Digital Anti-Aging Healthcare: An Overview of the Applications of Digital Technologies in Diet Management
Abstract
:1. Introduction
- Raise awareness of the use of digital technologies for diet management.
- Demonstrate the impact of diet management and digital technologies in anti-aging healthcare.
- Improve anti-aging outcomes for the betterment of the aging population.
2. Aging Healthcare
2.1. Impact of Aging on Health
2.2. Age-Related Diseases
2.3. Aging Biomarkers
2.4. Potential Interventions in the Aging Process
3. Role of Nutrition in Aging
4. Digital Anti-Aging Healthcare and Diet Management
4.1. Emergence of Digital Technologies in Healthcare
4.2. Digital Diet Management for Anti-Aging Healthcare
4.2.1. Mobile Applications for Diet Tracking (Food-Tracking Apps)
4.2.2. Web-Based Platforms and Virtual Coaching
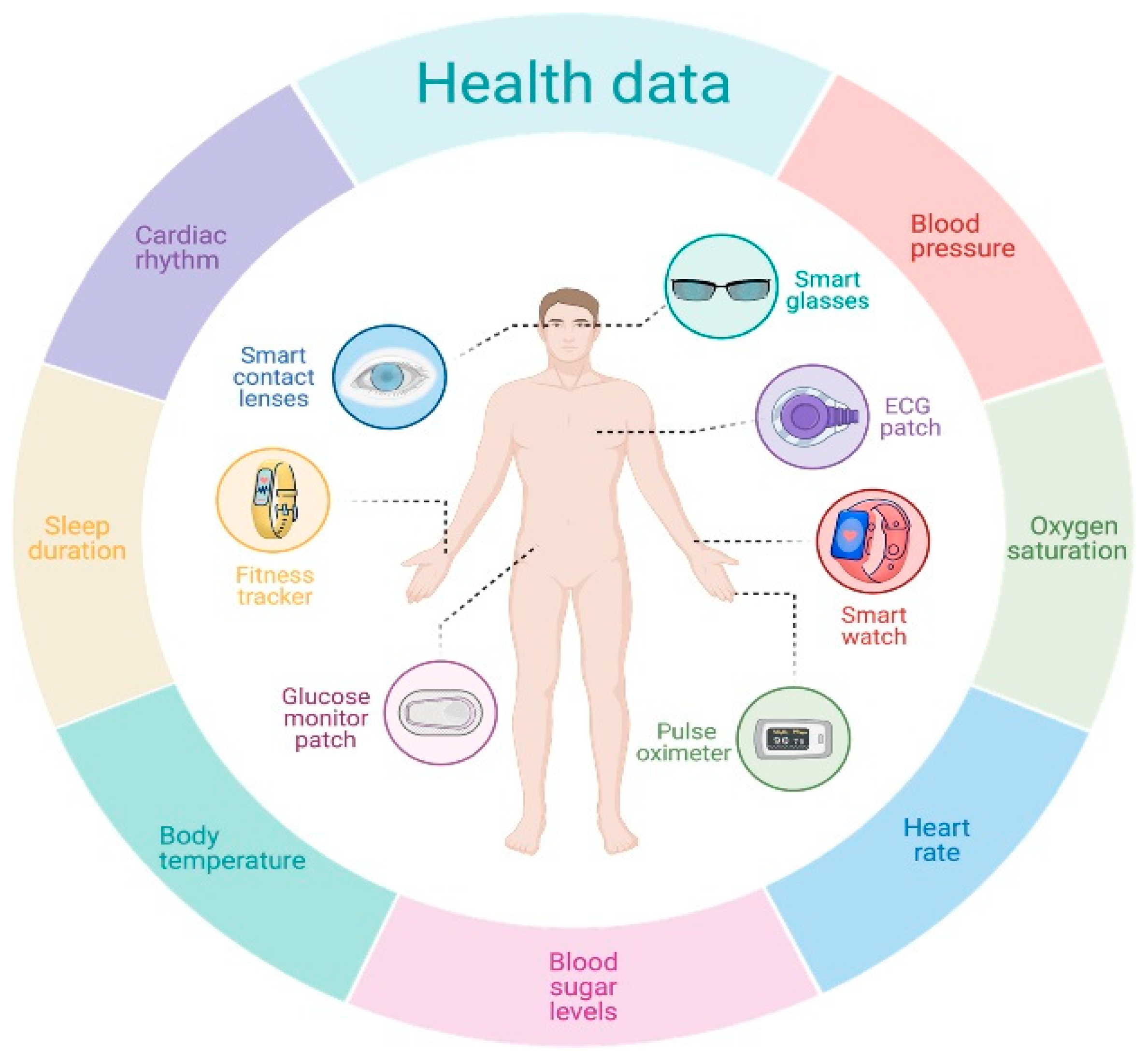
4.2.3. Wearable Devices, Sensors, and the IoT
4.2.4. Artificial Intelligence
4.2.5. Blockchain
- IBM Food Trust: A modular food traceability solution that is built using blockchain technology to operate across the food industry’s value chain [135]. IBM Food Trust provides real-time quality control, enabling increased visibility in the supply chain. Many businesses, including Walmart and Nestle, have adopted this platform, demonstrating significant potential for improving food safety.
