The Omics Complexity in Sepsis: The Limits of the Personalized Medicine Approach
Abstract
1. Introduction
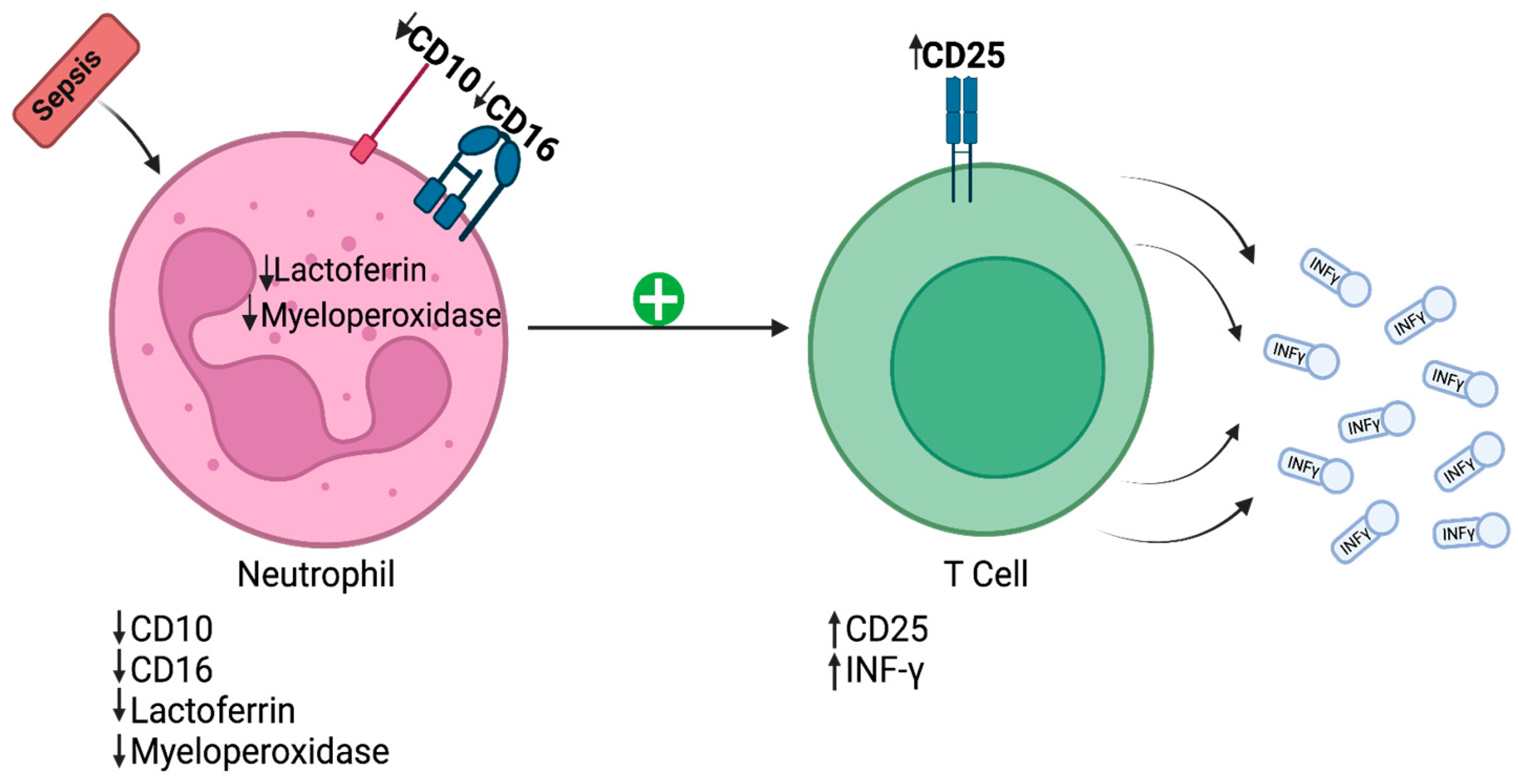
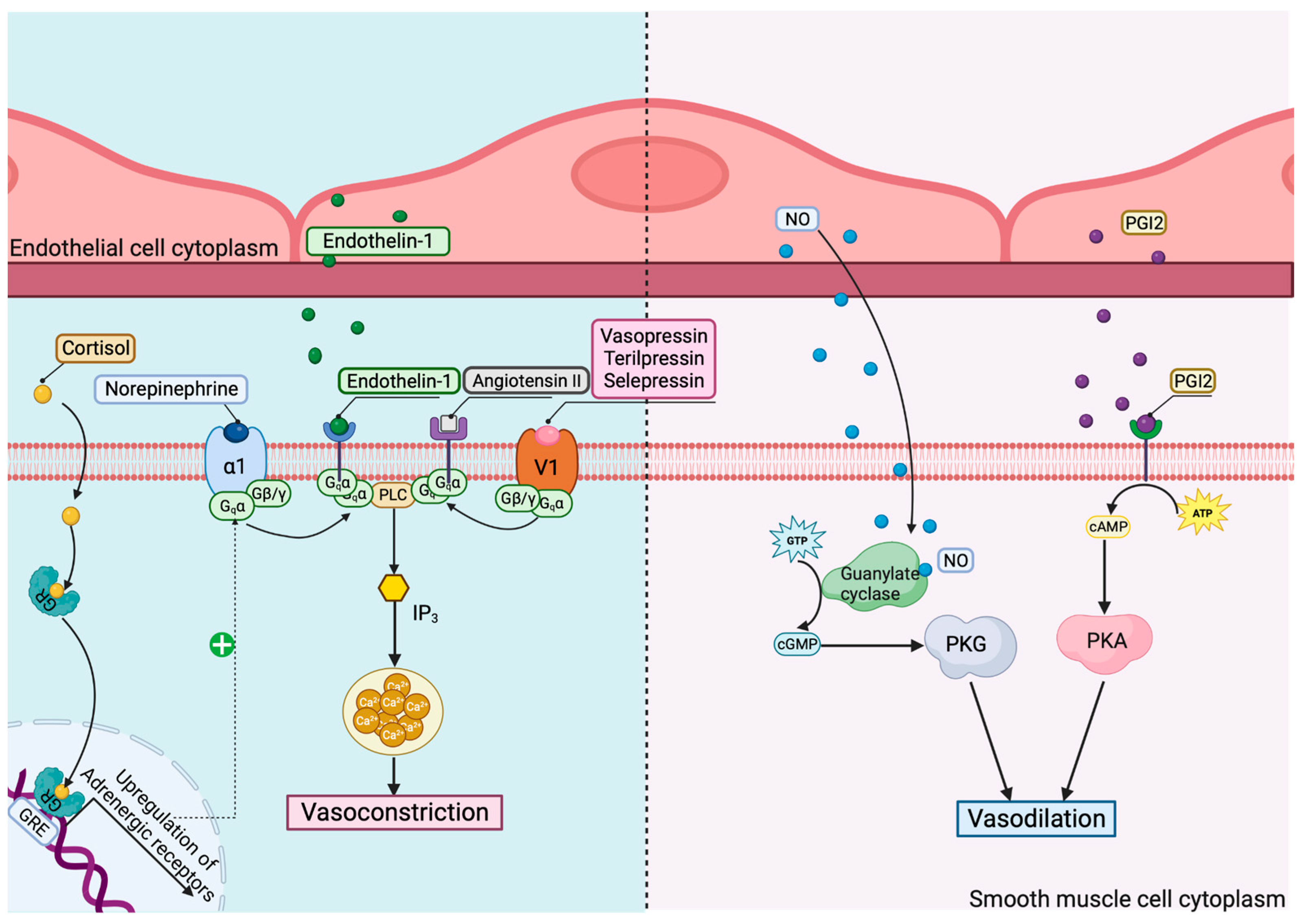
2. Cytomics
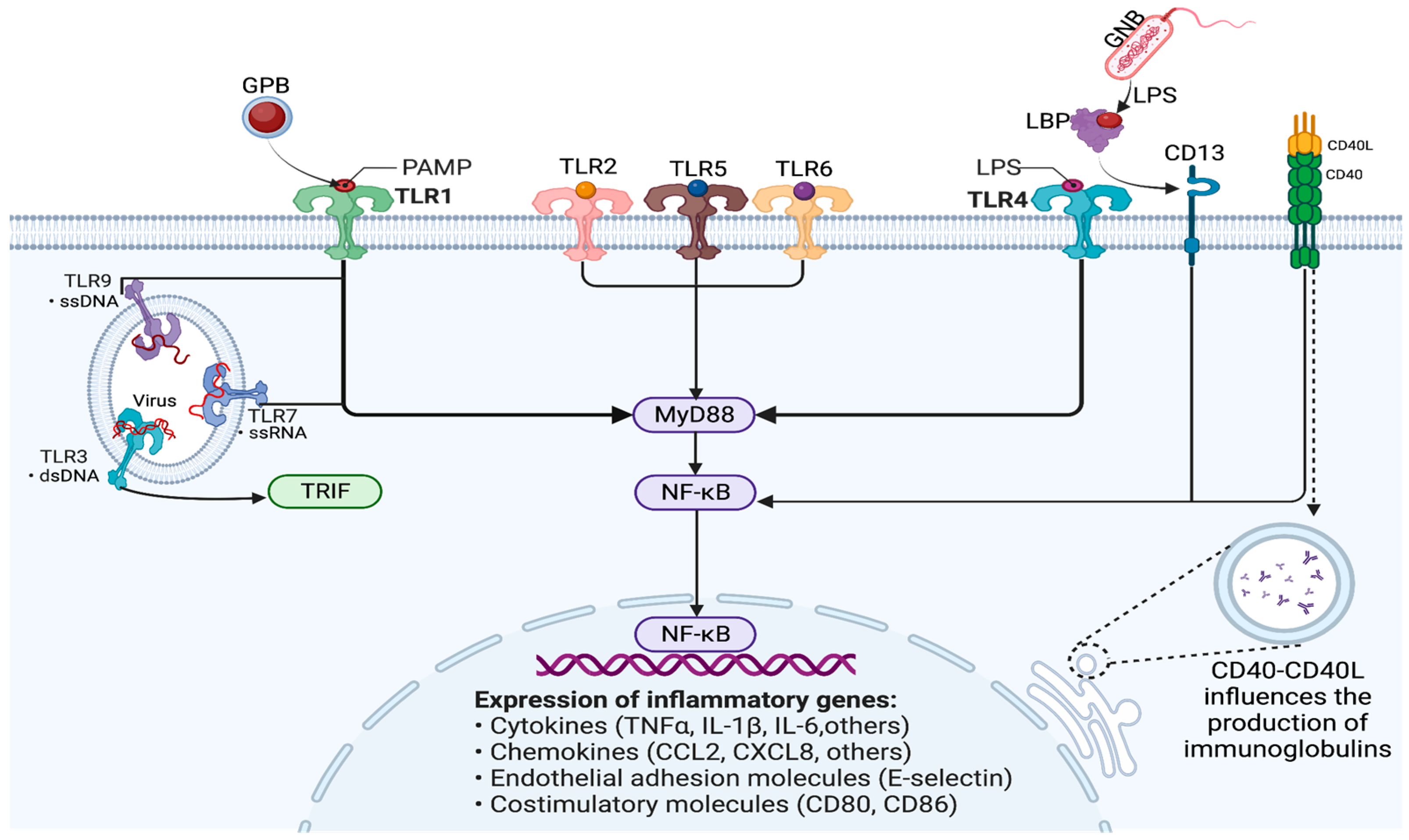
3. Genomics
4. Epigenomics
5. Transcriptomics
6. Proteomics
7. Metabolomics
8. Clinical Phenotypes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Su, L.; Cao, L.; Zhou, R.; Jiang, Z.; Xiao, K.; Kong, W.; Wang, H.; Deng, J.; Wen, B.; Tan, F.; et al. Identification of Novel Biomarkers for Sepsis Prognosis via Urinary Proteomic Analysis Using ITRAQ Labeling and 2D-LC-MS/MS. PLoS ONE 2013, 8, e54237. [Google Scholar] [CrossRef]
- Marshall, J.C.; Reinhart, K. Biomarkers of Sepsis. Crit. Care Med. 2009, 37, 2290–2298. [Google Scholar] [CrossRef]
- Lyle, N.H.; Pena, O.M.; Boyd, J.H.; Hancock, R.E.W. Barriers to the Effective Treatment of Sepsis: Antimicrobial Agents, Sepsis Definitions, and Host-Directed Therapies. Ann. N. Y. Acad. Sci. 2014, 1323, 101–114. [Google Scholar] [CrossRef]
- Islam, M.M.; Nasrin, T.; Walther, B.A.; Wu, C.C.; Yang, H.C.; Li, Y.C. Prediction of sepsis patients using machine learning approach: A meta-analysis. Comput. Methods Programs Biomed. 2019, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and Clinical Management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Huang, J.; Yang, Y.; Liu, L.; Shao, Y.; Li, L.; Sun, B. Dysfunction of Low-Density Neutrophils in Peripheral Circulation in Patients with Sepsis. Sci. Rep. 2022, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Lindell, R.B.; Meyer, N.J. Interrogating the Sepsis Host Immune Response Using Cytomics. Crit. Care 2023, 27, 93. [Google Scholar] [CrossRef] [PubMed]
- Bodinier, M.; Peronnet, E.; Brengel-Pesce, K.; Conti, F.; Rimmelé, T.; Textoris, J.; Vedrine, C.; Quemeneur, L.; Griffiths, A.D.; Tan, L.K.; et al. Monocyte Trajectories Endotypes Are Associated with Worsening in Septic Patients. Front. Immunol. 2021, 12, 795052. [Google Scholar] [CrossRef]
- Baghela, A.; Pena, O.M.; Lee, A.H.; Baquir, B.; Falsafi, R.; An, A.; Farmer, S.W.; Hurlburt, A.; Mondragon-Cardona, A.; Rivera, J.D.; et al. Predicting Sepsis Severity at First Clinical Presentation: The Role of Endotypes and Mechanistic Signatures. EbioMedicine 2022, 75, 103776. [Google Scholar] [CrossRef]
- Sutherland, A.M.; Walley, K.R.; Russell, J.A. Polymorphisms in CD14, Mannose-Binding Lectin, and Toll-like Receptor-2 Are Associated with Increased Prevalence of Infection in Critically Ill Adults. Crit. Care Med. 2005, 33, 638–644. [Google Scholar] [CrossRef]
