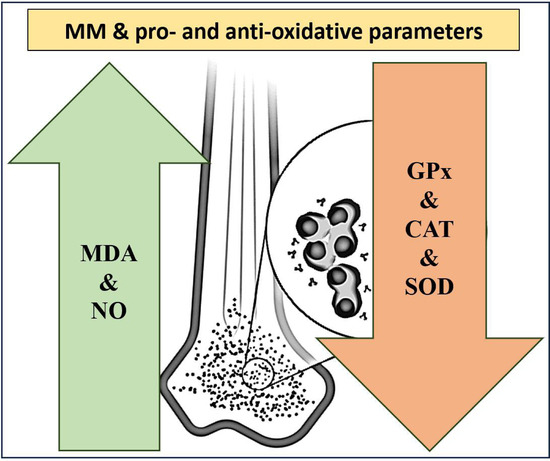
Multiple Myeloma from the Perspective of Pro- and Anti-Oxidative Parameters: Potential for Diagnostic and/or Follow-Up Purposes?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Setting and Participants
2.3. Data Gathering and Laboratory Analysis
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caillot, M.; Dakik, H.; Mazurier, F.; Sola, B. Targeting Reactive Oxygen Species Metabolism to Induce Myeloma Cell Death. Cancers 2021, 13, 2411. [Google Scholar] [CrossRef]
- Swamydas, M.; Murphy, E.V.; Ignatz-Hoover, J.J.; Malek, E.; Driscoll, J.J. Deciphering mechanisms of immune escape to inform immunotherapeutic strategies in multiple myeloma. J. Hematol. Oncol. 2022, 15, 1–17. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Al Kayyali, L.; Diak, Z.A.; Diak, O.A.; Krawczyk, J. Treatment and Disease-Related Complications in Multiple Myeloma; IntechOpen: London, UK, 2023. [Google Scholar]
- Lipchick, B.C.; Fink, E.E.; Nikiforov, M.A. Oxidative stress and proteasome inhibitors in multiple myeloma. Pharmacol. Res. 2016, 105, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Petrarca, C.; Di Gioacchino, M.; Casciaro, M.; Musolino, C.; Gangemi, S. Modulation of Cellular Redox Parameters for Improving Therapeutic Responses in Multiple Myeloma. Antioxidants 2022, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Sabuncuoglu, S.; Samadi, M.; Isikhan, S.Y.; Chirumbolo, S.; Peana, M.; Lay, I.; Yalcinkaya, A.; Bjørklund, G. A Comprehensive Review on Oxysterols and Related Diseases. Curr. Med. Chem. 2021, 28, 110–136. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, R.; Öz, F.N.; Durmuş, S.Y.; Fettah, A.; Kaman, A.; Teke, T.A.; Örün, U.A.; Tanır, G. Is There a Role for Laboratory Parameters in Predicting Coronary Artery Involvement in Kawasaki Disease? Klin. Padiatr. 2022, 234, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.-Y.; Jung, K.-H.; Lee, J.-H.; Park, J.-W.; Lee, K.-H. Reactive oxygen species-driven HIF1α triggers accelerated glycolysis in endothelial cells exposed to low oxygen tension. Nucl. Med. Biol. 2017, 45, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Santambrogio, P.; Fontana, F.; Frenquelli, M.; Cenci, S.; Marcatti, M.; Sitia, R.; Tonon, G.; Camaschella, C. Iron increases the susceptibility of multiple myeloma cells to bortezomib. Haematologica 2013, 98, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2017, 86, 749–775. [Google Scholar] [CrossRef]
- Hanschmann, E.M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 2013, 19, 1539–1605. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Cieslar, P.; Mášová, L.; Scheiner, T.; Ryšavá, J.; KříŽová, P.; Danzigová, Z.; Špička, I.; Tesař, V.R. Oxidative stress and platelet function in multiple myeloma and renal insufficiency: Clinical relations of different tests. Thromb. Res. 2002, 105, 277–283. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, M.; Satyam, A.; Kumar, L. Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma 2009, 50, 809–815. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, W.A.; Zainulabdeen, J.A.; Mehde, A.A. Investigation of the antioxidant status in multiple myeloma patients: Effects of therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Kuku, I.; Aydogdu, I.; Bayraktar, N.; Kaya, E.; Akyol, O.; Erkurt, M.A. Oxidant/antioxidant parameters and their relationship with medical treatment in multiple myeloma. Cell Biochem. Funct. 2005, 23, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, O.; Titova, N.; Elmanova, N. The relationship between the pro-oxidant and antioxidant system status of patients with multiple myeloma and the disease stage. Bull. Exp. Biol. Med. 2014, 157, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Zima, T.; Spicka, I.; Stipek, S.; Crkovska, J.; Platenik, J.; Merta, M.; Tesar, V. Antioxidant enzymes and lipid peroxidation in patients with multiple myeloma. Neoplasma 1996, 43, 69–73. [Google Scholar] [PubMed]