- TE-FOOD: This solution provides a permissioned blockchain for every food organization, regardless of size. Through its blockchain layers, TE-FOOD enhances the security of food traceability, providing real-time quality control to guarantee consumer trust. Migros, one of Switzerland’s largest supermarkets, uses this platform to achieve end-to-end product traceability [136].
- Ambrosus: This platform uses blockchain to enhance patients’ trust and confidence in food products. Ambrosus has achieved end-to-end traceability and ensures food safety and quality [137]. It has been adopted by several businesses, including the Swiss coffee producer Lattesso and the French wine producer Château Latour.
4.2.6. Other Technologies Involved in Digital Diet Monitoring
5. Technological Choices, User Experience, and Legal Compliance in Digital Diet Management
6. Effects of Digital Diet Control on Anti-Aging Healthcare
6.1. Improved Nutrition Monitoring and Compliance
6.2. Personalized Diet Recommendations
6.3. Behavior Change and Sustainable Habits
6.4. Impact on Longevity and Health Span
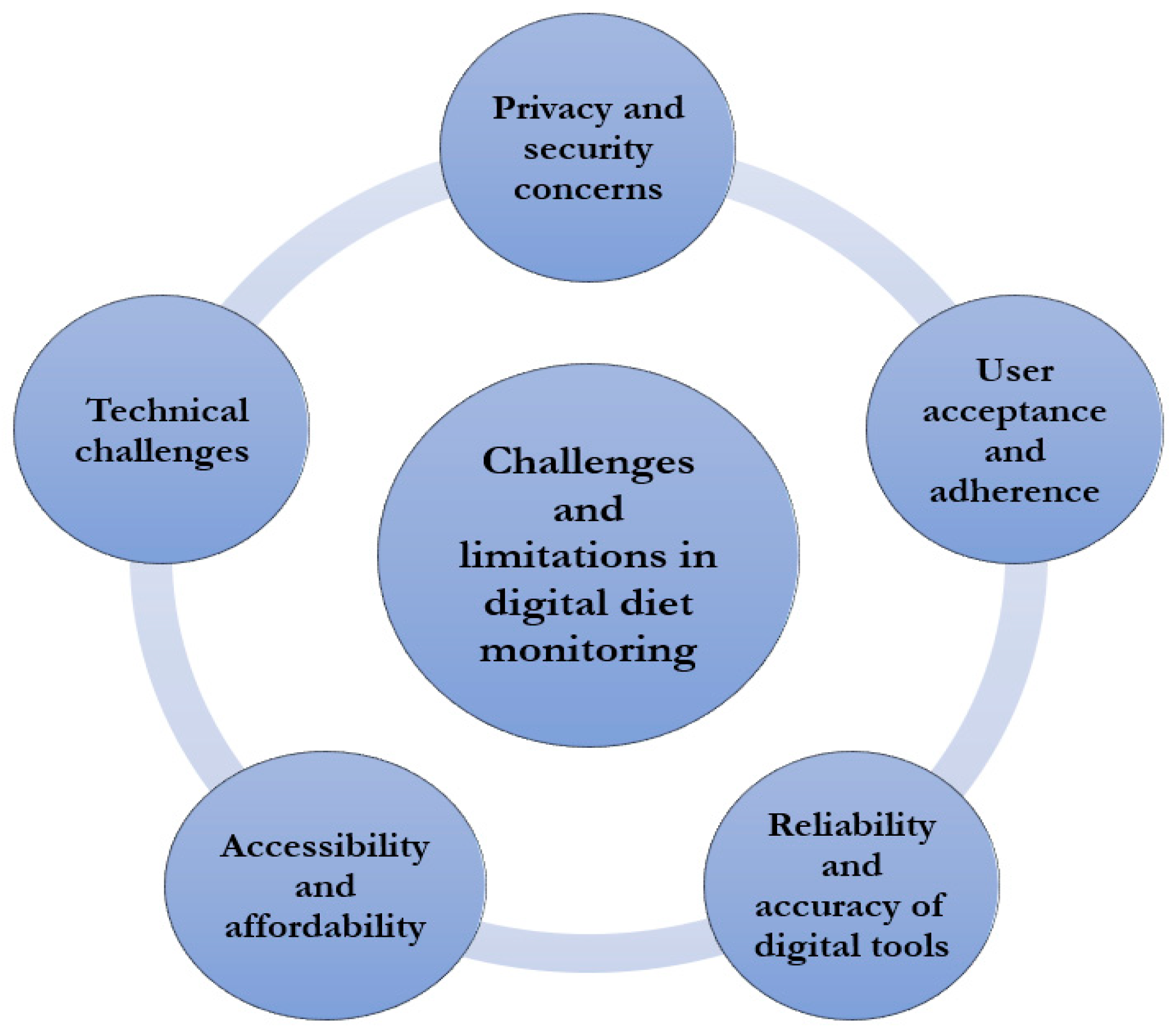
7. Challenges and Limitations in Digital Diet Management
7.1. Privacy and Security Concerns
7.2. User Acceptance and Adherence
7.3. Reliability and Accuracy of Digital Tools
7.4. Accessibility and Affordability
7.5. Technical Challenges
8. Future Directions and Recommendations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Sinclair, D.A.; Kligman, E.W. Do antiaging approaches promote longevity? How can you help your patients age gracefully with less disease? Exercise, good nutrition, and stress reduction are interventions backed by evidence, but what is the evidence behind hormonal treatments and dietary supplements? Patient Care Nurse Pract. 2005, 39, 10–18. [Google Scholar]
- American Academy of Anti Aging Medicine. Available online: https://www.a4m.com/ (accessed on 18 July 2023).
