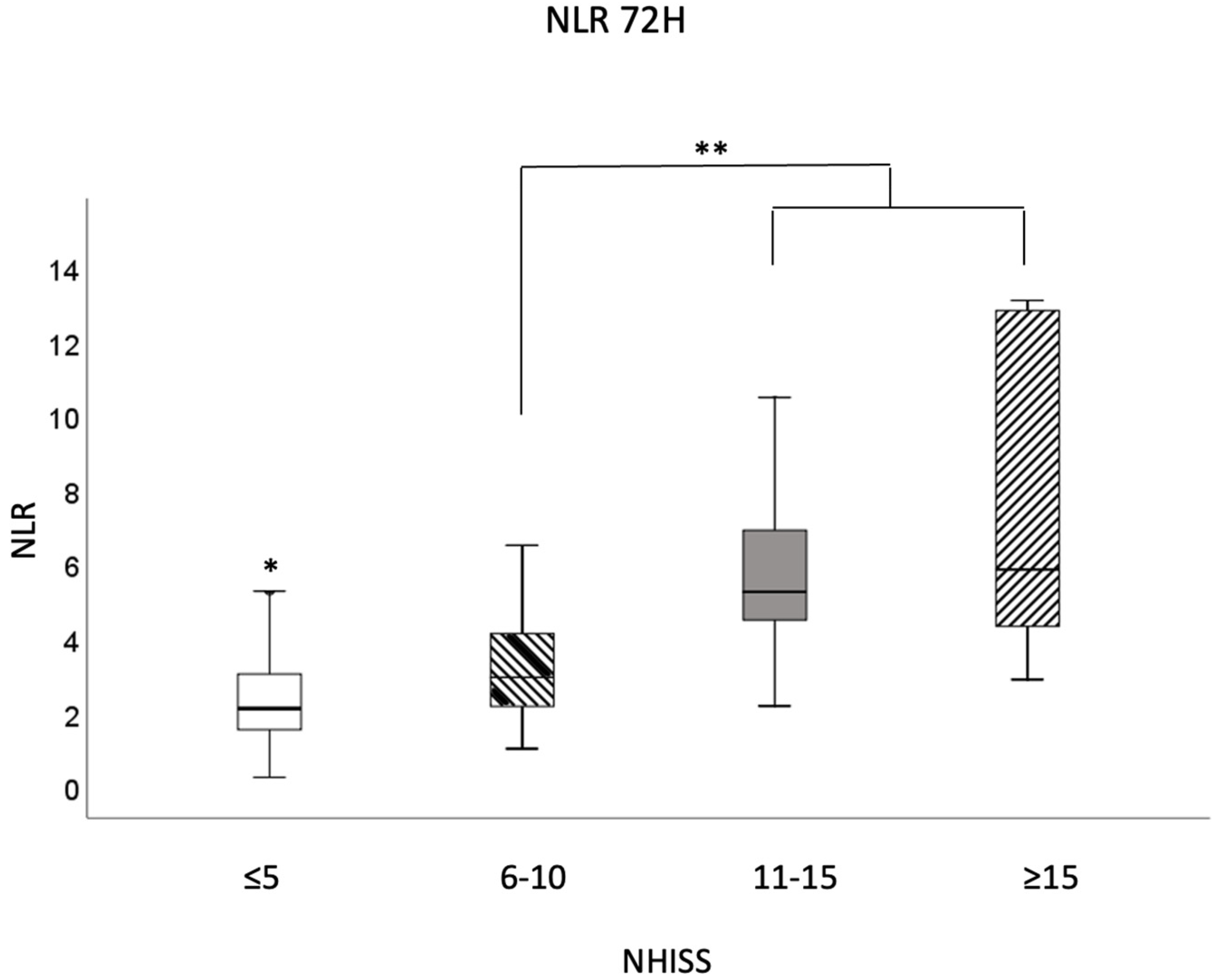
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO: World Health Organization. Stroke, Cerebrovascular Accident. Available online: https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html (accessed on 1 March 2023).