- Lorenz, E.; Mira, J.P.; Frees, K.L.; Schwartz, D.A. Relevance of Mutations in the TLR4 Receptor in Patients with Gram-Negative Septic Shock. Arch. Intern. Med. 2002, 162, 1028–1032. [Google Scholar] [CrossRef]
- Bronkhorst, M.W.G.A.; Lomax, M.A.Z.; Vossen, R.H.A.M.; Bakker, J.; Patka, P.; Van Lieshout, E.M.M. Risk of Infection and Sepsis in Severely Injured Patients Related to Single Nucleotide Polymorphisms in the Lectin Pathway. Br. J. Surg. 2013, 100, 1818–1826. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type Specific Roles of Nf-Kb Linking Inflamation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Liu, Z.L.; Hu, J.; Xiao, X.F.; Peng, Y.; Zhao, S.P.; Xiao, X.Z.; Yang, M.S. The CD40 Rs1883832 Polymorphism Affects Sepsis Susceptibility and SCD40L Levels. Biomed. Res. Int. 2018, 2018, 7497314. [Google Scholar] [CrossRef]
- Yu, L.; Fang, F.; Dai, X.; Xu, H.; Qi, X.; Fang, M.; Xu, Y. MKL1 Defines the H3K4Me3 Landscape for NF-ΚB Dependent Inflammatory Response. Sci. Rep. 2017, 7, 191. [Google Scholar] [CrossRef]
- Sun, J.; Cai, X.; Shen, J.; Jin, G.; Xie, Q. Correlation between Single Nucleotide Polymorphisms at the 3′-UTR of the NFKB1 Gene and Acute Kidney Injury in Sepsis. Genet. Test. Mol. Biomark. 2020, 24, 274–284. [Google Scholar] [CrossRef]
- Thair, S.A.; Walley, K.R.; Nakada, T.; McConechy, M.K.; Boyd, J.H.; Wellman, H.; Russell, J.A. A Single Nucleotide Polymorphism in NF-ΚB Inducing Kinase Is Associated with Mortality in Septic Shock. J. Immunol. 2011, 186, 2321–2328. [Google Scholar] [CrossRef]
- Arnalich, F.; Garcia-Palomero, E.; López, J.; Jiménez, M.; Madero, R.; Renart, J.; Vázquez, J.J.; Montiel, C. Predictive Value of Nuclear Factor B Activity and Plasma Cytokine Levels in Patients with Sepsis. Infect. Imun. 2000, 68, 1942–1945. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Z.; Zhang, H.; Wang, L. Correlation of Impaired NF-k B Activation in Sepsis-Induced Acute Lung Injury (ALI) in Diabetic Rats. J. Healthc. Eng. 2021, 2021, 5657284. [Google Scholar] [CrossRef]
- Jha, A.; Zilahi, G.; Rhodes, A. Vasoactive Therapy in Shock. BJA Educ. 2021, 21, 270–277. [Google Scholar] [CrossRef]
- Antonucci, E.; Giovini, M.; Agosta, S.; Sakr, Y.; Leone, M. Selepressin in Septic Shock. Shock 2022, 57, 172–179. [Google Scholar] [CrossRef]