- Kaeberlein, M.; Rabinovitch, P.S.; Martin, G.M. Healthy aging: The ultimate preventative medicine. Science 2015, 350, 1191–1193. [Google Scholar] [CrossRef]
- He, W.; Goodkind, D.; Kowal, P.R. An Aging World: 2015; U.S. Government Publishing Office: Washington, DC, USA, 2016.
- Fletcher, D.J. Age as Disease: Anti-Aging Technologies, Sites and Practices; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Cardona, B. ‘Healthy Ageing’policies and anti-ageing ideologies and practices: On the exercise of responsibility. Med. Health Care Philos. 2008, 11, 475–483. [Google Scholar] [CrossRef]
- Black, M.; Bowman, M. Nutrition and healthy aging. Clin. Geriatr. Med. 2020, 36, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Ortolá, R.; García-Esquinas, E.; García-Varela, G.; Struijk, E.A.; Rodríguez-Artalejo, F.; López-García, E. Influence of changes in diet quality on unhealthy aging: The seniors-ENRICA cohort. Am. J. Med. 2019, 132, 1091–1102. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.-S.H. Natural anti-aging skincare: Role and potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-S. A Exploratory study on the digital aging policies as solutions for a aging society. J. Digit. Converg. 2016, 14, 115–123. [Google Scholar] [CrossRef]
- Hall, A.K.; Bernhardt, J.M.; Dodd, V.; Vollrath, M.W. The digital health divide: Evaluating online health information access and use among older adults. Health Educ. Behav. 2015, 42, 202–209. [Google Scholar] [CrossRef]
- Chen, C.; Ding, S.; Wang, J. Digital health for aging populations. Nat. Med. 2023, 29, 1623–1630. [Google Scholar] [CrossRef]
- Mozumder, M.A.I.; Armand, T.P.T.; Imtiyaj Uddin, S.M.; Athar, A.; Sumon, R.I.; Hussain, A.; Kim, H.C. Metaverse for Digital Anti-Aging Healthcare: An Overview of Potential Use Cases Based on Artificial Intelligence, Blockchain, IoT Technologies, Its Challenges, and Future Directions. Appl. Sci. 2023, 13, 5127. [Google Scholar] [CrossRef]
- Mozumder, M.A.I.; Sumon, R.I.; Uddin, S.M.I.; Athar, A.; Kim, H.C. The Metaverse for Intelligent Healthcare using XAI, Blockchain, and Immersive Technology. In Proceedings of the 2023 IEEE International Conference on Metaverse Computing, Networking and Applications (MetaCom), Kyoto, Japan, 26–28 June 2023; pp. 612–616. [Google Scholar]
- Phillip, J.M.; Aifuwa, I.; Walston, J.; Wirtz, D. The mechanobiology of aging. Annu. Rev. Biomed. Eng. 2015, 17, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.N.; Brunet, A. The aging epigenome. Mol. Cell 2016, 62, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Di Benedetto, S.; Pawelec, G. The immune system and its dysregulation with aging. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Springer: Singapore, 2019; pp. 21–43. [Google Scholar]
- Nikolich-Žugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Piasecki, M.; Atherton, P. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef]
- Mental Health of Older Adults. World Health Organization, 12 December 2017. Available online: www.who.int/news-room/fact-sheets/detail/mental-health-of-older-adults (accessed on 17 August 2023).
