Infectious Complications in Laparoscopic Gynecologic Oncology Surgery within an ERAS-Compliant Setting
Abstract
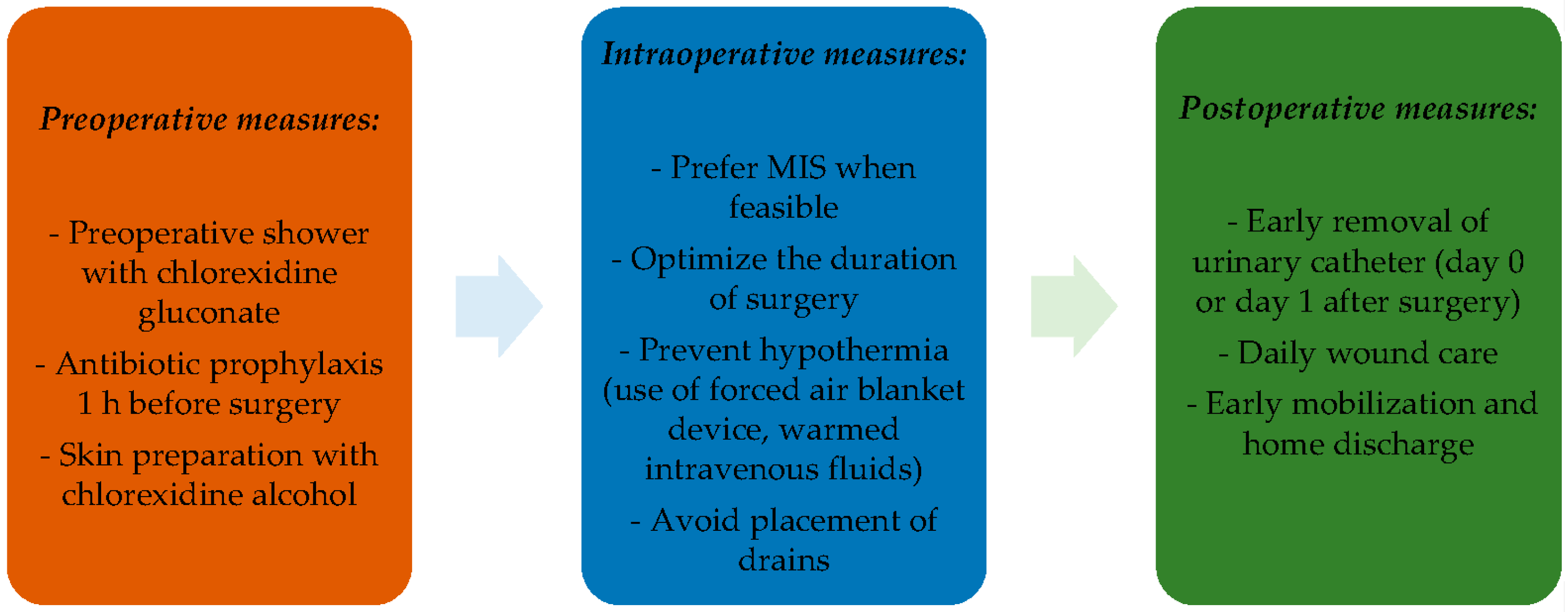
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
4.1. Results in the Context of Published Literature
4.2. Strengths and Weaknesses
4.3. Implications for Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, K.I.; Fader, A.N. New Developments in Minimally Invasive Gynecologic Oncology Surgery. Clin. Obstet. Gynecol. 2017, 60, 330–348. [Google Scholar] [CrossRef]
- Watrowski, R.; Kostov, S.; Alkatout, I. Complications in laparoscopic and robotic-assisted surgery: Definitions, classifications, incidence and risk factors—An up-to-date review. Videosurgery Other Miniinvasive Tech. 2021, 16, 501–525. [Google Scholar] [CrossRef]
- Ghezzi, F.; Cromi, A.; Uccella, S.; Siesto, G.; Giudici, S.; Serati, M.; Franchi, M. Laparoscopic versus open surgery for endometrial cancer: A minimum 3-year follow-up study. Ann. Surg. Oncol. 2010, 17, 271–278. [Google Scholar] [CrossRef]
- Díaz Feijoo, B.; Concin, N.; Matias Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.A.; Bosse, T.; Chargari, C. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. Available online: https://diposit.ub.edu/dspace/handle/2445/184431 (accessed on 5 July 2023).
