Long-Term Prognosis after ST-Elevation Myocardial Infarction in Patients with Premature Coronary Artery Disease
Abstract
:1. Introduction
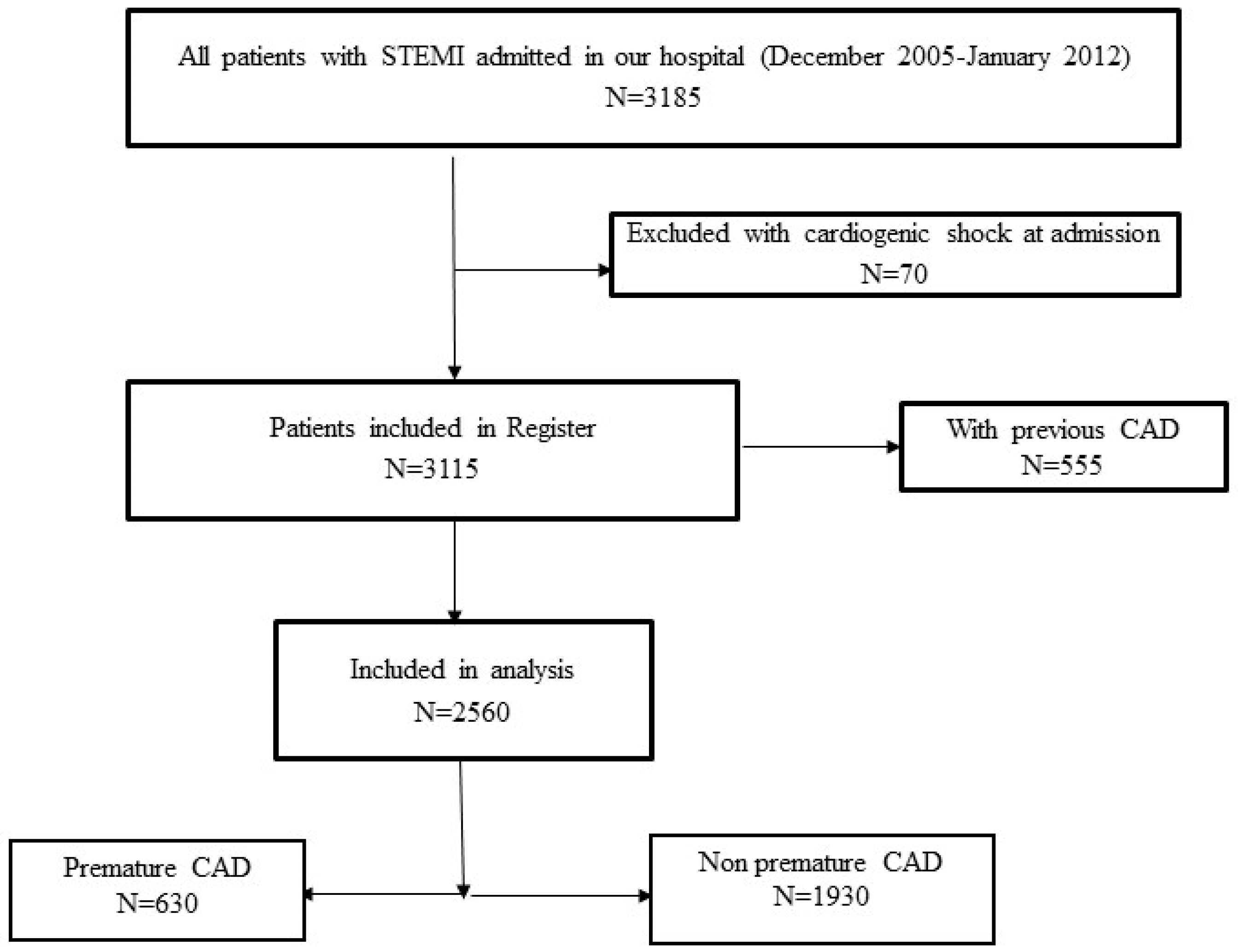
2. Materials and Methods
2.1. Study Population, Data Collection, and Definitions
2.2. Ethics
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. The Incidence and Risk Factors of Premature CAD
4.2. Prognosis in Patients with Premature CAD
4.3. Independent Predictors of Mortality and MACEs in Patients with Premature CAD
4.4. Clinical Implications
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collet, J.P.; Zeitouni, M.; Procopi, N.; Hulot, J.S.; Silvain, J.; Kerneis, M.; Thomas, D.; Lattuca, B.; Barthelemy, O.; Lavie-Badie, Y.; et al. ACTION Study Group. Long-Term Evolution of Premature Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 1868–1878. [Google Scholar] [CrossRef]
- Bugiardini, R.; Badimon, L.; ISACS-TC Investigators and Coordinators. The International Survey of Acute Coronary Syndromes in Transitional Countries (ISACS-TC): 2010–2015. Int. J. Cardiol. 2016, 217, S1–S6. [Google Scholar] [CrossRef]
- Wittlinger, T.; Seifert, C.; Simonis, G.; Gerlach, M.; Strasser, R.H. Prognosis in myocardial infarction of young patients: Results of a prospective registry. Int. J. Cardiol. 2020, 300, 1–6. [Google Scholar] [CrossRef]
- Vasić, A.; Vasiljević, Z.; Mickovski-Katalina, N.; Mandić-Rajčević, S.; Soldatović, I. Temporal Trends in Acute Coronary Syndrome Mortality in Serbia in 2005–2019: An Age-Period-Cohort Analysis Using Data from the Serbian Acute Coronary Syndrome Registry (RAACS). Int. J. Environ. Res. Public Health 2022, 19, 14457. [Google Scholar] [CrossRef]
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.M.; Qamar, A.; Vaduganathan, M.; Pandey, A.; Mph, D.L.B.; Caughey, M.C. Twenty Year Trends and Sex Differences in Young Adults Hospitalized with Acute Myocardial Infarction. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Ni, L.; Liu, K.; Gao, X.; Yang, J.; Zhang, X.; Ye, Y.; Dong, Q.; Fu, R.; Sun, H.; et al. Clinical Characteristics, Prognosis, and Gender Disparities in Young Patients with Acute Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 720378. [Google Scholar] [CrossRef] [PubMed]
- Pinxterhuis, T.H.; Ploumen, E.H.; Doggen, C.J.M.; Hartmann, M.; Schotborgh, C.E.; Anthonio, R.L.; Roguin, A.; Danse, P.W.; Benit, E.; Aminian, A.; et al. First myocardial infarction in patients with premature coronary artery disease: Insights into patient characteristics and outcome after treatment with contemporary stents. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 774–781. [Google Scholar] [CrossRef]