- Nakada, T.A.; Russell, J.A.; Boyd, J.H.; Aguirre-Hernandez, R.; Thain, K.R.; Thair, S.A.; Nakada, E.; McConechy, M.; Walley, K.R. Β2-Adrenergic Receptor Gene Polymorphism Is Associated with Mortality in Septic Shock. Am. J. Respir. Crit. Care Med. 2010, 181, 143–149. [Google Scholar] [CrossRef]
- Russell, J.A.; Walley, K.R.; Singer, J.; Gordon, A.C.; Hébert, P.C.; James Cooper, D.; Holmes, C.L.; Mehta, S.; Granton, J.T.; Storms, M.M.; et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock from the ICAPTURE Centre. N. Engl. J. Med. 2008, 358, 877–887. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, T.N.; Knight, J.K.; Goodwin, J.E. The Glucocorticoid Receptor in Cardiovascular Health and Disease. Cells 2019, 8, 1227. [Google Scholar] [CrossRef]
- Ronald De Kloet, E.; Derijk, R. Corticosteroid Receptor Genetic Polymorphisms and Stress Responsivity. Endocrine 2005, 28, 263–270. [Google Scholar] [CrossRef]
- Baker, A.C.; Chew, V.W.; Green, T.L.; Tung, K.; Lim, D.; Cho, K.; Greenhalgh, D.G. Single Nucleotide Polymorphisms and Type of Steroid Impact the Functional Response of the Human Glucocorticoid Receptor. J. Surg. Res. 2013, 180, 27–34. [Google Scholar] [CrossRef]
- Isac, T.; Isac, S.; Rababoc, R.; Cotorogea, M.; Iliescu, L. Epigenetics in Inflammatory Liver Diseases: A Clinical Perspective (Review). Exp. Ther. Med. 2022, 23, 366. [Google Scholar] [CrossRef]
- Falcão-Holanda, R.B.; Brunialti, M.K.C.; Jasiulionis, M.G.; Salomão, R. Epigenetic Regulation in Sepsis, Role in Pathophysiology and Therapeutic Perspective. Front. Med. 2021, 8, 685333. [Google Scholar] [CrossRef]
- Gao, M.; Yu, T.; Liu, D.; Shi, Y.; Yang, P.; Zhang, J.; Wang, J.; Liu, Y.; Zhang, X. Sepsis Plasma-Derived Exosomal MiR-1-3p Induces Endothelial Cell Dysfunction by Targeting SERP1. Clin. Sci. 2021, 135, 347–365. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Xia, H.; Yao, S. MicroRNA-155: Regulation of Immune Cells in Sepsis. Mediat. Inflamm. 2021, 2021, 8874854. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal MiR-30d-5p of Neutrophils Induces M1 Macrophage Polarization and Primes Macrophage Pyroptosis in Sepsis-Related Acute Lung Injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Yang, F.; Wang, D.; Lu, Y.; Liu, L. MicroRNA-326 Prevents Sepsis-Induced Acute Lung Injury via Targeting TLR4. Free Radic. Res. 2020, 54, 408–418. [Google Scholar] [CrossRef]
- Vasilescu, C.; Rossi, S.; Shimizu, M.; Tudor, S.; Veronese, A.; Ferracin, M.; Nicoloso, M.S.; Barbarotto, E.; Popa, M.; Stanciulea, O.; et al. MicroRNA Fingerprints Identify MiR-150 as a Plasma Prognostic Marker in Patients with Sepsis. PLoS ONE 2009, 4, e7405. [Google Scholar] [CrossRef]