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. SUCCOR study: An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Anchora, L.P.; Turco, L.C.; Bizzarri, N.; Capozzi, V.A.; Lombisani, A.; Chiantera, V.; De Felice, F.; Gallotta, V.; Cosentino, F.; Fagotti, A.; et al. How to Select Early-Stage Cervical Cancer Patients Still Suitable for Laparoscopic Radical Hysterectomy: A Propensity-Matched Study. Ann. Surg. Oncol. 2020, 27, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Radiother Oncol. 2023, 184, 109682. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; de Kroon, C.; Van Driel, W.; Tidy, J.; Marth, C.; et al. An international randomized phase III trial comparing radical hysterectomy and pelvic node dissection (RH) vs simple hysterectomy and pelvic node dissection (SH) in patients with low-risk early-stage cervical cancer (LRESCC): A Gynecologic Cancer Intergroup study led by the Canadian Cancer Trials Group (CCTG CX.5-SHAPE). J. Clin. Orthod. 2023, 41, LBA5511. [Google Scholar]
- Falconer, H.; Palsdottir, K.; Stalberg, K.; Dahm-Kähler, P.; Ottander, U.; Lundin, E.S.; Wijk, L.; Kimmig, R.; Jensen, P.T.; Eriksson, A.G.Z.; et al. Robot-assisted approach to cervical cancer (RACC): An international multi-center, open-label randomized controlled trial. Int. J. Gynecol. Cancer 2019, 29, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Kim, M.K.; Jung, Y.W.; Yun, B.S.; Seong, S.J.; Choi, C.H.; Kim, T.-J.; Lee, J.-W.; Bae, D.-S.; Kim, B.-G. Minimally invasive compared with open surgery in patients with borderline ovarian tumors. Gynecol. Oncol. 2017, 145, 508–512. [Google Scholar] [CrossRef]
- Maneo, A.; Vignali, M.; Chiari, S.; Colombo, A.; Mangioni, C.; Landoni, F. Are borderline tumors of the ovary safely treated by laparoscopy? Gynecol. Oncol. 2004, 94, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Candotti, G.; Peiretti, M.; Mangili, G.; Bergamini, A.; Candiani, M.; Cioffi, R.; Mais, V.; Rabaiotti, E.; Bocciolone, L. What women want: Fertility sparing surgery in Borderline ovarian tumours patients and pregnancy outcome. Eur. J. Surg. Oncol. 2020, 46, 888–892. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Falcetta, F.S.; Lawrie, T.A.; Medeiros, L.R.; da Rosa, M.I.; Edelweiss, M.I.; Stein, A.T.; Zelmanowicz, A.; Moraes, A.B.; Zanini, R.R.; Rosa, D. D Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst. Rev. 2016, 10, CD005344. [Google Scholar]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Garganese, G.; Vizzielli, G.; Carone, V.; Salerno, M.G.; Scambia, G. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am. J. Obstet. Gynecol. 2008, 199, 642.e1–642.e6. [Google Scholar] [CrossRef]
- Alletti, S.G.; Capozzi, V.A.; Rosati, A.; De Blasis, I.; Cianci, S.; Vizzielli, G.; Uccella, S.; Gallotta, V.; Fanfani, F.; Fagotti, A.; et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: A systematic review of the literature. Minerva Med. 2019, 110, 341–357. [Google Scholar]