- Smith, C.L.; Seigerman, M.; Adusumalli, S.; Giri, J.; Fiorilli, P.N.; Kolansky, D.M.; Kobayashi, T. Evolution and Outcomes of Premature Coronary Artery Disease. Curr. Cardiol. Rep. 2021, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Makkar, J.S.; Bana, A.; Sharma, K.; Kasliwal, A.; Sidana, S.K.; Degawat, P.R.; Bhagat, K.K.; Chaurasia, A.K.; Natani, V.; et al. Premature coronary artery disease, risk factors, clinical presentation, angiography and interventions: Hospital based registry. Indian Heart J. 2022, 74, 391–397. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Xenogiannis, I.; Brilakis, E.S.; Bhatt, D.L. Causes, Angiographic Characteristics, and Management of Premature Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2431–2449. [Google Scholar] [CrossRef]
- Khoja, A.; Andraweera, P.H.; Lassi, Z.S.; Ali, A.; Zheng, M.; Pathirana, M.M.; Aldridge, E.; Wittwer, M.R.; Chaudhuri, D.D.; Tavella, R.; et al. Risk Factors for Early Versus Late-Onset Coronary Heart Disease (CHD): Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, 1277–1311. [Google Scholar] [CrossRef]
- Abderrahman, H.A.; Al-Abdallat, I.M.; Idhair, A.K. Age threshold for proper definition of premature coronary artery disease in males. J. Forensic Leg. Med. 2018, 58, 45–49. [Google Scholar] [CrossRef]
- Pinxterhuis, T.H.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; Schotborgh, C.E.; Anthonio, R.L.; Roguin, A.; Danse, P.W.; Benit, E.; Aminian, A.; et al. Impact of premature coronary artery disease on adverse event risk following first percutaneous coronary intervention. Front. Cardiovasc. Med. 2023, 10, 1160201. [Google Scholar] [CrossRef] [PubMed]
- Vikulova, D.N.; Grubisic, M.; Zhao, Y.; Lynch, K.; Humphries, K.H.; Pimstone, S.N.; Brunham, L.R. Premature atherosclerotic cardiovascular disease: Trends in incidence, risk factors, and sex-related differences, 2000 to 2016. J. Am. Heart Assoc. 2019, 8, e012178. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Behfar, A.; Narula, J.; Kanwar, A.; Lerman, A.; Cooper, L.; Singh, M. Acute Myocardial Infarction in Young Individuals. Mayo Clin. Proc. 2020, 95, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Biery, D.W.; Singh, A.; Divakaran, S.; DeFilippis, E.M.; Wu, W.Y.; Klein, J.; Hainer, J.; Ramsis, M.; Natarajan, P.; et al. Risk Factors and Outcomes of Very Young Adults Who Experience Myocardial Infarction: The Partners YOUNG-MI Registry. Am. J. Med. 2020, 133, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.T.; Pang, Y.; Gao, L.L.; Han, L.J.; Yao, H.C. Clinical risk factors and outcomes of young patients with acute ST segment elevation myocardial infarction: A retrospective study. BMC Cardiovasc. Disord. 