- Liu, B.; Chen, F.; Cheng, N.T.; Tang, Z.; Wang, X.G.; Xu, M. MicroRNA-1224-5p Aggravates Sepsis-Related Acute Lung Injury in Mice. Oxidative Med. Cell. Longev. 2022, 2022, 9493710. [Google Scholar] [CrossRef]
- Zou, L.; He, J.; Gu, L.; Shahror, R.A.; Li, Y.; Cao, T.; Wang, S.; Zhu, J.; Huang, H.; Chen, F.; et al. Brain Innate Immune Response via MiRNA-TLR7 Sensing in Polymicrobial Sepsis. Brain Behav. Immun. 2022, 100, 10–24. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Tangtanatakul, P.; Trithiphen, O.; Soonthornchai, W.; Wongphoom, J.; Tachaboon, S.; Srisawat, N.; Leelahavanichkul, A. Plasma MiR-370-3P as a Biomarker of Sepsis-Associated Encephalopathy, the Transcriptomic Profiling Analysis of Microrna-Arrays from Mouse Brains. Shock 2020, 54, 347–357. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, X.Y.; Zhou, X.G.; Cheng, R.; Liu, H.Y.; Li, Y. Neuroprotective Effects of MicroRNA-210 on Hypoxic-Ischemic Encephalopathy. Biomed. Res. Int. 2013, 2013, 350419. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Shao, X.; Feng, J.; Yan, J.; Qin, Y. Inhibition of MicroRNA-155 Modulates Endotoxin Tolerance by Upregulating Suppressor of Cytokine Signaling 1 in Microglia. Exp. Ther. Med. 2018, 15, 4709–4716. [Google Scholar] [CrossRef]
- Russell, J.A.; Rush, B.; Boyd, J. Pathophysiology of Septic Shock. Crit. Care Clin. 2018, 34, 43–61. [Google Scholar] [CrossRef]
- Formosa, A.; Turgeon, P.; dos Santos, C.C. Role of MiRNA Dysregulation in Sepsis. Mol. Med. 2022, 28, 99. [Google Scholar] [CrossRef]
- Rajput, C.; Tauseef, M.; Farazuddin, M.; Yazbeck, P.; Amin, M.R.; Avin Br, V.; Sharma, T.; Mehta, D. MicroRNA-150 Suppression of Angiopoetin-2 Generation and Signaling Is Crucial for Resolving Vascular Injury. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 380–388. [Google Scholar] [CrossRef]
- Etzrodt, V.; Idowu, T.O.; Schenk, H.; Seeliger, B.; Prasse, A.; Thamm, K.; Pape, T.; Müller-Deile, J.; van Meurs, M.; Thum, T.; et al. Role of Endothelial MicroRNA 155 on Capillary Leakage in Systemic Inflammation. Crit. Care 2021, 25, 76. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Fan, H. Exosomes from Endothelial Progenitor Cells Improve the Outcome of a Murine Model of Sepsis. Mol. Ther. 2018, 26, 1375–1384. