Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review
Abstract
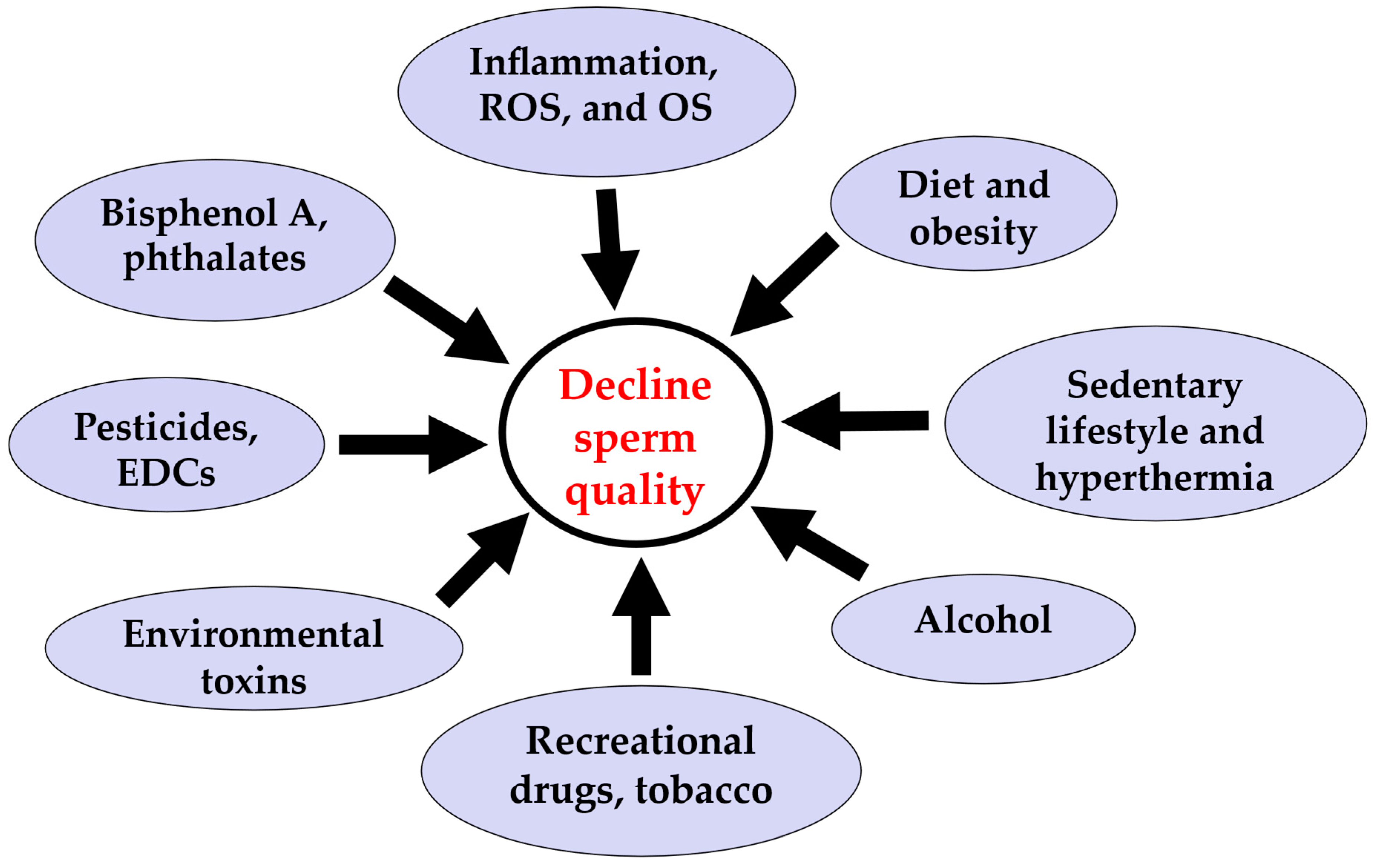
:1. The Global Human Sperm Decline
2. Unhealthy Lifestyles: Smoking and Other Lifestyle Factors
2.1. Smoking
2.2. Alcohol
2.3. Recreational Drugs
2.4. Stress and Poor Sleep
3. Environmental Factors and Sperm Quality
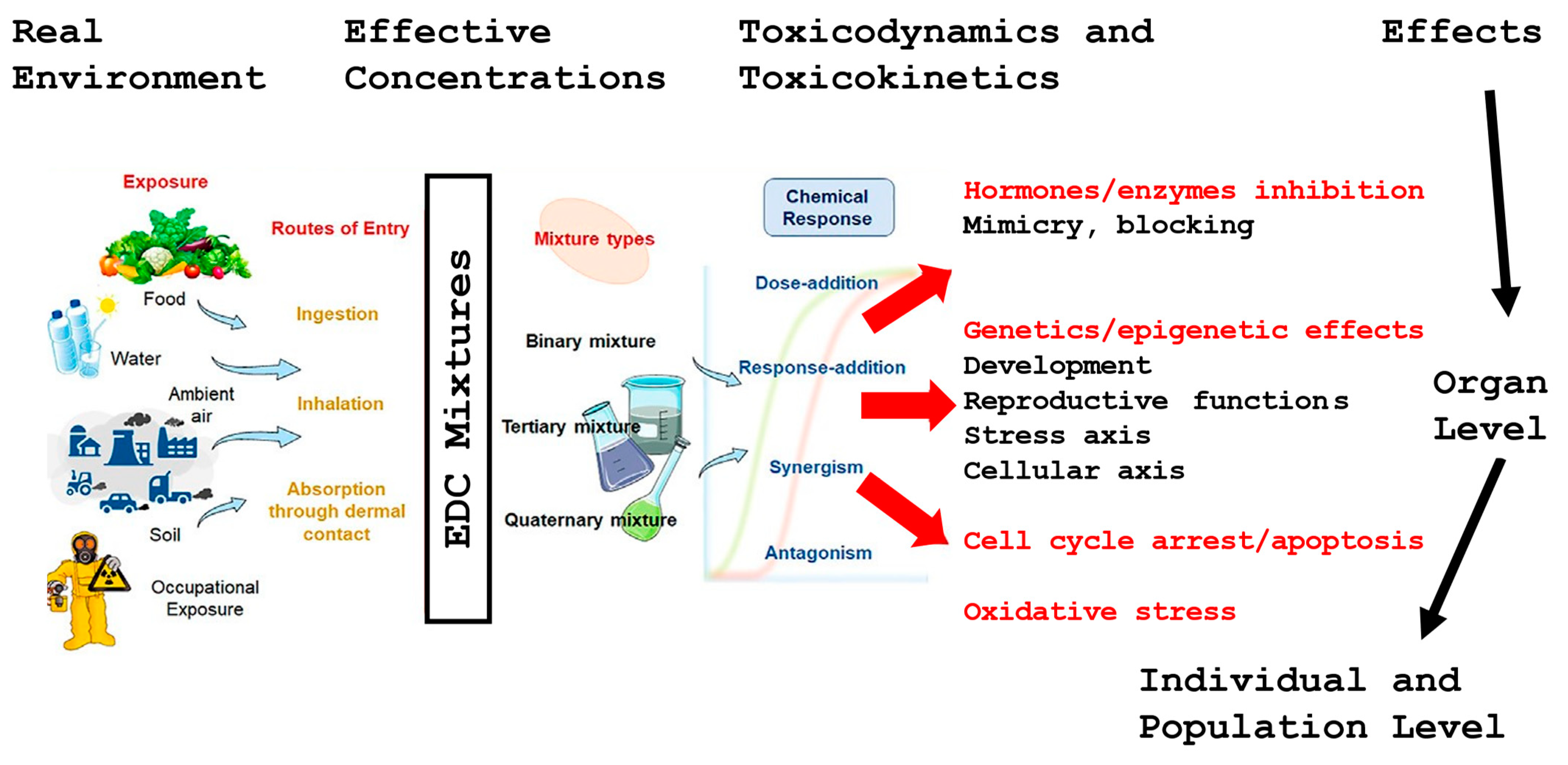
3.1. Endocrine-Disrupting Chemicals
3.2. Plasticisers
3.3. Effect of Endocrine Disruptors
4. Air Pollution
5. Diet, Sedentary Lifestyle, and Hyperthermia
5.1. Obesity
5.2. Metabolic Syndrome and Its Effect upon Sperm Parameters
5.3. Obesity and Inflammation Processes
6. Effect of Radiofrequency Radiation on Sperm Parameters
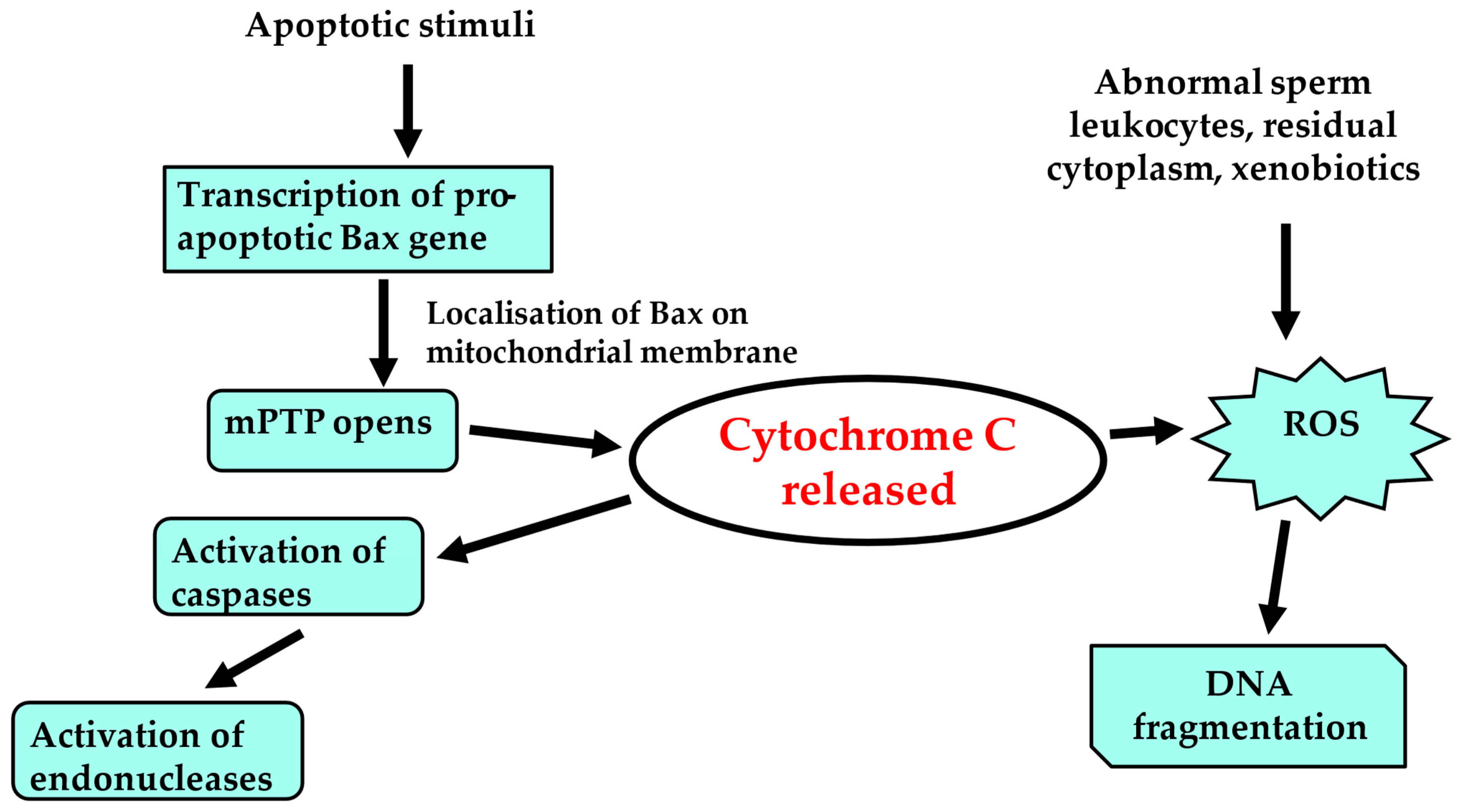
7. Oxidative Stress and Sperm Quality
8. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capogrosso, P.; Ventimiglia, E.; Boeri, L.; Cazzaniga, W.; Chierigo, F.; Montorsi, F.; Salonia, A. Male Infertility as a Proxy of the Overall Male Health Status. Minerva Urol. Nefrol. 2018, 70, 286–299. [Google Scholar] [CrossRef]
- De Jonge, C.; Barratt, C.L.R. The Present Crisis in Male Reproductive Health: An Urgent Need for a Political, Social, and Research Roadmap. Andrology 2019, 7, 762–768. [Google Scholar] [CrossRef]
- Kruger, T.F.; Menkveld, R.; Stander, F.S.; Lombard, C.J.; Van der Merwe, J.P.; van Zyl, J.A.; Smith, K. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil. Steril. 1986, 46, 1118–1123. [Google Scholar] [CrossRef]
- Levine, H.; Jorgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef]
- Huang, C.; Li, B.; Xu, K.; Liu, D.; Hu, J.; Yang, Y.; Nie, H.; Fan, L.; Zhu, W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 2017, 107, 83–88.e2. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H.; Krausz, C.; Oates, R.D. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017, 5, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Van Steirteghem, A.C.; Liu, J.; Joris, H.; Nagy, Z.; Janssenswillen, C.; Tournaye, H.; Derde, M.-P.; Van Assche, E.; Devroey, P. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum. Reprod. 1993, 8, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Fénichel, P.; Chevalier, N. Is testicular germ cell cancer estrogen dependent? The role of endocrine disrupting chemicals. Endocrinology 2019, 160, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Jorgensen, N.; Andersson, A.M.; Juul, A.; Main, K.M.; Kold Jensen, T.; Toppari, J. Populations, Decreasing Fertility, and Reproductive Health. Lancet 2019, 393, 1500–1501. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; Von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Aitken, R.J. The changing tide of human fertility. Hum. Reprod. 2022, 37, 629–638. [Google Scholar] [CrossRef]
- Chodick, G.; Epstein, S.; Shalev, V. Secular trends in testosterone—Findings from a large state-mandate care provider. Reprod. Biol. Endocrinol. 2020, 18, 19. [Google Scholar] [CrossRef]
- Hanson, H.A.; Mayer, E.N.; Anderson, R.E.; Aston, K.I.; Carrell, D.T.; Berger, J.; Lowrance, W.T.; Smith, K.R.; Hotaling, J.M. Risk of Childhood Mortality in Family Members of Men with Poor Semen Quality. Hum. Reprod. 2017, 32, 239–247. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Brooks, J.D.; Cullen, M.R.; Baker, L.C. Increased Risk of Cancer in Infertile Men: Analysis of U.S. Claims Data. J. Urol. 2015, 193, 1596–1601. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Cullen, M.R.; Baker, L.C. Increased Risk of Incident Chronic Medical Conditions in Infertile Men: Analysis of United States Claims Data. Fertil. Steril. 2016, 105, 629–636. [Google Scholar] [CrossRef]
- Latif, T.; Kold Jensen, T.; Mehlsen, J.; Holmboe, S.A.; Brinth, L.; Pors, K.; Skouby, S.O.; Jørgensen, N.; Lindahl-Jacobsen, R. Semen Quality as a Predictor of Subsequent Morbidity: A Danish Cohort Study of 4,712 Men with Long-Term Follow-up. Am. J. Epidemiol. 2017, 186, 910–917. [Google Scholar] [CrossRef]