2023, 23, 353. [Google Scholar] [CrossRef]
- Noaman, S.; Dinh, D.; Reid, C.M.; Brennan, A.L.; Clark, D.; Shaw, J.; Freeman, M.; Sebastian, M.; Oqueli, E.; Ajani, A.; et al. Comparison of Outcomes of Coronary Artery Disease Treated by Percutaneous Coronary Intervention in 3 Different Age Groups (<45, 46–65, and >65 Years). Am. J. Cardiol. 2021, 152, 19–26. [Google Scholar]
- Biswas, S.; Andrianopoulos, N.; Papapostolou, S.; Noaman, S.; Duffy, S.J.; Lefkovits, J.; Brennan, A.; Walton, A.; Shaw, J.A.; Ajani, A.; et al. Does the subtype of acute coronary syndrome treated by percutaneous coronary intervention predict long-term clinical outcomes? Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 318–327. [Google Scholar] [CrossRef]
- Mrdovic, I.; Savic, L.; Lasica, R.; Krljanac, G.; Asanin, M.; Brdar, N.; Djuricic, N.; Marinkovic, J.; Perunicic, J. Efficacy and safety of tirofiban-supported primary percutaneous coronary intervention in patients pretreated with 600 mg clopidogrel: Results of propensity analysis using the clinical center of serbia STEMI register. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 56–66. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Jortveit, J.; Pripp, A.H.; Langørgen, J.; Halvorsen, S. Incidence, risk factors and outcome of young patients with myocardial infarction. Heart 2020, 106, 1420–1426. [Google Scholar] [CrossRef]
- Zheng, R.; Liu, Y.; Hao, Z.; Liao, H.; Xiao, C. Clinical Characteristics and Prognosis of Young Patients with Coronary Heart Disease. Med. Sci. Monit. 2020, 26, e922957. [Google Scholar] [CrossRef] [PubMed]
- Savic, L.; Mrdovic, I.; Asanin, M.; Stankovic, S.; Krljanac, G.; Lasica, R. Prognostic impact of renal dysfunction on long-term mortality in patients with preserved, moderately impaired, and severely impaired left ventricular systolic function following myocardial infarction. Anatol. J. Cardiol. 2018, 20, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ. Res. 2019, 124, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.G.; Lansky, A.J.; Meller, S.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.; Shah, R.; Mehran, R.; Stone, G.W. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction:the HORIZONS-AMI trial. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]