- Murman, D.L. The impact of age on cognition. In Seminars in Hearing; Thieme Medical Publishers: New York, NY, USA, 2015; Volume 36, pp. 111–121. [Google Scholar]
- Brunet, A.; Berger, S.L. Epigenetics of aging and aging-related disease. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69 (Suppl. S1), S17–S20. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Dietary quality is related to frailty in community-dwelling older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; Van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): A practical tool for identification of nutritional status. JNHA-J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E. Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S. Too toxic to ignore. Nature 2012, 490, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Bai, X. Biomarkers of aging. In Aging and Aging-Related Diseases: Mechanisms and Interventions; Springer: Singapore, 2018; pp. 217–234. [Google Scholar]
- Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Boeve, B.F.; Christianson, T.J.; Pankratz, V.S.; Kullo, I.J.; Tangalos, E.G.; Ivnik, R.J.; Petersen, R.C. Association of C-reactive protein with mild cognitive impairment. Alzheimer’s Dement. 2009, 5, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Drew, L. Turning back time with epigenetic clocks. Nature 2022, 601, S20–S22. [Google Scholar] [CrossRef]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological age predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Fuellen, G.; Jansen, L.; Cohen, A.A.; Luyten, W.; Gogol, M.; Simm, A.; Saul, N.; Cirulli, F.; Berry, A.; Antal, P.; et al. Health and aging: Unifying concepts, scores, biomarkers and pathways. Aging Dis. 2019, 10, 883. [Google Scholar] [CrossRef]
- Yao, L.; Fang, H.; Leng, W.; Li, J.; Chang, J. Effect of aerobic exercise on mental health in older adults: A meta-analysis of randomized controlled trials. Front. Psychiatry 2021, 12, 748257. [Google Scholar] [CrossRef]
- Tully, M.A.; McMullan, I.; Blackburn, N.E.; Wilson, J.J.; Bunting, B.; Smith, L.; Kee, F.; Deidda, M.; Giné-Garriga, M.; Coll-Planas, L.; et al. Sedentary behavior, physical activity, and mental health in older adults: An isotemporal substitution model. Scand. J. Med. Sci. Sports 2020, 30, 1957–1965. [Google Scholar] [CrossRef]
- Juan, S.M.; Adlard, P.A. Ageing and cognition. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Springer: Singapore, 2019; pp. 107–122. [Google Scholar]
- Oschwald, J.; Guye, S.; Liem, F.; Rast, P.; Willis, S.; Röcke, C.; Jäncke, L.; Martin, M.; Mérillat, S. Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Rev. Neurosci. 2019, 31, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, P.; Kaeberlein, M.; Hansen, M. Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res. Rev. 2017, 39, 3–14. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Ren, R.; Ocampo, A.; Liu, G.-H.; Belmonte, J.C.I. Regulation of stem cell aging by metabolism and epigenetics. Cell Metab. 2017, 26, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bales, C.W.; Ritchie, C.S. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu. Rev. Nutr. 2002, 22, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative stress markers in inflammatory bowel diseases: Systematic review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of nutrients in human milk. Adv. Nutr. 2018, 9 (Suppl. S1), 278S–294S. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Chan, C.X.; Yang, Y.O.; Cheng, G.H.M.; Gera, S.K.; Mohammad, A.B.Z.; Oh, S.J.; Lee, J.K.; Shin, O.S. Aging and the immune system: The impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019, 11, e37. [Google Scholar]
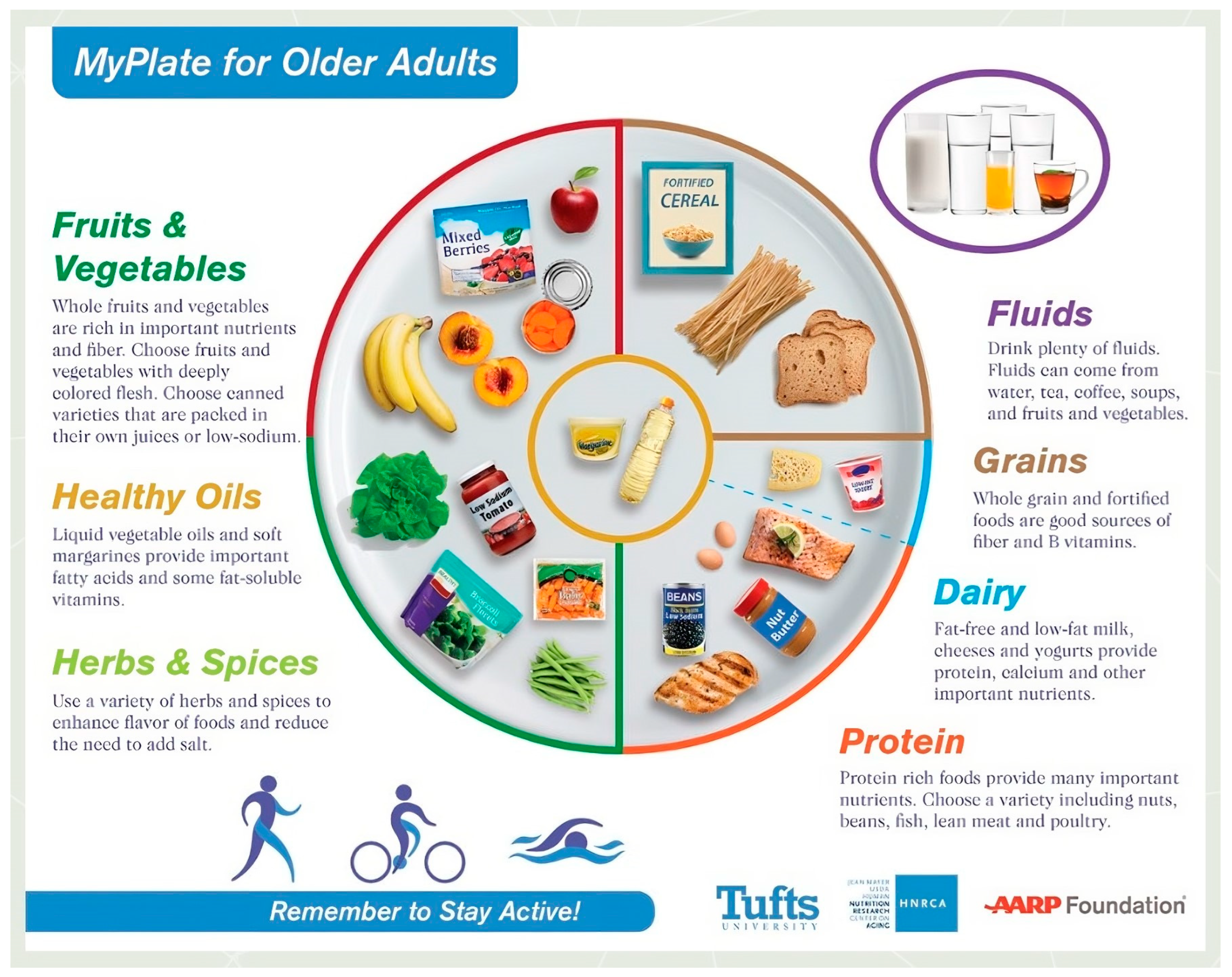
- Bobroff, L.B.; Gillen, M. Elder Nutrition: FCS2213/FY628, 3/2018. EDIS 2018, no. 2 (2018). Available online: https://edis.ifas.ufl.edu/publication/FY628 (accessed on 24 October 2023).