- Zima, T.; Spicka, I.; Stipek, S.; Crkovska, J.; Platenik, J.; Merta, M.; Nĕmecek, K.; Tesar, V. Lipid peroxidation and activity of antioxidative enzymes in patients with multiple myeloma. Cas. Lek. Ceskych 1996, 135, 14–17. [Google Scholar]
- Broch, K.; Popperud, T.; Gude, E.; Fløisand, Y.; Antal, E.A.; Bosse, G.; Jonsrud, C.; Hegard, T.; Skaara, S.; Elsais, A. A Middle-Aged Man Presenting with Progressive Heart Failure, Myopathy, and Monoclonal Gammopathy of Uncertain Significance. JACC Case Rep. 2020, 2, 785–789. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Unal, S.; Oztas, Y. Altered HDL particle in sickle cell disease: Decreased cholesterol content is associated with hemolysis, whereas decreased Apolipoprotein A1 is linked to inflammation. Lipids Health Dis. 2019, 18, 225. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Carmo De Carvalho E Martins, M.D.; Martins; Da Silva Santos Oliveira, A.S.; Da Silva, L.a.A.; Primo, M.G.S.; De Carvalho Lira, V.B. Biological indicators of oxidative stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and their application in nutrition. In Biomarkers in Nutrition; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–25. [Google Scholar]
- López-Sánchez, L.M.; Aranda, E.; Rodríguez-Ariza, A. Nitric oxide and tumor metabolic reprogramming. Biochem. Pharmacol. 2020, 176, 113769. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges. Antioxidants 2020, 9, 791. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Sarıkaya, E.; Doğan, S. Glutathione peroxidase in health and diseases. In Glutathione System and Oxidative Stress in Health and Disease; Bagatini, M.D., Ed.; IntechOpen: London, UK, 2020; pp. 49–65. [Google Scholar]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Gönenç, A.; Erten, D.; Aslan, S.; Akıncı, M.; Şimşek, B.; Torun, M. Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol. Int. 2006, 30, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Arsova-Sarafinovska, Z.; Sayal, A.; Eken, A.; Erdem, O.; Erten, K.; Özgök, Y.; Dimovski, A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin. Biochem. 2006, 39, 176–179. [Google Scholar] [CrossRef]
- Sharma, A.; Rajappa, M.; Saxena, A.; Sharma, M. Antioxidant status in advanced cervical cancer patients undergoing neoadjuvant chemoradiation. Br. J. Biomed. Sci. 2007, 64, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Lodh, M.; Goswami, B.; Gupta, N.; Patra, S.K.; Saxena, A. Assessment of oxidative stress and inflammatory process in patients of multiple myeloma. Indian J. Clin. Biochem. 2012, 27, 410–413. [Google Scholar] [CrossRef]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V. Prevalence of monoclo-nal gammopathy of undetermined significance. N. Engl. J. Med. 2006, 354, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 2002, 346, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Gupta, V.; Fonseca, R. Impact of primary molecularcytogenetic abnormalities and risk of progression in smoldering mul-tiple myeloma. Leukemia 2013, 27, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Neben, K.; Jauch, A.; Hielscher, T. Progression in smoldering mye-loma is independently determined by the chromosomal abnormali-ties del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J. Clin. Oncol. 2013, 31, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Choa, R.; Panaroni, C.; Bhatia, R.; Raje, N. It is worth the weight: Obesity and the transition from monoclonal gammopathy of undetermined significance to multiple myeloma. Blood Adv. 2023, 7, 5510–5523. [Google Scholar] [CrossRef] [PubMed]