- Latif, T.; Lindahl-Jacobsen, R.; Mehlsen, J.; Eisenberg, M.L.; Holmboe, S.A.; Pors, K.; Brinth, L.; Skouby, S.O.; Jørgensen, N.; Jensen, T.K. Semen Quality Associated with Subsequent Hospitalizations—Can the Effect be Explained by socio-Economic Status and Lifestyle Factors? Andrology 2018, 6, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A Paternal Environmental Legacy: Evidence for Epigenetic Inheritance Through the Male Germ Line. BioEssays 2014, 36, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Immler, S. The Sperm Factor: Paternal Impact Beyond Genes. Heredity 2018, 121, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Steger, K. Epigenetics in Male Reproduction: Effect of Paternal Diet on Sperm Quality and Offspring Health. Nat. Rev. Urol. 2016, 13, 584–595. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; Cairns, B.; Smith, A.; Carrell, D.T. Paternal Germ Line Aging: DNA Methylation Age Prediction from Human Sperm. BMC Genom. 2018, 19, 763. [Google Scholar] [CrossRef]
- Beal, M.A.; Yauk, C.L.; Marchetti, F. From Sperm to Offspring: Assessing the Heritable Genetic Consequences of Paternal Smoking and Potential Public Health Impacts. Mutat. Res. 2017, 773, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Linschooten, J.O.; Verhofstad, N.; Gutzkow, K.; Olsen, A.K.; Yauk, C.; Oligschlager, Y.; Brunborg, G.; van Schooten, F.J.; Godschalk, R.W.L. Paternal Lifestyle as a Potential Source of Germline Mutations Transmitted to Offspring. FASEB J. 2013, 27, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Heitmann, B.L.; Jensen, M.B.; Halldorsson, T.I.; Andersson, A.-M.; Skakkebæk, N.E.; Joensen, U.N.; Lauritsen, M.P.; Christiansen, P.; Dalgård, C.; et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am. J. Clin. Nutr. 2013, 97, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; Di Dato, C.; de Angelis, C.; Menafra, D.; Pozza, C.; Pivonello, R.; Isidori, A.; Gianfrilli, D. Smoke, alcohol and drug addiction and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Kumar, G.; Upadhyay, A.D.; Gupta, Y.K.M.; Chaturvedi, P.K. Correlation between lead and cadmium concentration and semen quality. Andrologia 2015, 47, 887–891. [Google Scholar] [PubMed]
- Frei, B.; Forte, T.M.; Ames, B.N.; Cross, C.E. Gaz phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effect of ascorbic acid. Biochem. J. 1991, 277, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kiziler, A.R.; Aydemir, B.; Onaran, I.; Alici, B.; Ozkara, H.; Gulyasar, T.; Akyolcu, M.C. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol. Trace Elem. Res. 2007, 120, 82–91. [Google Scholar] [CrossRef]
- Oldereid, N.B.; Thomassen, Y.; Attramadal, A.; Olaisen, B.; Purvis, K. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J. Reprod. Fertil. 1993, 99, 421–425. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; McLachlan, R.I. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B.; Aitken, R.J. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-29-deoxyguanosine, a marker of oxidative stress. Biol. Reprod. 2009, 81, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J. Cell Sci. 2013, 126 Pt. 6, 1488–1497. [Google Scholar] [CrossRef]
- Jensen, T.K.; Swan, S.; Jorgensesn, N.; Toppari, J.; Redmon, B.; Punab, M.; Drobnis, E.Z.; Haugen, T.B.; Zilaitiene, B.; Sparks, A.E.; et al. Alcohol and male reproductive health: A cross-sectional study of 8344 healthy men from Europe and the USA. Hum. Reprod. 2014, 29, 1801–1809. [Google Scholar] [CrossRef]
- Sen, E.; Tunali, Y.; Erkan, M. Testicular development of male mice offsprings exposed to acrylamide and alcohol during the gestation and lactation period. Hum. Exp. Toxicol. 2015, 34, 401–414. [Google Scholar] [CrossRef]
- Emanuele, M.A.; Emanuele, N.V. Alcohol’s effects on male reproduction. Alcohol. Health Res. World 1998, 22, 195–201. [Google Scholar]
- La Vignera, S.; Condorelli, R.A.; Balercia, G.; Vicari, E.; Calogero, A.E. Does alcohol have any effect on male reproductive function? A review of literature. Asian J. Androl. 2013, 15, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; La Vignera, S. Chronic consumption of alcohol and sperm parameters: Our experience and the main evidences. Andrologia 2015, 47, 368–379. [Google Scholar] [CrossRef]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 328. [Google Scholar] [CrossRef]
- Kucheria, K.; Saxena, R.; Mohan, D. Semen analysis in alcohol dependence syndrome. Andrologia 1985, 17, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Salonen, I.; Pakarinen, P.; Huhtaniemi, I. Effect of chronic ethanol diet in expression of gonadotropin genes in the male rat. J. Pharmacol. Exp. Ther. 1992, 260, 463–467. [Google Scholar]