- MyPlate for Older Adults Adjusts Eating Guidelines. Lisa Esposito, 25 May 2016. Available online: https://health.usnews.com/wellness/articles/2016-05-25/myplate-for-older-adults-adjusts-eating-guidelines (accessed on 24 October 2023).
- Awad, A.; Trenfield, S.J.; Pollard, T.D.; Ong, J.J.; Elbadawi, M.; McCoubrey, L.E.; Goyanes, A.; Gaisford, S.; Basit, A.W. Connected healthcare: Improving patient care using digital health technologies. Adv. Drug Deliv. Rev. 2021, 178, 113958. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, A.I.; Kitsios, F.; Talias, M.A. Digital Transformation in Healthcare: Technology Acceptance and Its Applications. Int. J. Environ. Res. Public Health 2023, 20, 3407. [Google Scholar] [CrossRef]
- Rahaman, A.; Islam; Sadi, M.; Nooruddin, S. Developing IoT Based Smart Health Monitoring Systems: A Review. Rev. D’intelligence Artif. 2019, 33, 435–440. [Google Scholar] [CrossRef]
- Stepnov, I. Advantages and Challenges of Digital Technology. In Technology and Business Strategy: Digital Uncertainty and Digital Solutions; Palgrave Macmillan: Cham, Switzerland, 2021; pp. 295–308. [Google Scholar]
- Bashshur, R.L.; Shannon, G.W.; Smith, B.R.; Alverson, D.C.; Antoniotti, N.; Barsan, W.G.; Bashshur, N.; Brown, E.M.; Coye, M.J.; Doarn, C.R.; et al. The empirical foundations of telemedicine interventions for chronic disease management. Telemed. e-Health 2014, 20, 769–800. [Google Scholar] [CrossRef]
- Bashshur, R.; Doarn, C.R.; Frenk, J.M.; Kvedar, J.C.; Woolliscroft, J.O. Telemedicine and the COVID-19 pandemic, lessons for the future. Telemed. e-Health 2020, 26, 571–573. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef] [PubMed]
- Armand, T.P.T.; Mozumder, M.A.I.; Ali, S.; Amaechi, A.O.; Kim, H.C. Developing a Low-Cost IoT-Based Remote Cardiovascular Patient Monitoring System in Cameroon. Healthcare 2023, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.D.; He, W.; Li, S. Internet of things in industries: A survey. IEEE Trans. Ind. Inform. 2014, 10, 2233–2243. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Emanuel, E.J. Predicting the future—Big data, machine learning, and clinical medicine. N. Engl. J. Med. 2016, 375, 1216. [Google Scholar] [CrossRef]
- Shaik, T.; Tao, X.; Higgins, N.; Li, L.; Gururajan, R.; Zhou, X.; Acharya, U.R. Remote patient monitoring using artificial intelligence: Current state, applications, and challenges. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2023, 13, e1485. [Google Scholar] [CrossRef]
- Aceto, G.; Persico, V.; Pescapé, A. The role of Information and Communication Technologies in healthcare: Taxonomies, perspectives, and challenges. J. Netw. Comput. Appl. 2018, 107, 125–154. [Google Scholar] [CrossRef]
- Holmgren, A.J.; Esdar, M.; Hüsers, J.; Coutinho-Almeida, J. Health Information Exchange: Understanding the Policy Landscape and Future of Data Interoperability. Yearb. Med. Inform. 2023, 32, 184–194. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition strategies promoting healthy aging: From improvement of cardiovascular and brain health to prevention of age-associated diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef]
- Amoutzopoulos, B.; Steer, T.; Roberts, C.; Cade, J.E.; Boushey, C.J.; Collins, C.E.; Trolle, E.; de Boer, E.J.; Ziauddeen, N.; van Rossum, C.; et al. Traditional methods v. new technologies–dilemmas for dietary assessment in large-scale nutrition surveys and studies: A report following an international panel discussion at the 9th International Conference on Diet and Activity Methods (ICDAM9), Brisbane, 3 September 2015. J. Nutr. Sci. 2018, 7, e11. [Google Scholar]
- Sau-wa Mak, V. Technologies and dietary change: The pharmaceutical nexus and the marketing of anti-aging functional food in a Chinese society. Food Foodways 2021, 29, 309–330. [Google Scholar] [CrossRef]
- i Alizadehsaravi, Niousha. BARRIERS AND FACILITATORS TO RECEIVING ADEQUATE NUTRITION IN LONG-TERM CARE RESIDENTS LIVING WITH MODERATE TO SEVERE DEMENTIA. Available online: http://hdl.handle.net/10222/82825 (accessed on 19 December 2023).