- Graça, S.C.; Mosca, T.; Gagliardi, R.J.; Forte, W.C.N. Neutrophilic inflammation in stroke. Rev. AMB 2021, 67, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Stroke. Lancet 2016, 389, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.B.; Yu, Z.M.; Guo, P.; Wang, Q.Q.; Qi, R.M.; Shan, M.J.; Lv, J.; Gong, T. Neutrophils and neutrophil-lymphocyte ratio: Inflammatory markers associated with intimal-media thickness of atherosclerosis. Thromb. Res. 2018, 170, 45–52. [Google Scholar] [CrossRef]
- Kocaturk, O.; Besli, F.; Gungoren, F.; Kocaturk, M.; Tanriverdi, Z. The relationship among neutrophil to lymphocyte ratio, stroke territory, and 3-month mortality in patients with acute ischemic stroke. Neurol. Sci. 2019, 40, 139–146. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wu, G. Relationship of Neutrophil-to-Lymphocyte Ratio with Carotid Plaque Vulnerability and Occurrence of Vulnerable Carotid Plaque in Patients with Acute Ischemic Stroke. Biomed. Res. Int. 2021, 2021, 6894623. [Google Scholar] [CrossRef]
- Otxoa, A.A.; Miró, M.F.; Pedragosa, J.; Gallizioli, M.; Justicia, C.; Gaja Capdevila, N.; Ruíz-Jaen, F.; Salas-Perdomo, A.; Bosch, A.; Calvo, M.; et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019, 137, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Akter, F.; Qin, L.; Cheng, J.; Guo, M.; Yao, S.; Jian, Z.; Liu, R.; Wu, S. Adaptive Immunity Regulation and Cerebral Ischemia. Front. Immunol. 2020, 11, 689. [Google Scholar] [CrossRef]
- Corriere, C.; Marca, S.D.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef]
- Stowe, A.M.; Adair-Kirk, T.L.; Gonzales, E.R.; Perez, R.S.; Shah, A.R.; Park, T.S.; Gidday, J.M. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol. Dis. 2009, 35, 82–90. [Google Scholar] [CrossRef]
- Vallés, J.; Lago, A.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Moscardó, A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: Prognostic significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef]
- Forte, W.C.N. Órgãos linfoides e subpopulações de linfócitos. In Imunologia do Básico ao Aplicado, 4th ed.; Forte, W.C.N., Ed.; Editora Atheneu: Rio de Janeiro, Brazil, 2023; pp. 35–50. [Google Scholar]
- McColl, B.W.; Rothwell, N.J.; Allan, S.M. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J. Neurosci. 2007, 18, 4403–4412. [Google Scholar] [CrossRef]
- Protti, G.; Gagliardi, R.J.; Forte, W.C.; Sprovieri, S.R. Interleukin-10 may protect against progressing injury during the acute phase of ischemic stroke. Arq. Neuropsiquiatr. 2013, 71, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Menni, A.E.; Tzikos, G.; Fyntanidou, B.; Ioannidis, A.; Loukipoudi, L.; Grosomanidis, V.; Chorti, A.; Shrewsbury, A.; Stavrou, G.; Kotzampassi, K. The Effect of Probiotics on the Prognostication of the Neutrophil-to-Lymphocyte Ratio in Severe Multi-Trauma Patients. J. Pers. Med. 2024, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Mahe, J.; Wang, L.; Guo, K.; Liu, X.; Zeng, X.; Jing, L. High leukocyte-to-lymphocyte ratio is associated with acute relapse in multiple sclerosis patients. Neurol. Res. 2022, 44, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Pikija, S.; Sztriha, L.K.; Killer-Oberpfalzer, M.; Weymayr, F.; Hecker, C.; Ramesmayer, C.; Hauer, L.; Sellner, J. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J. Neuroinflamm. 2018, 15, 319. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Koton, S.; Patole, S.; Carlson, J.M.; Haight, T.; Johansen, M.; Schneider, A.L.C.; Pike, J.R.; Gottesman, R.F.; Coresh, J. Methods for stroke severity assessment by chart review in the Atherosclerosis Risk in Communities study. Sci. Rep. 2022, 12, 12338. [Google Scholar] [CrossRef]
- Koton, S.; Pike, J.R.; Johansen, M.; Knopman, D.S.; Lakshminarayan, K.; Mosley, T.; Patole, S.; Rosamond, W.D.; Schneider, A.L.C.; Sharrett, A.R.; et al. Association of Ischemic Stroke Incidence, Severity, and Recurrence with Dementia in the Atherosclerosis Risk in Communities Cohort Study. JAMA Neurol. 2022, 79, 271–280. [Google Scholar] [CrossRef]
- Garcia, J.H. The neuropathology of stroke. Hum. Pathol. 1975, 6, 583–598. [Google Scholar] [CrossRef]
- Bivard, A.; Garcia-Esperon, C.; Churilov, L.; Spratt, N.; Russell, M.; Campbell, B.C.; Choi, P.; Kleinig, T.; Ma, H.; Markus, H.; et al. Tenecteplase versus alteplase for stroke thrombolysis evaluation (TASTE): A multicentre, prospective, randomized, open-label, blinded-endpoint, controlled phase III non-inferiority trial protocol. Int. J. Stroke 2023, 18, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Med. J. 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Gosav, E.M.; Tanase, D.M.; Buliga-Finis, O.N.; Rezuș, I.-I.; Morariu, P.C.; Floria, M.; Rezus, C. The Prognostic Role of the Neutrophil-to-Lymphocytes Ratio in the Most Frequent Cardiovascular Diseases: An Update. Life 2024, 14, 985. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cai, L.; Yi, T.; Yi, X.; Hu, Y. Neutrophil-to-lymphocyte ratio is associated with stroke progression and functional outcome in patients with ischemic stroke. Brain Behav. 2023, 13, 3261. [Google Scholar] [CrossRef]