- Carroll, K.; Pottinger, A.M.; Wynter, S.; DaCosta, V. Marijuana use and its influence on sperm morphology and motility: Identified risk for fertility among Jamaican men. Andrology 2019, 18, 844–847. [Google Scholar] [CrossRef]
- Payne, K.S.; Mazur, D.J.; Hotaling, J.M.; Pastuszak, A.W. Cannabis and male fertility: A systematic review. J. Urol. 2019, 202, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Fronczak, C.M.; Kim, E.D.; Barqawi, A.B. The insults of illicit drug use on male fertility. J. Androl. 2012, 33, 515–528. [Google Scholar] [CrossRef]
- Yuan, H.F.; Shangguan, H.F.; Zheng, Y.; Meng, T.-Q.; Xiong, C.-L. Decline in semen concentration of healthy Chinese adults: Evidence from 9357 participants from 2010 to 2015. Asian J. Androl. 2018, 20, 379–386. [Google Scholar]
- Zou, P.; Sun, L.; Chen, Q.; Zhang, G.; Yang, W.; Zeng, Y.; Zhou, N.; Li, Y.; Liu, J.; Ao, L.; et al. Social support modifies an association between work stress and semen quality—Results from 384 Chinese male workers. J. Psychosom. Res. 2019, 117, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, E.; Szabo, G.; Szigeti, F.; Balog, P. Relationships between psychological well-being, lifestyle factors and fertility. Orv. Hetil. 2015, 156, 483–492. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Viganò, P.; Chiaffarino, F.; Bonzi, V.; Salonia, A.; Ricci, E.; Papaleo, E.; Mauri, P.A.; Parazzini, F. Sleep disturbances and semen quality in an Italian cross sectional study. Basic. Clin. Androl. 2017, 27, 4–9. [Google Scholar] [CrossRef]
- Patel, P.; Shiff, B.; Kohn, T.P.; Ramasamy, R. Impaired sleep is associated with low testosterone in US adult males: Results from the National Health and Nutrition Examination Survey. World J. Urol. 2018, 37, 1449–1453. [Google Scholar] [CrossRef]
- Demirkol, M.K.; Yıldırım, A.; Gıca, Ş.; Doğan, N.T.; Resim, S. Evaluation of the effect of shift working and sleep quality on semen parameters in men attending infertility clinic. Andrologia 2021, 53, e14116. [Google Scholar] [CrossRef]
- Green, A.; Barak, S.; Shine, L.; Kahane, A.; Dagan, Y. Exposure by males to light emitted from media devices at night is linked with decline of sperm quality and correlated with sleep quality measures. Chronobiol. Int. 2020, 37, 414–424. [Google Scholar] [CrossRef]
- Gold, H.B.; Jung, Y.H.; Corces, V.G. Not Just Heads and Tails: The Complexity of the Sperm Epigenome. J. Biol. Chem. 2018, 293, 13815–13820. [Google Scholar] [CrossRef]
- Castillo, J.; Jodar, M.; Oliva, R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum. Reprod. Update 2018, 24, 535–555. [Google Scholar] [CrossRef]
- Predieri, B.; Iughetti, L.; Bernasconi, S.; Street, M.E. Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn? Int. J. Mol. Sci. 2022, 23, 11899. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Hasan, N.; Soto, A.; Sonnenschein, C. Environmental endocrine disruptors: Effects on the human male reproductive system. Rev. Endocr. Metab. Disord. 2015, 16, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Potashnik, G. A four-year reassessment of workers with dichlorobromopropane-induced testicular dysfunction. Andrologia 1983, 15, 164–170. [Google Scholar] [CrossRef]
- Slutsky, M.; Levin, J.L.; Levy, B.S. Azoospermia and oligospermia among a large cohort of of DCBP applicators in 12 countries. Int. J. Occup. Environ. Health 1999, 5, 116–122. [Google Scholar] [CrossRef]
- Sadler-Riggleman, I.; Klukovich, R.; Nilsson, E.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Epigenetic transgenerational inheritance of testis pathology and Sertoli cell epimutations: Generational origins of male infertility. Environ. Epigenetics 2019, 5, dvz013. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Totaro, M.; Parisi, A.; D’Andrea, S.; Lucente, L.; Cordeschi, G.; Francavilla, S.; Francavilla, F.; Barbonetti, A. Bisphenol A and male fertility: Myths and realities. Front. Endocrinol. 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Dziewirska, E.; Radwan, M.; Hanke, W. Air pollution from natural and anthropic sources and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tang, E.I.; Cheng, C.Y. Regulation of blood-testis barrier by actin binding proteins and protein kinases. Reproduction 2016, 151, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Ligocka, D.; Radwan, P.; Bochenek, M.; Hawuła, W.; Jakubowski, L.; Hanke, W. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod. Toxicol. 2013, 42, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef]