- Nitecki, R.; Rauh-Hain, J.A.; Melamed, A.; Scambia, G.; Pareja, R.; Coleman, R.L.; Ramirez, P.T.; Fagotti, A. Laparoscopic cytoreduction After Neoadjuvant ChEmotherapy (LANCE). Int. J. Gynecol. Cancer 2020, 30, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, V.; Bizzarri, N.; Guida, F.; Vascone, C.; Costantini, B.; Scambia, G.; Fagotti, A. Role of surgery in gynaecological sarcomas. Oncotarget 2019, 10, 2561–2575. [Google Scholar] [CrossRef]
- Bogani, G.; Chiappa, V.; Ditto, A.; Martinelli, F.; Donfrancesco, C.; Indini, A.; Lorusso, D.; Raspagliesi, F. Morcellation of undiagnosed uterine sarcoma: A critical review. Crit. Rev. Oncol. Hematol. 2016, 98, 302–308. [Google Scholar] [CrossRef]
- DiNapoli, M.N.; Truong, M.D.; Halfon, J.K.; Burke, W.M. Unsuspected Uterine Sarcoma in an Urban Hospital: Does Surgical Approach Matter? J. Minim. Invasive Gynecol. 2018, 25, 491–497. [Google Scholar] [CrossRef]
- Lee, M.S.; Venkatesh, K.K.; Growdon, W.B.; Ecker, J.L.; York-Best, C.M. Predictors of 30-day readmission following hysterectomy for benign and malignant indications at a tertiary care academic medical center. Am. J. Obstet. Gynecol. 2016, 214, 607.e1–607.e12. [Google Scholar] [CrossRef]
- Radosa, M.P.; Meyberg-Solomayer, G.; Radosa, J.; Vorwergk, J.; Oettler, K.; Mothes, A.; Baum, S.; Juhasz-Boess, I.; Petri, E.; Solomayer, E.F.; et al. Standardised Registration of Surgical Complications in Laparoscopic-Gynaecological Therapeutic Procedures Using the Clavien-Dindo Classification. Geburtshilfe Frauenheilkd. 2014, 74, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, S.; Suarez-Salvador, E.; Buras, M.; Magtibay, P.; Magrina, J. Mortality Rates in Laparoscopic and Robotic Gynecologic Oncology Surgery: A Systemic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2019, 26, 1253–1267.e4. [Google Scholar] [CrossRef]
- Jaiyeoba, O. Postoperative infections in obstetrics and gynecology. Clin. Obstet. Gynecol. 2012, 55, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Lachiewicz, M.P.; Moulton, L.J.; Jaiyeoba, O. Infection Prevention and Evaluation of Fever after Laparoscopic Hysterectomy. J. Soc. Laparoendosc. Surg. 2015, 19, e2015.00065. [Google Scholar] [CrossRef]
- Clarke-Pearson, D.L.; Geller, E.J. Complications of hysterectomy. Obstet. Gynecol. 2013, 121, 654–673. [Google Scholar] [CrossRef]
- Seaman, S.J.; Han, E.; Arora, C.; Kim, J.H. Surgical site infections in gynecology: The latest evidence for prevention and management. Curr. Opin. Obstet. Gynecol. 2021, 33, 296–304. [Google Scholar] [CrossRef]