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, D.; Gao, J.; Xiang, X.; Hu, X.; Li, S.; Wu, W.; Cai, J.; Tang, C.; Zhang, D.; et al. Discovery and Validation of MiR-452 as an Effective Biomarker for Acute Kidney Injury in Sepsis. Theranostics 2020, 10, 11963–11975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, H.; Liu, W.; Liu, S.; Wang, X.Y.; Diao, Z.L.; Zhang, A.H.; Guo, W.; Han, X.; Dong, X.; et al. Endothelial Progenitor Cells-Derived Exosomal MicroRNA-21-5p Alleviates Sepsis-Induced Acute Kidney Injury by Inhibiting RUNX1 Expression. Cell Death Dis. 2021, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Jin, Y.; Lin, M.; Xia, X.; Chen, X.; Huang, A. Down-Regulation of Xist and MiR-7a-5p Improves Lps-Induced Myocardial Injury. Int. J. Med. Sci. 2020, 17, 2570–2577. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.L.; Qin, L.J. The LncRNA XIST/MiR-150-5p/c-Fos Axis Regulates Sepsis-Induced Myocardial Injury via TXNIP-Modulated Pyroptosis. Lab. Investig. 2021, 101, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Chen, Y.F.; Wang, H.D.; Gao, F.H. Expression of Plasma MiRNA-497 in Children with Sepsis-Induced Myocardial Injury and Its Clinical Significance. Chin. J. Contemp. Pediatr. 2018, 20, 32–36. [Google Scholar]
- Bosmann, M.; Ward, P.A. The Inflammatory Response in Sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging Role of Exosomes in Cancer Progression and Tumor Microenvironment Remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, C.; Qiao, K.; Li, Z.; Han, J.; Han, X.; Li, K.; Lai, K.; Liu, N.; et al. Biomimetic Immunosuppressive Exosomes That Inhibit Cytokine Storms Contribute to the Alleviation of Sepsis. Adv. Mater. 2022, 34, e2108476. [Google Scholar] [CrossRef]
- Wu, D.; Shi, Y.; Zhang, H.; Miao, C. Epigenetic Mechanisms of Immune Remodeling in Sepsis: Targeting Histone Modification. Cell Death Dis. 2023, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- von Knethen, A.; Brüne, B. Histone Deacetylation Inhibitors as Therapy Concept in Sepsis. Int. J. Mol. Sci. 2019, 20, 346. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.; Li, B.; Chong, Y.; Zheng, G.; Sun, S.; Feng, F. SIRT1 Alleviates Isoniazid-Induced Hepatocyte Injury by Reducing Histone Acetylation in the IL-6 Promoter Region. Int. Immunopharmacol. 2019, 67, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Yu, W.D.; Chen, X.P. SirT1 Activator Represses the Transcription of TNF-α in THP-1 Cells of a Sepsis Model via Deacetylation of H4K16. Mol. Med. Rep. 2016, 14, 5544–5550. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Fukudome, E.Y.; Lu, J.; Chong, W.; Jin, G.; Liu, Z.; Velmahos, G.C.; Demoya, M.; King, D.R.; et al. Identification of Citrullinated Histone H3 as a Potential Serum Protein Biomarker in a Lethal Model of Lipopolysaccharide-Induced Shock. Surgery 2011, 150, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Di, C.; Chang, P.; Li, L.; Feng, Z.; Xiao, S.; Yan, X.; Xu, X.; Li, H.; Qi, R.; et al. Lactylated Histone H3K18 as a Potential Biomarker for the Diagnosis and Predicting the Severity of Septic Shock. Front. Immunol. 2022, 12, 786666. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, T.; Lang, X.; Jia, S.; Yang, Y.; Zhu, C.; Wang, Y.; Feng, S.; Wang, C.; Zhang, P.; et al. Inhibiting DNA Methylation Improves Survival in Severe Sepsis by Regulating NF-ΚB Pathway. Front. Immunol. 2020, 11, 1360. [Google Scholar] [CrossRef]
- Pelaia, T.M.; Shojaei, M.; McLean, A.S. The Role of Transcriptomics in Redefining Critical Illness. Crit. Care 2023, 27, 89. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.S.; et al. Genomic Landscape of the Individual Host Response and Outcomes in Sepsis: A Prospective Cohort Study. Lancet Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.L.; Davenport, E.E.; Radhakrishnan, J.; Humburg, P.; Gordon, A.C.; Hutton, P.; Svoren-Jabalera, E.; Garrard, C.; Hill, A.V.S.; Hinds, C.J.; et al. Shared and Distinct Aspects of the Sepsis Transcriptomic Response to Fecal Peritonitis and Pneumonia. Am. J. Respir. Crit. Care Med. 2017, 196, 328–339. [Google Scholar] [CrossRef]
- Scicluna, B.P.; van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of Patients with Sepsis according to Blood Genomic Endotype: A Prospective Cohort Study. Lancet Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis across Multiple Datasets Reveals Three Robust Clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.-P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med. 2018, 378, 809–818. [Google Scholar] [CrossRef]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids from the VaNISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef]
- Gómez, H.; Anderko, R.R.; Carcillo, J.A. Identifying Inflammatory Phenotypes to Target Mechanism-Specific Treatments in Sepsis. Cell Rep. Med. 2022, 3, 100823. [Google Scholar] [CrossRef]
- Sinha, P.; Calfee, C.S. Phenotypes in Acute Respiratory Distress Syndrome: Moving towards Precision Medicine. Curr. Opin. Crit. Care 2019, 25, 12–20. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. Acute Respiratory Distress Syndrome Subphenotypes and Differential Response to Simvastatin: Secondary Analysis of a Randomised Controlled Trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef]