- Pollard, S.H.; Cox, K.J.; Blackburn, B.E.; Wilkins, D.G.; Carrell, D.T.; Stanford, J.B.; Porucznik, C.A. Male exposure to bisphenol A (BPA) and semen quality in the Home Observation of Periconceptional Exposures (HOPE) cohort. Reprod. Toxicol. 2019, 90, 82–87. [Google Scholar] [CrossRef]
- Radwan, M.; Wielgomas, B.; Dziewirska, E.; Radwan, P.; Kałużny, P.; Klimowska, A.; Hanke, W.; Jurewicz, J. Urinary Bisphenol A Levels and Male Fertility. Am. J. Men’s Health 2018, 12, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Shi, L.; Zhu, Y.; Tian, F.; Shi, M.; Li, Q.; Ge, R.S. Bisphenol F blocks Leydig cell maturation and steroidogenesis in pubertal male rats through suppressing androgen receptor signaling and activating G-protein coupled estrogen receptor 1 (GPER1) signaling. Food Chem. Toxicol. 2022, 167, 113268. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Mahalingam, S.; Flaws, J.A. Environmental Contaminants Affecting Fertility and Somatic Health. Semin. Reprod. Med. 2017, 35, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Imamovic-Kumalic, S.; Pinter, B. From Oxidative Stress to Male Infertility: Review of the Associations of Endocrine-Disrupting Chemicals (Bisphenols, Phthalates, and Parabens) with Human Semen Quality. Antioxidants 2022, 11, 1617. [Google Scholar] [CrossRef]
- Kiwitt-Cárdenas, J.; Adoamnei, E.; Arense-Gonzalo, J.J.; Sarabia-Cos, L.; Vela-Soria, F.; Fernández, M.F.; Gosálvez, J.; Mendiola, J.; Torres-Cantero, A.M. Associations between urinary concentrations of bisphenol A and sperm DNA fragmentation in young men. Environ. Res. 2021, 199, 111289. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Gül, M.; Rambhatla, A.; Agarwal, A. Temporal decline of sperm concentration: Role of endocrine disruptors. Endocrine 2023, 79, 1–16. [Google Scholar] [CrossRef]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Chang, W.H.; Li, S.S.; Wu, M.H.; Pan, H.A.; Lee, C.C. Phthalates might interfere with testicular function by reducing testosterone and insulin-like factor 3 levels. Hum. Reprod. 2015, 30, 2658–2670. [Google Scholar] [CrossRef]
- Ješeta, M.; Navrátilová, J.; Franzová, K.; Fialková, S.; Kempisty, B.; Ventruba, P.; Žáková, J.; Crha, I. Overview of the mechanisms of action of selected bisphenols and perfluoroalkyl chemicals on the male reproductive axes. Front. Genet. 2021, 12, 692897. [Google Scholar] [CrossRef]
- Sikka, S.C.; Gurbuz, N. Reproductive toxicity of organophosphate and carbamate pesticides. In Toxicology of Organophosphate & Carbamate Compounds; Gupta, R.C., Ed.; Elsevier Academic Press: London, UK, 2006; pp. 447–462. [Google Scholar]
- Jeng, H.A.; Yu, L. Alteration of sperm quality and hormone levels by polycyclic aromatic hydrocarbons on airborne particulate particles. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2008, 43, 675–681. [Google Scholar] [CrossRef]
- Hammoud, A.; Carrell, D.T.; Gibson, M.; Sanderson, M.; Parker-Jones, K.; Peterson, C.M. Decreased sperm motility is associated with air pollution in salt Lake City. Fertil. Steril. 2010, 93, 1875–1879. [Google Scholar] [CrossRef]
- Hammoud, S.S.; Purwar, J.; Pflueger, C.; Cairns, B.R.; Carrell, D.T. Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil. Steril. 2016, 94, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Olooto, W.E.; Adeleye, A.O.; Amballi, A.A.; Mosuro, A.O.; Banjo, T.A. Thyroid stimulating hormone assay as the first line biochemical parameter to determine thyroid gland abnormalities. Pak. J. Biol. Sci. 2014, 17, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Lie, P.P.; Cheng, C.Y.; Mruk, D.D. Signalling pathways regulating the blood-testis barrier. Int. J. Biochem. Cell Biol. 2013, 45, 621–625. [Google Scholar] [CrossRef]