- Nelson, G.; Ramirez, P.T.; Dowdy, S.C.; Douglas Wilson, R.; Scott, M.J. The ERAS® Society Handbook for Obstetrics & Gynecology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Nelson, G.; Fotopoulou, C.; Taylor, J.; Glaser, G.; Bakkum-Gamez, J.; Meyer, L.; Stone, R.; Mena, G.; Elias, K.; Altman, A.; et al. Enhanced recovery after surgery (ERAS®) society guidelines for gynecologic oncology: Addressing implementation challenges—2023 update. Gynecol. Oncol. 2023, 173, 58–67. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Pickett, C.M.; Seeratan, D.D.; Mol, B.W.J.; Nieboer, T.E.; Johnson, N.; Bonestroo, T.; Aarts, J.W. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst. Rev. 2023, 8, CD003677. [Google Scholar]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651. [Google Scholar] [CrossRef]
- Dedden, S.J.; Derix, M.M.P.; Geomini, P.M.A.J.; Maas, J.W.M.; Bongers, M.Y. Immediate catheter removal after laparoscopic hysterectomy: A retrospective analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 76–79. [Google Scholar] [CrossRef]
- Catanzarite, T.; Saha, S.; Pilecki, M.A.; Kim, J.Y.S.; Milad, M.P. Longer Operative Time During Benign Laparoscopic and Robotic Hysterectomy Is Associated with Increased 30-Day Perioperative Complications. J. Minim. Invasive Gynecol. 2015, 22, 1049–1058. [Google Scholar] [CrossRef]
- Singh, S.; Swarer, K.; Resnick, K. Longer operative time is associated with increased post-operative complications in patients undergoing minimally-invasive surgery for endometrial cancer. Gynecol. Oncol. 2017, 147, 554–557. [Google Scholar] [CrossRef]
- Kaya, A.C.; Radosa, M.P.; Zimmermann, J.S.M.; Stotz, L.; Findeklee, S.; Hamza, A.; Sklavounos, P.; Takacs, F.Z.; Wagenpfeil, G.; Radosa, C.G.; et al. Intraoperative and postoperative complications of gynecological laparoscopic interventions: Incidence and risk factors. Arch. Gynecol. Obstet. 2021, 304, 1259–1269. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, B.P.-H.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef]
- Nugent, E.K.; Hoff, J.T.; Gao, F.; Massad, L.S.; Case, A.; Zighelboim, I.; Mutch, D.G.; Thaker, P.H. Wound complications after gynecologic cancer surgery. Gynecol. Oncol. 2011, 121, 347–352. [Google Scholar] [CrossRef]
- Marra, A.R.; Puig-Asensio, M.; Edmond, M.B.; Schweizer, M.L.; Bender, D. Infectious complications of laparoscopic and robotic hysterectomy: A systematic literature review and meta-analysis. Int. J. Gynecol. Cancer 2019, 29, 518–530. [Google Scholar] [CrossRef]
- Chi, D.S.; Abu-Rustum, N.R.; Sonoda, Y.; Awtrey, C.; Hummer, A.; Venkatraman, E.S.; Franklin, C.C.; Hamilton, F.; Gemignani, M.L.; Barakat, R.R. Ten-year experience with laparoscopy on a gynecologic oncology service: Analysis of risk factors for complications and conversion to laparotomy. Am. J. Obstet. Gynecol. 2004, 191, 1138–1145. [Google Scholar] [CrossRef]
| Total | Complication n; % | No Complication n; % | |
|---|---|---|---|
| 260; 100 | 15; 5.8 | 245; 94.2 | |
| Parity | 214 | 14; 93.3 | 200; 81.6 |
| Age median (years) | 63 | 60 | 63 |
| BMI median (kg/m2) | 27 | 28 | 26 |