| Characteristics | Premature CAD | Non-Premature CAD | p-Value |
|---|---|---|---|
| N = 630 | N = 1930 | ||
| Age, years med (IQR) | 45 (41, 48) | 62 (56, 71) | <0.001 |
| Female, n (%) | 162 (25.7) | 522 (27) | 0.177 |
| Hypertension, n (%) | 293 (46.5) | 1346 (69.7) | <0.001 |
| HLP, n (%) | 413 (65.5) | 1116 (57.8) | <0.001 |
| Smoking, n (%) | 485 (76.9) | 922 (47.8) | <0.001 |
| Family history, n (%) | 314 (49.8) | 542 (28) | <0.001 |
| Pain duration, hours med (IQR) | 2 (1, 4) | 3 (1.5, 4) | <0.001 |
| Atrial fibrillation on initial ECG, n (%) | 13 (2.1) | 145 (7.5) | <0.001 |
| Complete AV block, n (%) | 13 (2.1) | 101 (5.2) | 0.005 |
| Killip class > 1, n (%) | 48 (7.6) | 254 (13.2) | 0.001 |
| Systolic BP at admission, med (IQR) | 130 (120, 150) | 140 (120, 150) | <0.001 |
| Heart rate at admission med (IQR) | 80 (70, 90) | 76 (68, 89) | 0.059 |
| BBB on initial ECG, n (%) | 16 (2.6) | 71 (3.6) | 0.559 |
| Multivessel disease, n (%) | 229 (36.3) | 1130 (58.5) | <0.001 |
| 3-vessel disease, n (%) | 75 (11.9) | 520 (26.9) | <0.001 |
| LM stenosis, n (%) | 13 (2.1) | 137 (7.1) | <0.001 |
| LAD culprit vessel, n (%) | 249 (39.5) | 765 (39.7) | 0.583 |
| Preprocedural flow TIMI 0, n (%) | 440 (69.8) | 1328 (68.8) | 0.379 |
| Postprocedural flow TIMI < 3, n (%) | 16 (2.5) | 81 (4.2) | 0.067 |
| Stent implanted, n (%) | 610 (96.7) | 1835 (95.1) | 0.067 |
| Total stent length, med (IQR) | 23 (18, 25) | 24 (19, 26) | 0.204 |
| Acute stent thrombosis, n (%) | 10 (1.5) | 18 (0.9) | 0.162 |
| CK-MB med (IQR) | 2254 (1051, 3898) | 1925 (1067, 3391) | <0.001 |
| Troponin I, med (IQR) | 93.2 (34.1, 183.1) | 90.7 (33.7, 172) | 0.001 |
| EF, med (IQR) | 50 (42, 58) | 47 (40, 55) | <0.001 |
| EF < 40%, n (%) | 63 (10%) | 620 (24.2) | <0.001 |
| EF 40–49%, n (%) | 155 (24.6) | 953 (37.2) | <0.001 |
| EF ≥ 50%, n (%) | 402 (63.8) | 986 (38.5) | <0.001 |
| Diabetes, n (%) | 57 (9.1) | 400 (20.7) | <0.001 |
| CKD, n (%) | 8 (1.2) | 358 (18.5) | <0.001 |
| Obesity, n (%) | 124 (19.7) | 287 (14.9) | 0.018 |
| Anemia, n (%) | 18 (2.8) | 164 (8.5) | <0.001 |
| Previous stroke, n (%) | 8 (1.3) | 81 (4.2) | <0.001 |
| Therapy at discharge * | |||
| Beta blockers, n (%) | 611 (93) | 1848 (95.7) | 0.150 |
| ACE inhibitors, n (%) | 539 (85.6) | 1739 (90.1) | 0.003 |
| Statin, n (%) | 607 (96.3) | 1902 (98.5) | 0.139 |