- Xu, R.; Zhong, Y.; Li, R.; Li, Y.; Zhong, Z.; Liu, T.; Wang, Q.; Lv, Z.; Huang, S.; Duan, Y.G.; et al. Association between exposure to ambient air pollution and semen quality: A systematic review and meta-analysis. Sci. Total Environ. 2023, 870, 161892. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Torres-Cantero, A.M.; Moreno-Grau, J.M.; Ten, J.; Roca, M.; Moreno-Grau, S.; Bernabeu, R. Exposure to environmental toxins in males seeking infertility treatment: A case-controlled study. Reprod. Biomed. Online 2008, 16, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Selevan, S.G.; Borkovec, L.; Slott, V.L.; Zudova, Z.; Rubes, J.; Evenson, D.P.; Perreault, S.D. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ. Health Perspect. 2000, 108, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, F.; Zhang, M.; Lan, L.; Qiao, Z.; Cui, Y.; An, J.; Wang, N.; Fan, Z.; Zhao, X.; et al. Association between air pollution and sperm quality: A systematic review and meta-analysis. Environ. Pollut. 2016, 208, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Sokol, R.Z.; Kraft, P.; Fowler, I.M.; Mamet, R.; Kim, E.; Berhane, K.T. Exposure to environmental ozone alters semen quality. Environ. Health Perspect. 2006, 114, 360–365. [Google Scholar] [CrossRef]
- Rubes, J.; Selevan, S.G.; Evenson, D.P.; Zudova, D.; Vozdova, M.; Zudova, Z.; Robbins, W.A.; Perreault, S.D. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum. Reprod. 2005, 20, 2776–2783. [Google Scholar] [CrossRef]
- Robbins, W.A.; Rubes, J.; Selevan, S.G.; Perreault, S.D. Air pollution and sperm aneuploidy in healthy young men. Environ. Epidemiol. Toxicol. 1999, 1, 125–131. [Google Scholar]
- Moldan, B.; Schnoor, J.L. Czechoslovakia: Examining a critically ill environment. Environ. Sci. Technol. 1992, 26, 14–21. [Google Scholar] [CrossRef]
- Radwan, M.; Jurewicz, J.; Polańska, K.; Sobala, W.; Radwan, P.; Bochenek, M.; Hanke, W. Exposure to ambient air pollution—Does it affect semen quality and the level of reproductive hormones? Ann. Hum. Biol. 2016, 43, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Bagchi, S.; Chhikara, B.S.; Pavlík, A.; Sláma, P.; Roychoudhury, S. Reproductive toxicity of combined effects of endocrine disruptors on human reproduction. Front. Cell Dev. Biol. 2023, 11, 1162015. [Google Scholar] [CrossRef] [PubMed]
- Efrat, M.; Stein, A.; Pinkas, H.; Unger, R.; Birk, R. Dietary patterns are positively associated with semen quality. Fertil. Steril. 2018, 109, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Montano, L.; Ceretti, E.; Donato, F.; Bergamo, P.; Zani, C.; Viola, G.C.V.; Notari, T.; Pappalardo, S.; Zani, D.; Ubaldi, S.; et al. Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in Italy: The FASt randomized controlled trial. Eur. Urol. Focus. 2022, 8, 351–359. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematicreview of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Budzinska, M.; Kamieniczna, M.; Wojnar, L.; Gill, K.; Piasecka, M.; Kups, M.; Fraczek, M. The role of the intrinsic pathway of apoptosis in human ejaculated sperm damage under a state of scrotal heat stress. J. Assist. Reprod. Genet. 2023, 41, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Lewandowska, A.; Budzinska, M.; Kamieniczna, M.; Wojnar, L.; Gill, K.; Piasecka, M.; Kups, M.; Havrylyuk, A.; Chopyak, V.; et al. The Role of Seminal Oxidative Stress Scavenging System in the Pathogenesis of Sperm DNA Damage in Men Exposed and Not Exposed to Genital Heat Stress. Int. J. Environ. Res. Public Health 2022, 19, 2713. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Lane, M.; Owens, J.A.; Bakos, H.W. Paternal Obesity Negatively Affects Male Fertility and Assisted Reproduction Outcomes: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2015, 31, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of Obese Parents have Altered DNA Methylation Patterns at Imprinted Genes. Int. J. Obes. 2015, 39, 650–657. [Google Scholar] [CrossRef]
- Jing, J.; Peng, Y.; Fan, W.; Han, S.; Peng, Q.; Xue, C.; Qin, X.; Liu, Y.; Ding, Z. Obesity-induced oxidative stress and mitochondrial dysfunction negatively affect sperm quality. FEBS Open Bio 2023, 13, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, Z.; Qian, Q.; Wang, Y.; Xiu, X.; Ou, P.; Fang, J.; Li, G. Effects of paternal obesity on maternal-neonatal outcomes and long-term prognosis in adolescents. Front. Endocrinol. 2023, 14, 1114250. [Google Scholar] [CrossRef]