- Mortazavi, B.J.; Gutierrez-Osuna, R. A review of digital innovations for diet monitoring and precision nutrition. J. Diabetes Sci. Technol. 2023, 17, 217–223. [Google Scholar] [CrossRef]
- Samoggia, A.; Riedel, B. Assessment of nutrition-focused mobile apps’ influence on consumers’ healthy food behaviour and nutrition knowledge. Food Res. Int. 2020, 128, 108766. [Google Scholar] [CrossRef] [PubMed]
- Lieffers, J.R.L.; Hanning, R.M. Dietary assessment and self-monitoring: With nutrition applications for mobile devices. Can. J. Diet. Pract. Res. 2012, 73, e253–e260. [Google Scholar] [CrossRef] [PubMed]
- Suganyadevi, S.; Shamia, D.; Balasamy, K. An IoT-based diet monitoring healthcare system for women. In Smart Healthcare System Design: Security and Privacy Aspects; Wiley Online Library: Online, United States; 2022; pp. 167–202. [Google Scholar]
- Sun, H.; Zhang, Z.; Hu, R.Q.; Qian, Y. Wearable communications in 5G: Challenges and enabling technologies. IEEE Veh. Technol. Mag. 2018, 13, 100–109. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zeng, X.; Cao, J.; Jiang, W. Application of blockchain technology in food safety control: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 2800–2819. [Google Scholar] [CrossRef]
- Wilkinson, S.A.; Fjeldsoe, B.; Willcox, J.C. Evaluation of the Pragmatic Implementation of a Digital Health Intervention Promoting Healthy Nutrition, Physical Activity, and Gestational Weight Gain for Women Entering Pregnancy at a High Body Mass Index. Nutrients 2023, 15, 588. [Google Scholar] [CrossRef] [PubMed]
- Health and Wellness Apps in Asia. January 2021. Available online: https://www.asia-research.net/health-and-wellness-apps-in-asia/ (accessed on 8 December 2023).
- Asia-Pacific Diet and Nutrition Apps Market—Industry Trends and Forecast to 2028. Available online: https://www.databridgemarketresearch.com/reports/asia-pacific-diet-and-nutrition-apps-market (accessed on 8 December 2023).
- Divya, R.; Vithiya Lakshmi, S.; JayaLakshmi, S.L. Diet Monitoring and Health Analysis Using Artificial Intelligence. Int. J. Adv. Netw. Appl. 2019, 202–204. Available online: https://www.proquest.com/openview/fcf40edba2c0235b536341a8a9755a6b/1?pq-origsite=gscholar&cbl=886380 (accessed on 19 December 2023).
- Wang, J.; Sereika, S.M.; Chasens, E.R.; Ewing, L.J.; Matthews, J.T.; Burke, L.E. Effect of adherence to self-monitoring of diet and physical activity on weight loss in a technology-supported behavioral intervention. Patient Prefer. Adherence 2012, 6, 221–226. [Google Scholar] [CrossRef]
- Helander, E.; Kaipainen, K.; Korhonen, I.; Wansink, B. Factors related to sustained use of a free mobile app for dietary self-monitoring with photography and peer feedback: Retrospective cohort study. J. Med. Internet Res. 2014, 16, e3084. [Google Scholar] [CrossRef]
- Kiernan, M.; Moore, S.D.; Schoffman, D.E.; Lee, K.; King, A.C.; Taylor, C.B.; Kiernan, N.E.; Perri, M.G. Social support for healthy behaviors: Scale psychometrics and prediction of weight loss among women in a behavioral program. Obesity 2012, 20, 756–764. [Google Scholar] [CrossRef]
- Computer Aided Nutritional Analysis Program (CAN-Pro). Available online: http://canpro6.kns.or.kr/ (accessed on 21 December 2023).
- Vu, T.; Lin, F.; Alshurafa, N.; Xu, W. Wearable food intake monitoring technologies: A comprehensive review. Computers 2017, 6, 4. [Google Scholar] [CrossRef]
- Farooq, M.; Sazonov, E. Automatic measurement of chew count and chewing rate during food intake. Electronics 2016, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Montiel, V.R.-V.; Vargas, E.; Teymourian, H.; Wang, J. Wearable and mobile sensors for personalized nutrition. ACS Sens. 2021, 6, 1745–1760. [Google Scholar] [CrossRef]
- Amft, O.; Troster, G. On-body sensing solutions for automatic dietary monitoring. IEEE Pervasive Comput. 2009, 8, 62–70. [Google Scholar] [CrossRef]
- Hassannejad, H.; Matrella, G.; Ciampolini, P.; De Munari, I.; Mordonini, M.; Cagnoni, S. Automatic diet monitoring: A review of computer vision and wearable sensor-based methods. Int. J. Food Sci. Nutr. 2017, 68, 656–670. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- de Moraes Lopes, M.H.B.; Ferreira, D.D.; Ferreira, A.C.B.H.; da Silva, G.R.; Caetano, A.S.; Braz, V.N. Use of artificial intelligence in precision nutrition and fitness. In Artificial Intelligence in Precision Health; Academic Press: Cambridge, MA, USA, 2020; pp. 465–496. [Google Scholar]
- Malik, D.; Narayanasamy, N.; Pratyusha, V.A.; Thakur, J.; Sinha, N. Understanding Nutrition. In Textbook of Nutritional Biochemistry; Springer Nature: Singapore, 2023; pp. 43–64. [Google Scholar]
- U.S. Department of Agriculture. Download FoodData Central Data. Available online: https://fdc.nal.usda.gov/download-datasets.html (accessed on 7 December 2023).