- Xue, W.; Li, Y.; Xia, H.; Yu, T.; Sun, S.; Zhang, M. Influence of neutrophil-to-lymphocyte ratio and mean platelet volume on severity and short-term prognosis of acute ischemic stroke. Am. J. Transl. Res. 2022, 14, 4066–4073. [Google Scholar]
- Ying, Y.; Yu, F.; Luo, Y.; Feng, X.; Liao, D.; Wei, M.; Li, X.; Huang, Q.; Liu, Z.; Zhang, L.; et al. Neutrophil-to-Lymphocyte Ratio as a Predictive Biomarker for Stroke Severity and Short-Term Prognosis in Acute Ischemic Stroke with Intracranial Atherosclerotic Stenosis. Front. Neurol. 2021, 12, 705949. [Google Scholar] [CrossRef]
- Chojdak-Łukasiewicz, J.; Dziadkowiak, E.; Zimny, A.; Paradowski, B. Cerebral small vessel disease: A review. Adv. Clin. Exp. Med. 2021, 30, 349–356. [Google Scholar] [CrossRef]
- Tortora, G.J.; Derrickson, B. Encéfalo e nervos cranianos. In Princípios de Anatomia e Fisiologia, 10th ed.; Editora Guanabara Koogan: Rio de Janeiro, Brazil, 2016; pp. 478–526. [Google Scholar]
- Przykaza, L. Understanding the Connection Between Common Stroke Comorbidities, Their Associated Inflammation, and the Course of the Cerebral Ischemia/Reperfusion Cascade. Front. Immunol. 2021, 12, 782569. [Google Scholar] [CrossRef]
- Swan, G.E.; Lessov-Schlaggar, C.N. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007, 17, 259–273. [Google Scholar] [CrossRef]
- De La Monte, S.M.; Kril, J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014, 127, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Montecucco, F.; Dallegri, F. Update on the effects of treatment with recombinant tissue-type plasminogen activator (rt-PA) in acute ischemic stroke. Expert Opin. Biol. Ther. 2016, 16, 1323–1340. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, M.G.; Paciaroni, M. Treatments in Ischemic Stroke: Current and Future. Eur. Neurol. 2022, 85, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheng, J.; Ye, Q.; Ye, Z.; Zhang, Y.; Liu, Y.; Huang, G.; Chen, F.; Yang, M.; Wang, C.; et al. Day 1 neutrophil-to-lymphocyte ratio (NLR) predicts stroke outcome after intravenous thrombolysis and mechanical thrombectomy. Front. Neurol. 2022, 13, 941251. [Google Scholar] [CrossRef]


| 24 h | 72 h | ||
|---|---|---|---|
| Subtype of stroke | Cardioembolic (n = 66) | 3.82 (3.99–5.46) | 2.96 (3.47–5.80) |
| Atherothrombotic (n = 54) | 3.69 (4.17–6.45) | 3.04 (3.16–5.84) | |
| Small vases (n = 26) | 2.53 * (2.47–4.16) | 1.97 * (1.75–2.65) | |
| Cryptogenic (n = 48) | 4.10 (3.87–6.59) | 2.57 (2.19–4.27) | |
| Stroke topography | Supratentorial (n = 161) | 3.9 (0.4–18.6) | 2.9 (0.3–31.0) |
| Infratentorial (n = 33) | 3.0 (1.3–16.3) | 2.2 (0.9–11.3) | |
| Risk factors | Systemic Arterial hypertension (n = 128) | 3.6 (0.4–18.1) | 2.8 (0.3–31.0) |
| Diabetes mellitus (n = 63) | 3.6 (1.3–18.1) | 2.9 (0.9–31.0) | |
| Smoking (n = 36) | 3.6 (0.4–10.1) | 2.6 (0.3–13.1) | |
| Previous stroke (n = 31) | 3.8 (1.3–10.6) | 2.9 (0.9–7.0) | |
| Alcoholism (n = 29) | 3.6 (1.3–10.1) | 2.8 (0.9–6.1) | |
| Dyslipidemia (n = 22) | 4.9 (1.3–16.4) | 4.3 (1.1–12.8) | |
| Treatment | Thrombolyzed (n = 73) | 3.9 (1.3–18.6) | 2.9 (0.9–31.0) |
| Not thrombolyzed (n = 116) | 3.7 (0.4–16.3) | 2.6 (0.3–16.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, S.C.; Mosca, T.; Gagliardi, V.D.B.; Forte, W.C.N.; Gagliardi, R.J. Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Ischemic Stroke. J. Pers. Med. 2024, 14, 1149. https://doi.org/10.3390/jpm14121149
Graça SC, Mosca T, Gagliardi VDB, Forte WCN, Gagliardi RJ. Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Ischemic Stroke. Journal of Personalized Medicine. 2024; 14(12):1149. https://doi.org/10.3390/jpm14121149
Chicago/Turabian StyleGraça, Santhiago Calvelo, Tainá Mosca, Vivian Dias Baptista Gagliardi, Wilma Carvalho Neves Forte, and Rubens José Gagliardi. 2024. "Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Ischemic Stroke" Journal of Personalized Medicine 14, no. 12: 1149. https://doi.org/10.3390/jpm14121149
APA StyleGraça, S. C., Mosca, T., Gagliardi, V. D. B., Forte, W. C. N., & Gagliardi, R. J. (2024). Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Ischemic Stroke. Journal of Personalized Medicine, 14(12), 1149. https://doi.org/10.3390/jpm14121149