- Lin, J.; Gu, W.; Huang, H. Effects of Paternal Obesity on Fetal Development and Pregnancy Complications: A Prospective Clinical Cohort Study. Front. Endocrinol. 2022, 13, 826665. [Google Scholar] [CrossRef]
- Dupont, C.; Faure, C.; Daoud, F.; Gautier, B.; Czernichow, S.; Lévy, R. Metabolic syndrome and smoking are independent risk factors of male idiopathic infertility. Basic Clin. Androl. 2019, 29, 9. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kao, T.W.; Peng, T.C.; Yang, H.F.; Chen-Jung, W.U.; Chen, W.L. Metabolic syndrome and semen quality in adult population. J. Diabetes 2019, 12, 294–304. [Google Scholar] [CrossRef]
- Venigalla, G.; Ila, V.; Dornbush, J.; Bernstein, A.; Loloi, J.; Pozzi, E.; Miller, D.; Ramasamy, R. Male obesity: Associated effects on fertility and the outcomes of offspring. Andrology 2023. [Google Scholar] [CrossRef]
- Geronikolou, S.; Pavlopoulou, A.; Lambrou, G.I.; Koutelekos, J.; Cokkinos, D.; Albanopoulos, K.; Chrousos, G.P. Kisspeptin and the genetic obesity interactome. Adv. Exp. Med. Biol. 2021, 1339, 111–117. [Google Scholar]
- Guo, D.; Wu, W.; Tang, Q.; Qiao, S.; Chen, Y.; Chen, M.; Teng, M.; Lu, C.; Ding, H.; Xia, Y.; et al. The impact of BMI on sperm parameters and the metabolite changes of seminal plasma concomitantly. Oncotarget 2017, 8, 48619–48634. [Google Scholar] [CrossRef]
- Roushandeh, M.A.; Salehi, I.; Mortazavi, M. Protective effects of restricted diet and antioxidants on testis tissue in rats fed with high-fat diet. Iran. Biomed. J. 2015, 19, 96–101. [Google Scholar]
- Meng, M.; Zhang, W.; Zhang, J.; Liang, Z.; Kuang, Y.; Wang, Y. Effect of paternal body mass index on neonatal outcomes of singletons after frozen-thawed embryo transfer cycles: Analysis of 7908 singleton newborns. Fertil. Steril. 2020, 113, 1215–1223.e1. [Google Scholar]
- Håkonsen, L.B.; Thulstrup, A.M.; Aggerholm, A.S.; Olsen, J.; Bonde, J.P.; Andersen, C.Y.; Bungum, M.; Ernst, E.H.; Hansen, M.L.; Ernst, E.H.; et al. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod. Health 2011, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 2017, 154, R123–R131. [Google Scholar] [CrossRef] [PubMed]
- Samavat, J.; Natali, I.; Degl’Innocenti, S.; Filimberti, E.; Cantini, G.; Di Franco, A.; Danza, G.; Seghieri, G.; Lucchese, M.; Baldi, E.; et al. Acrosome reaction is impaired in spermatozoa of obese men: A preliminary study. Fertil. Steril. 2014, 102, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Heidenreich, F.; Pursche, T.; Kuhlisch, E.; Kettner, K.; Grunewald, S.; Kratzsch, J.; Dittmar, G.; Glander, H.J.; Hoflack, B.; et al. Identification of increased amounts of eppin protein complex components in sperm cells of diabetic and obese individuals by difference gel electrophoresis. Mol. Cell. Proteom. 2011, 10, M110.007187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Y.; Song, N.; Fan, Y.; Li, K.; Teng, X.; Guo, Q.; Ding, Z. Proteomic pattern changes associated with obesity-induced asthenozoospermia. Andrology 2015, 3, 247–259. [Google Scholar] [CrossRef]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef]
- Griffin, C.; Lanzetta, N.; Eter, L.; Singer, K. Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R211–R216. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, F.; Liang, H.; Yan, J.; Xu, H.; Li, Z.; Wen, X.; Weng, J. GLP-1receptor agonist exenatide attenuates the detrimental effects of obesity on inflammatory profile in testis and sperm quality in mice. Am. J. Reprod. Immunol. 2015, 74, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yuan, M.; Zhang, J.; Li, J.; Gong, D.; Li, Y.; Zhang, J.; Lin, P.; Huang, L. IL-6 mediates differentiation disorder during spermatogenesis in obesity-associated inflammation by affecting the expression of Zfp637 through the SOCS3/STAT3 pathway. Sci. Rep. 2016, 6, 28012. [Google Scholar] [CrossRef]
- Tsatsanis, C.; Dermitzaki, E.; Avgoustinaki, P.; Malliaraki, N.; Mytaras, V.; Margioris, A.N. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Hormones 2015, 14, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Murakami, M.; Kinouchi, R.; Ogata, R.; Kuwahara, A.; Yasui, T.; Irahara, M. Neonatal lipopolysaccharide exposure attenuates the homotypic stress-induced suppression of LH secretion in adulthood in male rat. Int. J. Dev. Neurosci. 2009, 27, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, Y.; Wang, G.; Liu, Z.; Liu, L.; Sun, F. Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep. 2014, 4, 4260. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Bilinska, B.; Mruk, D.D. Interleukin 1alpha-induced disruption of the Sertoli cell cytoskeleton affects gap junctional communication. Cell. Signal. 2016, 28, 469–480. [Google Scholar] [CrossRef]