| High blood pressure | 94 | 7; 46.7 | 87; 35.5 |
| Diabetes | 20 | 1; 6.7 | 19; 7.8 |
| Hypothyroidism | 32 | 4; 26.7 | 28; 11.4 |
| Cardiovascular disease | 27 | 3; 20 | 24; 9.8 |
| Psychiatric disorders | 5 | 1; 6.7 | 4; 1.6 |
| ASA Status | |||
| 1 | 12 | 0; | 12; 4.9 |
| 2 | 204 | 10; 66.7 | 194; 79.2 |
| 3 | 44 | 5; 33.3 | 39; 15.9 |
| Total n; % | Complication n; % | No Complication n; % | |
|---|---|---|---|
| 260; 100 | 15; 5.8% | 245; 94.2% | |
| Primary disease | |||
| Endometrial Cancer | 180; 69.2 | 11; 73.3 | 169; 68.9 |
| Cervical Cancer | 18; 6.9 | 1; 6.7 | 17; 6.9 |
| Ovarian Cancer | 9; 3.5 | 3; 20 | 6; 2.4 |
| BOT | 51; 19.6 | 0 | 51; 20.8 |
| Uterine sarcoma | 2; 0.8 | 0 | 2; 0.8 |
| FIGO stage | |||
| Endometrial Cancer (2009) | |||
| IA | 110; 42.3 | 7; 46.8 | 103; 42 |
| IB | 34; 13.1 | 0 | 34; 13.9 |
| II | 3; 1.2 | 1; 6.6 | 2; 0.8 |
| IIIA | 7; 2.7 | 0 | 7; 2.9 |
| IIIB | 1; 0.4 | 0 | 1; 0.4 |
| IIIC | 24; 9.2 | 3; 20 | 21; 9.3 |
| IVB | 1; 0.4 | 0 | 1; 0.4 |
| Cervical Cancer (2018) | |||
| IA1 | 6; 2.3 | 0 | 6; 2.5 |
| IA2 | 2; 0.8 | 1; 6.6 | 1; 0.4 |
| IB1 | 4; 1.5 | 0 | 4; 1.5 |
| IB2 | 3; 1.3 | 0 | 3; 1.2 |
| IB3 | 1; 0.4 | 0 | 1; 0.4 |
| IIB | 1; 0.4 | 0 | 1; 0.4 |
| IIIC | 1; 0.4 | 0 | 1; 0.4 |
| Ovarian Tumor (2013) | |||
| IA | 45; 17.3 | 2; 13.4 | 43; 17.5 |
| IB | 2; 0.8 | 0 | 2; 0.8 |
| IC | 5; 1.9 | 0 | 5; 2.0 |
| IIA | 2; 0.8 | 0 | 2; 0.8 |
| IIB | 1; 0.4 | 0 | 1; 0.4 |
| IIIA | 1; 0.4 | 0 | 1; 0.4 |
| IIIB | 1; 0.4 | 0 | 1; 0.4 |
| IIIC | 2; 0.8 | 1; 6.6 | 1; 0.4 |
| Uterine Sarcoma (2017) | |||
| IB | 2; 0.8 | 0 | 2; 0.8 |
| LVSI | 37; 14.2 | 4; 26.7 | 33; 13.5 |
| Grading | |||
| G1 | 143; 55.0 | 6; 40 | 137; 55.9 |
| G2 | 63; 24.2 | 6; 40 | 57; 23.3 |
| G3 | 54; 20.8 | 3; 20 | 51; 20.8 |
| Simple Hysterectomy | 82; 31.5 | 6; 40 | 76; 31 |
| SLN | 115; 44.2 | 6; 40 | 109; 44.5 |
| Pelvic LND | 58; 22.3 | 5; 33.3 | 53; 21.6 |
| Aortic LND | 38; 14.6 | 3; 20 | 35; 14.3 |
| C1 Hysterectomy | 51; 19.6 | 7; 46.7 | 44; 17.9 |
| Adjuvant treatment | 87; 33.5 | 5; 33.3 | 82; 33.5 |
| Type | Total n | Total % | Treatment | Clavien-Dindo |
|---|---|---|---|---|
| UTIs | 9 | 3.5 | Antibiotic therapy | II |
| Pneumonia | 1 | 0.4 | Antibiotic therapy | II |
| Infected abdominal hematoma | 3 | 1.15 | Antibiotic therapy | II |
| Infected subfascial hematoma | Antibiotic therapy | II | ||
| Infected pelvic lymphocele | Antibiotic therapy Surgical decontamination followed by post-operative intensive care admission. | IV | ||
| Vaginal cuff abscess | 1 | 0.4 | Surgical decontamination of the abscess | IIIB |
| Skin scar infection | 1 | 0.4 | Surgical decontamination | IIIA |
| Total | Infectious Complications | ||||
|---|---|---|---|---|---|
n; % | Yes n; % | No n; % | p-Value | OR (Confidence Interval 95%) | |
| 260 | 15; 5.8 | 245; 94.2 | - | - | |
| Previous pregnancy | 203; 78.1 | 14; 6.9 | 189; 93.1 | 0.436 | - |