- Bos, L.D.; Schouten, L.R.; Van Vught, L.A.; Wiewel, M.A.; Ong, D.S.Y.; Cremer, O.; Artigas, A.; Martin-Loeches, I.; Hoogendijk, A.J.; Van Der Poll, T.; et al. Identification and Validation of Distinct Biological Phenotypes in Patients with Acute Respiratory Distress Syndrome by Cluster Analysis. Thorax 2017, 72, 876–883. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic Changes in Amino Acid Concentration Profiles in Patients with Sepsis. PLoS ONE 2015, 10, e0121933. [Google Scholar] [CrossRef]
- Zeden, J.-P.; Fusch, G.; Holtfreter, B.; Schefold, J.C.; Reinke, P.; Domanska, G.; Haas, J.-P.; Gruendling, M.; Westerholt, A.; Schuett, C. Excessive Tryptophan Catabolism along the Kynurenine Pathway Precedes Ongoing Sepsis in Critically Ill Patients. Anaesth. Intensive Care 2010, 38, 307–316. [Google Scholar] [CrossRef]
- Kerksick, C.; Willoughby, D. The Antioxidant Role of Glutathione and N-Acetyl-Cysteine Supplements and Exercise-Induced Oxidative Stress. J. Int. Soc. Sports Nutr. 2005, 2, 38. [Google Scholar] [CrossRef]
- Ekremoǧlu, M.; Türközkan, N.; Erdamar, H.; Kurt, Y.; Yaman, H. Protective Effect of Taurine on Respiratory Burst Activity of Polymorphonuclear Leukocytes in Endotoxemia. Amino Acids 2007, 32, 413–417. [Google Scholar] [CrossRef]
- Reisinger, A.C.; Posch, F.; Hackl, G.; Marsche, G.; Sourij, H.; Bourgeois, B.; Eller, K.; Madl, T.; Eller, P. Branched-chain Amino Acids Can Predict Mortality in Icu Sepsis Patients. Nutrients 2021, 13, 3106. [Google Scholar] [CrossRef]
- Dalli, J.; Colas, R.A.; Quintana, C.; Barragan-Bradford, D.; Hurwitz, S.; Levy, B.D.; Choi, A.M.; Serhan, C.N.; Baron, R.M. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations with Survival and Clinical Outcomes. Crit. Care Med. 2017, 45, 58–68. [Google Scholar] [CrossRef]
- Walley, K.R.; Boyd, J.H.; Kong, H.J.J.; Russell, J.A. Low Low-Density Lipoprotein Levels Are Associated with, but Do Not Causally Contribute to, Increased Mortality in Sepsis. Crit. Care Med. 2019, 47, 463–466. [Google Scholar] [CrossRef]
- Hussain, H.; Vutipongsatorn, K.; Jiménez, B.; Antcliffe, D.B. Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response. Metabolites 2022, 12, 376. [Google Scholar] [CrossRef]
- Eckerle, M.; Ambroggio, L.; Puskarich, M.A.; Winston, B.; Jones, A.E.; Standiford, T.J.; Stringer, K.A. Metabolomics as a Driver in Advancing Precision Medicine in Sepsis. Pharmacotherapy 2017, 37, 1023–1032. [Google Scholar] [CrossRef]