- Global Dietary Database, Gerald J. and Dorothy R. Friedman. Available online: https://www.globaldietarydatabase.org/ (accessed on 7 December 2023).
- European Food Information Resource. Available online: https://www.eurofir.org/ (accessed on 7 December 2023).
- Bossard, L.; Guillaumin, M.; Gool, L.V. Food-101—Mining discriminative components with random forests. In European Conference on Computer Vision; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Matsuda, Y.; Hoashi, H.; Yanai, K. Recognition of multiple-food images by detecting candidate regions. In Proceedings of the IEEE International Conference on Multimedia and Expo (ICME), Melbourne, VIC, Australia, 9–13 July 2012. [Google Scholar]
- Kawano, Y.; Yanai, K. Automatic expansion of a food image dataset leveraging existing categories with domain adaptation. In Proceedings of the ECCV Workshop on Transferring and Adapting Source Knowledge in Computer Vision (TASK-CV), Zurich, Switzerland, 6–7 September 2014. [Google Scholar]
- Chen, M.; Dhingra, K.; Wu, W.; Yang, L.; Sukthankar, R.; Yang, J. PFID: Pittsburgh fast-food image dataset. In Proceedings of the 2009 16th IEEE International Conference on Image Processing (ICIP), Cairo, Egypt, 7–10 November 2009. [Google Scholar]
- Okamoto, K.; Yanai, K. UEC-FoodPIX Complete: A large-scale food image segmentation dataset. In Pattern Recognition. ICPR International Workshops and Challenges: Virtual Event, 10–15 January 2021, Proceedings, Part V; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Rippin, H.L.; Hutchinson, J.; Jewell, J.; Breda, J.J.; Cade, J.E. Adult nutrient intakes from current national dietary surveys of European populations. Nutrients 2017, 9, 1288. [Google Scholar] [CrossRef]
- National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Label Database [Database]. 2022. Available online: https://www.dsld.nlm.nih.gov/dsld/index.jsp (accessed on 7 December 2023).
- Korea National Health and Nutrition Examination Survey. Available online: https://knhanes.kdca.go.kr/knhanes/main.do (accessed on 15 December 2023).
- Toledo, R.Y.; Alzahrani, A.A.; Martinez, L. A food recommender system considering nutritional information and user preferences. IEEE Access 2019, 7, 96695–96711. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; Liu, F.; Qiu, Z.; He, Y. Application of deep learning in food: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1793–1811. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.M.; Khanna, R. Application of artificial intelligence in smart kitchen. Int. J. Innov. Technol. Interdiscip. Sci. 2018, 1, 1–8. [Google Scholar]
- Kocaballi, A.B.; Sezgin, E.; Clark, L.; Carroll, J.M.; Huang, Y.; Huh-Yoo, J.; Kim, J.; Kocielnik, R.; Lee, Y.-C.; Mamykina; et al. Design and evaluation challenges of conversational agents in health care and well-being: Selective review study. J. Med. Internet Res. 2022, 24, e38525. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Valdés-Mas, R.; Elinav, E. The Role of Artificial Intelligence in Deciphering Diet–Disease Relationships: Case Studies. Annu. Rev. Nutr. 2023, 43, 225–250. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Mamoshina, P.; Vanhaelen, Q.; Scheibye-Knudsen, M.; Moskalev, A.; Aliper, A. Artificial intelligence for aging and longevity research: Recent advances and perspectives. Ageing Res. Rev. 2019, 49, 49–66. [Google Scholar] [CrossRef]
- Anselma, L.; Mazzei, A.; De Michieli, F. An artificial intelligence framework for compensating transgressions and its application to diet management. J. Biomed. Inform. 2017, 68, 58–70. [Google Scholar] [CrossRef]
- Begum, N.; Goyal, A.; Sharma, S. Artificial Intelligence-Based Food Calories Estimation Methods in Diet Assessment Research. In Artificial Intelligence Applications in Agriculture and Food Quality Improvement; IGI Global: Hershey, PA, USA, 2022; pp. 276–290. [Google Scholar]
- Dutta, K.; Rajput, A.; Srivastava, S.; Chidambaram, A.; Srivastava, A. Design of AI-Enabled Application to Detect Ayurvedic Nutritional Values of Edible Items and Suggest a Diet. In International Conference on Human-Computer Interaction; Springer International Publishing: Cham, Switzerland, 2022; pp. 245–256. [Google Scholar]
- Aslan, S.; Ciocca, G.; Schettini, R. Semantic food segmentation for automatic dietary monitoring. In Proceedings of the 2018 IEEE 8th International Conference on Consumer Electronics-Berlin (ICCE-Berlin), Berlin, Germany, 2–5 September 2018; pp. 1–6. [Google Scholar]
- Wang, W.; Min, W.; Li, T.; Dong, X.; Li, H.; Jiang, S. A review on vision-based analysis for automatic dietary assessment. Trends Food Sci. Technol. 2022, 122, 223–237. [Google Scholar] [CrossRef]
- Zhang, J.; Oh, Y.J.; Lange, P.; Yu, Z.; Fukuoka, Y. Artificial intelligence chatbot behavior change model for designing artificial intelligence chatbots to promote physical activity and a healthy diet. J. Med. Internet Res. 2020, 22, e22845. [Google Scholar] [CrossRef]
- Hezarjaribi, N.; Mazrouee, S.; Hemati, S.; Chaytor, N.S.; Perrigue, M.; Ghasemzadeh, H. Human-in-the-loop learning for personalized diet monitoring from unstructured mobile data. ACM Trans. Interact. Intell. Syst. (TiiS) 2019, 9, 1–24. [Google Scholar] [CrossRef]
- George, M.P. An Innovative Cloud Based Digital Health Application integrated with Artificial Intelligence Modules for User Oriented Medical Nutrition Therapy. 2022. Available online: http://hdl.handle.net/20.500.14146/10622 (accessed on 18 December 2023).