| Hypertension | 93; 35.8 | 7; 7.5 | 86; 92.5 | 0.521 | - |
| Diabetes | 20; 7.7 | 1; 5 | 19; 95 | 0.843 | - |
| Hypothyroidism | 32; 12.3 | 4; 12.5 | 28; 87.5 | 0.526 | - |
| Cardiovascular disease | 27; 10.4 | 3; 11.1 | 24; 88.9 | 0.787 | - |
| ASA Status | 0.144 | - | |||
| 1 2 3 | 12; 4.6 204; 78.5 44; 16.9 | 0; - 10; 4.9 5; 11.4 | 12; 194; 95.1 39; 88.6 | - - - | |
| Previous cesarean section | 30; 11.5 | 3; 10 | 27; 90 | 0.216 | - |
| Radical Hysterectomy | 51; 19.6 | 7; 13.7 | 44; 86.3 | 0.010 | OR 3.977 (95%CI 1.370–11.544) |
| Sentinel lymph nodes | 114; 43.8 | 6; 5.3 | 108; 94.7 | 0.926 | - |
| Pelvic lymphadenectomy | 58; 22.3 | 5; 8.6 | 53; 91.4 | 0.231 | - |
| Aortic lymphadenectomy | 38; 14.6 | 3; 7.9 | 35; 92.1 | 0.894 | - |
| Bowel Resection | 1; 0.4 | 1; 100 | 0; 0 | 0.014 | OR 1.071 (95% CI 0.936–1.227) |
| Appendicectomy | 19; 7.3 | 2; 10.5 | 17; 89.5 | 0.405 | - |
| Postoperative Intensive Care Unit | 10; 3.8 | 1; 10 | 9; 90 | 0.761 | - |
| Intraoperative blood transfusion | 1; 0.4 | 0 0 | 1; 100 | 0.736 | - |
| Median (Range) | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Age (years) | 63 (20–89) | 0.983 | 0.941–1.028 | 0.458 |
| BMI (kg/m2) | 27 (18–46) | 1.064 | 0.978–1.158 | 0.149 |
| Operation time (min) | 110 (40–407) | 1.009 | 1.000–1.017 | 0.045 |
| EBL (mL) | 70 (50–1000) | 1.002 | 0.999–1.006 | 0.246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, V.A.; De Finis, A.; Scarpelli, E.; Gallinelli, A.; Monfardini, L.; Cianci, S.; Gulino, F.A.; Rotondella, I.; Celora, G.M.; Martignon, G.; et al. Infectious Complications in Laparoscopic Gynecologic Oncology Surgery within an ERAS-Compliant Setting. J. Pers. Med. 2024, 14, 147. https://doi.org/10.3390/jpm14020147
Capozzi VA, De Finis A, Scarpelli E, Gallinelli A, Monfardini L, Cianci S, Gulino FA, Rotondella I, Celora GM, Martignon G, et al. Infectious Complications in Laparoscopic Gynecologic Oncology Surgery within an ERAS-Compliant Setting. Journal of Personalized Medicine. 2024; 14(2):147. https://doi.org/10.3390/jpm14020147
Chicago/Turabian StyleCapozzi, Vito Andrea, Alessandra De Finis, Elisa Scarpelli, Asya Gallinelli, Luciano Monfardini, Stefano Cianci, Ferdinando Antonio Gulino, Isabella Rotondella, Gabriella Maria Celora, Giulia Martignon, and et al. 2024. "Infectious Complications in Laparoscopic Gynecologic Oncology Surgery within an ERAS-Compliant Setting" Journal of Personalized Medicine 14, no. 2: 147. https://doi.org/10.3390/jpm14020147
APA StyleCapozzi, V. A., De Finis, A., Scarpelli, E., Gallinelli, A., Monfardini, L., Cianci, S., Gulino, F. A., Rotondella, I., Celora, G. M., Martignon, G., Ghi, T., & Berretta, R. (2024). Infectious Complications in Laparoscopic Gynecologic Oncology Surgery within an ERAS-Compliant Setting. Journal of Personalized Medicine, 14(2), 147. https://doi.org/10.3390/jpm14020147