- Pan, T.; Sun, S.; Chen, Y.; Tian, R.; Chen, E.; Tan, R.; Wang, X.; Liu, Z.; Liu, J.; Qu, H. Immune Effects of PI3K/Akt/HIF-1α-Regulated Glycolysis in Polymorphonuclear Neutrophils during Sepsis. Crit. Care 2022, 26, 29. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. J. Am. Med. Assoc. 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Cotorogea-Simion, M.; Pavel, B.; Isac, S.; Telecan, T.; Matache, I.M.; Bobirca, A.; Bobirca, F.T.; Rababoc, R.; Droc, G. What Is Different in Acute Hematologic Malignancy-Associated ARDS? An Overview of the Literature. Medicina 2022, 58, 1215. [Google Scholar] [CrossRef]
- Bhavani, S.V.; Semler, M.; Qian, E.T.; Verhoef, P.A.; Robichaux, C.; Churpek, M.M.; Coopersmith, C.M. Development and Validation of Novel Sepsis Subphenotypes Using Trajectories of Vital Signs. Intensive Care Med. 2022, 48, 1582–1592. [Google Scholar] [CrossRef]
- Kudo, D.; Goto, T.; Uchimido, R.; Hayakawa, M.; Yamakawa, K.; Abe, T.; Shiraishi, A.; Kushimoto, S. Coagulation Phenotypes in Sepsis and Effects of Recombinant Human Thrombomodulin: An Analysis of Three Multicentre Observational Studies. Crit. Care 2021, 25, 114. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isac, S.; Isac, T.; Tanasescu, M.D.; Pavel, B.; Andreescu, C.V.; Badea, A.-G.; Ojog, D.; Teodorescu, G.-D.; Laceanu, A.; Trifan, C.-B.; et al. The Omics Complexity in Sepsis: The Limits of the Personalized Medicine Approach. J. Pers. Med. 2024, 14, 225. https://doi.org/10.3390/jpm14030225
Isac S, Isac T, Tanasescu MD, Pavel B, Andreescu CV, Badea A-G, Ojog D, Teodorescu G-D, Laceanu A, Trifan C-B, et al. The Omics Complexity in Sepsis: The Limits of the Personalized Medicine Approach. Journal of Personalized Medicine. 2024; 14(3):225. https://doi.org/10.3390/jpm14030225
Chicago/Turabian StyleIsac, Sebastian, Teodora Isac, Maria Daniela Tanasescu, Bogdan Pavel, Cristina Veronica Andreescu, Andrada-Georgiana Badea, Damiana Ojog, Geani-Danut Teodorescu, Anca Laceanu, Cristian-Bogdan Trifan, and et al. 2024. "The Omics Complexity in Sepsis: The Limits of the Personalized Medicine Approach" Journal of Personalized Medicine 14, no. 3: 225. https://doi.org/10.3390/jpm14030225
APA StyleIsac, S., Isac, T., Tanasescu, M. D., Pavel, B., Andreescu, C. V., Badea, A.-G., Ojog, D., Teodorescu, G.-D., Laceanu, A., Trifan, C.-B., & Droc, G. (2024). The Omics Complexity in Sepsis: The Limits of the Personalized Medicine Approach. Journal of Personalized Medicine, 14(3), 225. https://doi.org/10.3390/jpm14030225