- Stanton, P.G. Regulation of the blood-testis barrier. Semin. Cell Dev. Biol. 2016, 59, 166–173. [Google Scholar] [CrossRef]
- Sullivan, R. Epididymosomes: A heterogeneous population of microvesicles with multiple functions in sperm maturation and storage. Asian J. Androl. 2015, 17, 726–729. [Google Scholar] [CrossRef]
- Sciorio, R.; Tramontano, L.; Esteves, S.C. Effects of mobile phone radiofrequency radiation on sperm quality. Zygote 2022, 30, 159–168. [Google Scholar] [CrossRef]
- Adams, J.A.; Galloway, T.S.; Mondal, D.; Esteves, S.C.; Mathews, F. Effect of mobile telephones on sperm quality: A systematic review and meta-analysis. Environ. Int. 2014, 70, 106–112. [Google Scholar] [CrossRef]
- Dasdag, S.; Tas, M.; Akdag, M.Z.; Yegin, K. Effect of long-term exposure of 2.4 GHz radiofrequency radiation emitted from Wi-Fi equipment on testes functions. Electromagn. Biol. Med. 2015, 34, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RFEMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef]
- Kim, S.; Han, D.; Ryu, J.; Kim, K.; Kim, Y.H. Effects of mobile phone usage on sperm quality—No time-dependent relationship on usage: A systematic review and updated meta-analysis. Environ. Res. 2021, 202, 111784. [Google Scholar] [CrossRef]
- Othman, H.; Ammari, M.; Sakly, M.; Abdelmelek, H. Effects of prenatal exposure to WIFI signal (2.45 GHz) on postnatal development and behavior in rat: Influence of maternal restraint. Behav. Brain Res. 2017, 326, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.; Colombi, D. Thermal response of tissue to RF exposure from canonical dipoles at frequencies for future mobile communication systems. Electron. Lett. 2017, 53, 360–362. [Google Scholar] [CrossRef]
- Deepinder, F.; Makker, K.; Agarwal, A. Cell phones and male infertility: Dissecting the relationship. Reprod. Biomed. Online 2007, 15, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Agarwal, A.A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.M.; Rizzoto, G.; Kastelic, J.P. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology 2020, 158, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, F.R.; Swerdloff, R.S. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil. Steril. 1988, 49, 1–23. [Google Scholar] [PubMed]
- Carlsen, E.; Andersson, A.M.; Petersen, J.H.; Skakkebaek, N.E. History of febrile illness and variation in semen quality. Hum. Reprod. 2003, 18, 2089–2092. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Rao, M.; Hu, S.F.; Ke, D.D.; Zhu, C.H.; Xia, W. Effect of transient scrotal hyperthermia on human sperm: An iTRAQ-based proteomic analysis. Reprod. Biol. Endocrinol. 2020, 18, 83. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Xiao, W.; Chen, S.R.; Zhang, M.H.; Qiu, Y.; Liu, Y.X. Biologic response of sperm and seminal plasma to transient testicular heating. Front. Biosci. 2019, 24, 1401–1425. [Google Scholar]
- Rizzoto, G.; Boe-Hansen, G.; Klein, C.; Thundathil, J.C.; Kastelic, J.P. Acute mild heat stress alters gene expression in testes and reduces sperm quality in mice. Theriogenology 2020, 158, 375–381. [Google Scholar] [CrossRef]
- Singh, R.; Nath, R.; Mathur, A.K.; Sharma, R.S. Effect of radiofrequency radiation on reproductive health. Indian J. Med. Res. 2018, 148, S92–S99. [Google Scholar]
- Lewis, S.E.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.; D’Agata, R.; Calogero, A.E. Effects of the exposure to mobile phones on male reproduction: A review of the literature. J. Androl. 2012, 33, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hawkins, K.L.; DeWolf, W.C.; Morgentaler, A. Heat stress causes testicular germ cell apoptosis in adult mice. J. Androl. 1997, 18, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, S.; Susskind, C. Effects of chronic microwave irradiation on mice. IRE Trans. Biomed. Electron. 1962, 9, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, G.; Liu, J.; Cao, J.; Ao, L.; Zhang, S. Association between mobile phone use and semen quality: A systemic review and meta-analysis. Andrology 2014, 2, 491–501. [Google Scholar] [CrossRef]