| Diuretic, n (%) | 62 (9.9) | 306 (15.8) | <0.001 |
| Calcium antagonist, n (%) | 11 (1.7) | 70 (3.6) | 0.009 |
| Amiodarone, n (%) | 13 (2) | 52 (3.1) | 0.133 |
| In-hospital mortality, n (%) | 4 (0.6) | 80 (4.1) | <0.001 |
| Event | Premature CAD | Non-Premature CAD | p-Value |
|---|---|---|---|
| N = 630 | N = 1930 | ||
| 30 days | |||
| All-cause mortality, n (%) | 5 (0.7) | 93 (4.8) | <0.001 |
| MACE, n (%) | 30 (4.7) | 157 (8.1) | <0.001 |
| Cardiovascular death, n (%) | 5 (0.7) | 93 (4.8) | <0.001 |
| Non-fatal recurrent infarction, n (%) | 19 (3) | 64 (3.3) | 0.540 |
| TVR, n (%) | 19 (3) | 64 (3.3) | 0.540 |
| Non-fatal stroke, n (%) | 0 | 15 (0.7) | 0.085 |
| 1 year | |||
| All-cause mortality, n (%) | 9 (1.4) | 126 (6.5) | <0.001 |
| MACE, n (%) | 36 (5.6) | 205 (10.6) | 0.022 |
| Cardiovascular death, n (%) | 9 (1.4) | 105 (5.4) | <0.001 |
| Non-fatal recurrent infarction, n (%) | 27 (4.3) | 98 (5.1) | 0.362 |
| TVR, n (%) | 34 (5.4) | 106 (5.5) | 0.360 |
| Non-fatal stroke, n (%) | 2 (0.3) | 28 (1.4) | 0.080 |
| MACE, n (%) | 36 (5.6) | 205 (10.6) | 0.022 |
| 8 years | |||
| All-cause death, n (%) | 15 (2.4) | 157 (8.1) | <0.001 |
| MACE, n (%) | 51 (8) | 340 (17.6) | <0.001 |
| Cardiovascular death, n (%) | 13 (2) | 146 (7.6) | <0.001 |
| Non-fatal recurrent infarction, n (%) | 51 (8.1) | 168 (8.7) | 0.726 |
| TVR, n (%) | 58 (9.2) | 169 (8.6) | 0.585 |
| Non-fatal stroke, n (%) | 7 (1.1) | 51 (2.6) | 0.089 |
| All-Cause Mortality | MACE | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Univariable analysis | ||||
| Premature CAD | 0.50 (0.28–0.91) | <0.001 | 0.27 (0.15–0.47) | <0.001 |
| Killip > 1 at admission | 5.04 (3.78–6.89) | <0.001 | 7.02 (5.21–9.45) | <0.001 |
| Atrial fibrillation at admission | 4.58 (3.12–6.59) | <0.001 | 4.69 (3.22–6.83) | <0.001 |
| CKD | 4.90 (3.59–6.69) | <0.001 | 5.36 (3.88–7.41) | <0.001 |
| Postprocedural TIMI < 3 | 3.36 (2.43–5.42) | <0.001 | 7.52 (5.14–11.01) | <0.001 |
| 3-vessel disease | 1.56 (1.14–2.14) | 0.005 | 2.05 (1.49–2.83) | <0.001 |
| Ejection fraction % | 0.89 (0.87–0.90) | <0.001 | 0.88 (0.87–0.89) | <0.001 |
| Systolic blood pressure at admission, mmHg | 0.99 (0.98–0.99) | 0.024 | ||
| Complete AV block at admission | 2.80 (1.93–4.07) | <0.001 | ||
| Diabetes mellitus | 2.0 (1.42–2.82) | <0.001 | ||
| Multivariable analysis | ||||
| Premature CAD | 0.48 (0.26–0.83) | 0.001 | 0.50 (0.27–0.91) | 0.026 |
| CKD | 2.36 (1.68–3.31) | <0.001 | 2.74 (1.94–3.89) | <0.001 |