- Tavori, H.; Ormseth, M.J.; Lilley, J.S.; Papen, C.R.; May-Zhang, L.S.; Davies, S.S. Progressively decreasing plasma high-density lipoprotein cholesterol levels preceding diagnosis of smoldering myeloma. J. Clin. Lipidol. 2020, 14, 293–296. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 719–734. [Google Scholar] [CrossRef]

| Groups | p | ||
|---|---|---|---|
| Controls (n = 30) | Patients (n = 30) | ||
| Age (years) | 65.13 ± 8.80 | 63.87 ± 10.93 | 0.623 |
| Sex | |||
| Male | 17 (56.67%) | 18 (60.00%) | 1.000 |
| Female | 13 (43.33%) | 12 (40.00%) | |
| Comorbidities | |||
| Diabetes mellitus | 5 (16.67%) | 7 (23.33%) | 0.747 |
| Hypertension | 12 (40.00%) | 11 (36.67%) | 1.000 |
| Ischemic heart disease | 3 (10.00%) | 7 (23.33%) | 0.299 |
| COPD | 2 (6.67%) | 3 (10.00%) | 1.000 |
| Hypothyroidism | 1 (3.33%) | 2 (6.67%) | 1.000 |
| MDA (nmol/mL) | 3.80 (3.04–4.12) | 8.49 (6.94–10.34) | <0.001 |
| GSH-Px (ng/mL) | 78.36 (60.42–85.12) | 57.30 (50.73–72.04) | <0.001 |
| CAT (ng/mL) | 85.45 (69.95–93.73) | 59.53 (55.52–71.05) | <0.001 |
| SOD (ng/mL) | 31.94 ± 9.80 | 22.89 ± 9.40 | 0.001 |
| NO (µmol/L) | 27.85 (13.04–36.49) | 54.60 (49.46–64.89) | <0.001 |
| Cut-Off | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC (95% CI) | p | |
|---|---|---|---|---|---|---|---|---|
| MDA (nmol/mL) | >4.35 | 96.67% | 100.00% | 98.33% | 100.00% | 96.77% | 0.967 (0.902–1.000) | <0.001 |
| GSH-Px (ng/mL) | <59.8 | 70.00% | 90.00% | 80.00% | 87.50% | 75.00% | 0.773 (0.645–0.901) | <0.001 |
| CAT (ng/mL) | <67.2 | 73.33% | 86.67% | 80.00% | 84.62% | 76.47% | 0.850 (0.755–0.945) | <0.001 |
| SOD (ng/mL) | <21.2 | 56.67% | 86.67% | 71.67% | 80.95% | 66.67% | 0.760 (0.639–0.881) | 0.001 |
| NO (µmol/L) | >38.5 | 96.67% | 90.00% | 93.33% | 90.63% | 96.43% | 0.960 (0.895–1.000) | <0.001 |
| Unadjusted | Adjusted (1) | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| MDA (nmol/mL) | 5.679 (1.755–18.380) | 0.004 | 6.336 (1.886–21.289) | 0.003 |
| GSH-Px (ng/mL) | 0.937 (0.900–0.974) | 0.001 | 0.931 (0.892–0.972) | 0.001 |
| CAT (ng/mL) | 0.915 (0.874–0.958) | <0.001 | 0.910 (0.865–0.957) | <0.001 |
| SOD (ng/mL) | 0.898 (0.838–0.963) | 0.003 | 0.895 (0.833–0.962) | 0.003 |
| NO (µmol/L) | 1.217 (1.087–1.362) | 0.001 | 1.217 (1.084–1.366) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kul, A.N.; Ozturk Kurt, B. Multiple Myeloma from the Perspective of Pro- and Anti-Oxidative Parameters: Potential for Diagnostic and/or Follow-Up Purposes? J. Pers. Med. 2024, 14, 221. https://doi.org/10.3390/jpm14030221
Kul AN, Ozturk Kurt B. Multiple Myeloma from the Perspective of Pro- and Anti-Oxidative Parameters: Potential for Diagnostic and/or Follow-Up Purposes? Journal of Personalized Medicine. 2024; 14(3):221. https://doi.org/10.3390/jpm14030221
Chicago/Turabian StyleKul, Ayse Nilgun, and Bahar Ozturk Kurt. 2024. "Multiple Myeloma from the Perspective of Pro- and Anti-Oxidative Parameters: Potential for Diagnostic and/or Follow-Up Purposes?" Journal of Personalized Medicine 14, no. 3: 221. https://doi.org/10.3390/jpm14030221