- Dasdag, S.; Zulkuf Akdag, M.; Aksen, F.; Yilmaz, F.; Bashan, M.; Mutlu Dasdag, M.M.; Salih Celik, M. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics 2003, 24, 182–188. [Google Scholar] [CrossRef]
- Sciorio, R.; Smith, G.D. Embryo culture at a reduced oxygen concentration of 5%: A mini review. Zygote 2019, 27, 355–361. [Google Scholar] [CrossRef]
- Aitken, R.J. Sperm DNA integrity: A special issue exploring the causes, consequences, and treatment of DNA damage in human spermatozoa. Andrology 2023, 11, 1541–1544. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef]
- Moazamian, A.; Gharagozloo, P.; Aitken, R.J.; Drevet, J.R. Oxidative stress and reproductive function: Sperm telomeres, oxidative stress, and infertility. Reproduction 2022, 164, F125–F133. [Google Scholar] [CrossRef]
- Roque, M.; Esteves, S.C. Effect of varicocele repair on sperm DNA fragmentation: A review. Int. Urol. Nephrol. 2018, 50, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Santi, D.; Simoni, M. An update on clinical and surgical interventions to reduce sperm DNA fragmentation in infertile men. Andrology 2020, 8, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Homa, S.T.; Vessey, W.; Perez-Miranda, A.; Riyait, T.; Agarwal, A. Reactive Oxygen Species (ROS) in human semen: Determination of a reference range. J. Assist. Reprod. Genet. 2015, 32, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ayaz, A.; Samanta, L.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Sabanegh, E. Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clin. Proteom. 2015, 12, 23. [Google Scholar] [CrossRef]
- Lai, T.C.; Roychoudhury, S.; Cho, C.L. Oxidative Stress and Varicocele-Associated Male Infertility. Adv. Exp. Med. Biol. 2022, 1358, 205–235. [Google Scholar]
- Ribas-Maynou, J.; Benet, J. Single and doublestrand sperm DNA damage: Different reproductive effects on male fertility. Genes 2019, 10, 105. [Google Scholar] [CrossRef]
- Desai, N.; Sharma, R.; Makker, K.; Sabanegh, E.; Agarwal, A. Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil. Steril. 2009, 92, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Adrologia 2018, 50, e13012. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Pelliccione, F.; Micillo, A.; Cordeschi, G.; D’Angeli, A.; Necozione, S.; Gandini, L.; Lenzi, A.; Francavilla, F.; Francavilla, S. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil. Steril. 2011, 95, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Awanti, S.M.; Ingin, J.B.; Jeevangi, S.R.; Patil, G.A.; Awanti, B.S. The effect of radio-frequency radiation emitted from mobile phones on plasma oxidants and antioxidants in mobile phone users. J. Clin. Diagn. Res. 2010, 4, 2758–2761. [Google Scholar]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernanded, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef]
| WHO Edition | Volume (mL) | Sperm Concentration (106/mL)/Total Number (106/Ejaculate) | Total/Progressive Sperm Motility (%) | Normal Forms (%) |
|---|---|---|---|---|
| 2nd | ≥2 | ≥20/≥40 | ≥50/≥25 | ≥50 |
| 3rd | ≥2 | ≥20/≥40 | ≥50/≥25 | ≥30 |
| 4th | ≥2 | ≥20/≥40 | ≥50/≥25 | Normal forms <15 might be associated with decreased in vitro fertilisation success |
| 5th | ≥1.5 | ≥15/≥39 | ≥40/≥32 | ≥4 * |
| 6th | ≥1.4 | ≥16/≥39 | ≥42/≥30 | ≥4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciorio, R.; Tramontano, L.; Adel, M.; Fleming, S. Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. J. Pers. Med. 2024, 14, 198. https://doi.org/10.3390/jpm14020198
Sciorio R, Tramontano L, Adel M, Fleming S. Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. Journal of Personalized Medicine. 2024; 14(2):198. https://doi.org/10.3390/jpm14020198
Chicago/Turabian StyleSciorio, Romualdo, Luca Tramontano, Mohammed Adel, and Steven Fleming. 2024. "Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review" Journal of Personalized Medicine 14, no. 2: 198. https://doi.org/10.3390/jpm14020198
APA StyleSciorio, R., Tramontano, L., Adel, M., & Fleming, S. (2024). Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. Journal of Personalized Medicine, 14(2), 198. https://doi.org/10.3390/jpm14020198