| Postprocedural TIMI < 3 | 2.14 (1.40–3.27) | <0.001 | 2.41 (1.56–3.72) | <0.001 |
| Killip > 1 at admission | 1.89 (1.26–2.58) | 0.001 | 1.96 (1.35–2.85) | <0.001 |
| Atrial fibrillation at admission | 1.61 (1.08–2.41) | 0.019 | 1.58 (1.04–2.38) | 0.031 |
| 3-vessel disease | 1.50 (1.04–1.90) | 0.045 | ||
| Ejection fraction % | 0.91 (0.90–0.93) | <0.001 | 0.90 (0.88–0.92) | <0.001 |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| All-cause mortality | ||||
| EF < 40% | 8.28 (5.25–12.35) | <0.001 | 5.59 (2.18–8.52) | <0.001 |
| EF 40–49% | 2.04 (1.17–9.12) | 0.025 | ||
| LM stenosis | 7.26 (1.69–12.12) | 0.009 | ||
| Baseline CKD | 6.91 (3.25–16.8) | 0.035 | ||
| Killip class > 1 at admission | 6.29 (3.14–12.59) | <0.001 | ||
| Postprocedural TIMI < 3 | 6.09 (1.35–20.9) | 0.019 | ||
| Atrial fibrillation at admission | 3.58 (1.27–13.5) | 0.001 | ||
| MACE | ||||
| EF < 40% | 4.79 (2.39–8.74) | <0.001 | 4.18 (1.98–8.13) | <0.001 |
| EF 40–49% | 1.59 (1.11–2.91) | 0.050 | ||
| Baseline CKD | 3.79 (2.45–5.61) | 0.001 | ||
| Postprocedural TIMI < 3 | 2.39 (1.17–7.49) | 0.021 | ||
| Smoking | 2.16 (1.19–5.08) | 0.059 | ||
| Killp class > 1 at admission | 1.99 (1.09–4.43) | 0.049 | ||
| 3-vessel disease | 1.51 (1.01–2.67) | 0.050 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savic, L.; Mrdovic, I.; Asanin, M.; Stankovic, S.; Lasica, R.; Krljanac, G.; Simic, D.; Matic, D. Long-Term Prognosis after ST-Elevation Myocardial Infarction in Patients with Premature Coronary Artery Disease. J. Pers. Med. 2024, 14, 231. https://doi.org/10.3390/jpm14030231
Savic L, Mrdovic I, Asanin M, Stankovic S, Lasica R, Krljanac G, Simic D, Matic D. Long-Term Prognosis after ST-Elevation Myocardial Infarction in Patients with Premature Coronary Artery Disease. Journal of Personalized Medicine. 2024; 14(3):231. https://doi.org/10.3390/jpm14030231
Chicago/Turabian StyleSavic, Lidija, Igor Mrdovic, Milika Asanin, Sanja Stankovic, Ratko Lasica, Gordana Krljanac, Damjan Simic, and Dragan Matic. 2024. "Long-Term Prognosis after ST-Elevation Myocardial Infarction in Patients with Premature Coronary Artery Disease" Journal of Personalized Medicine 14, no. 3: 231. https://doi.org/10.3390/jpm14030231
APA StyleSavic, L., Mrdovic, I., Asanin, M., Stankovic, S., Lasica, R., Krljanac, G., Simic, D., & Matic, D. (2024). Long-Term Prognosis after ST-Elevation Myocardial Infarction in Patients with Premature Coronary Artery Disease. Journal of Personalized Medicine, 14(3), 231. https://doi.org/10.3390/jpm14030231