- Celis-Morales, C.A.; Livingstone, K.M.; Marsaux, C.F.M.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of personalized nutrition on health-related behaviour change: Evidence from the Food4Me European randomized controlled trial. Int. J. Epidemiol. 2017, 46, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Laroiya, C.; Saxena, D.; Komalavalli, C. Applications of blockchain technology. In Handbook of Research on Blockchain Technology; Academic Press: Cambridge, MA, USA, 2020; pp. 213–243. [Google Scholar]
- Onik, M.M.H.; Aich, S.; Yang, J.; Kim, C.S.; Kim, H.C. Blockchain in healthcare: Challenges and solutions. In Big Data Analytics for Intelligent Healthcare Management; Academic Press: Cambridge, MA, USA, 2019; pp. 197–226. [Google Scholar]
- Chakraborty, S.; Aich, S.; Kim, H.C. A secure healthcare system design framework using blockchain technology. In Proceedings of the 2019 21st International Conference on Advanced Communication Technology (ICACT), Pyeongchang, Republic of Korea, 17–20 February 2019; pp. 260–264. [Google Scholar]
- Lei, M.; Xu, L.; Liu, T.; Liu, S.; Sun, C. Integration of privacy protection and blockchain-based food safety traceability: Potential and challenges. Foods 2022, 11, 2262. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, L.; Zheng, Z.; Liu, S.; Li, X.; Cao, L.; Li, J.; Sun, C. Smart contract-based agricultural food supply chain traceability. IEEE Access 2021, 9, 9296–9307. [Google Scholar] [CrossRef]
- Kamilaris, A.; Fonts, A.; Prenafeta-Boldύ, F.X. The rise of blockchain technology in agriculture and food supply chains. Trends Food Sci. Technol. 2019, 91, 640–652. [Google Scholar] [CrossRef]
- Blockchain in Food Supply Chain: A Transparent Revolution. Available online: https://blog.emb.global/blockchain-in-food-supply-chain/ (accessed on 18 February 2024).
- Ellahi, R.M.; Wood, L.C.; Bekhit, A.E.-D.A. Blockchain-based frameworks for food traceability: A systematic review. Foods 2023, 12, 3026. [Google Scholar] [CrossRef] [PubMed]
- IBM Food Trust. Available online: https://www.ibm.com/products/supply-chain-intelligence-suite/food-trust (accessed on 18 February 2024).
- Switzerland’s Largest Supermarket Chain follows the Pack with Blockchain Food Tracking. Available online: https://www.forbes.com/sites/darrynpollock/2019/08/30/switzerlands-largest-supermarket-chain-follows-the-pack-with-blockchain-food-tracking/?sh=205b977b5d2c (accessed on 18 February 2024).
- Ambrosus Looks at Blockchain-Based Platform to Improve Supply Chain. Available online: https://www.foodnavigator.com/Article/2017/07/28/Ambrosus-drives-change-in-food-supply-chain-ecosystem?utm_source=copyright&utm_medium=OnSite&utm_campaign=copyright (accessed on 18 February 2024).
- Gkouskou, K.; Vlastos, I.; Karkalousos, P.; Chaniotis, D.; Sanoudou, D.; Eliopoulos, A.G. The “virtual digital twins” concept in precision nutrition. Adv. Nutr. 2020, 11, 1405–1413. [Google Scholar] [CrossRef]
- Bedoya, M.G.; Montoya, D.R.; Tabilo-Munizaga, G.; Pérez-Won, M.; Lemus-Mondaca, R. Promising perspectives on novel protein food sources combining artificial intelligence and 3D food printing for food industry. Trends Food Sci. Technol. 2022, 128, 38–52. [Google Scholar] [CrossRef]
- Wu, M.Y.-C.; Hsu, M.-Y.; Chen, S.-J.; Hwang, D.-K.; Yen, T.-H.; Cheng, C.-M. Point-of-care detection devices for food safety monitoring: Proactive disease prevention. Trends Biotechnol. 2017, 35, 288–300. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging point-of-care technologies for food safety analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef]
- Macovei, D.-G.; Irimes, M.-B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2023, 415, 1033–1063. [Google Scholar] [CrossRef]
- Srinivasan, B.; Lee, S.; Erickson, D.; Mehta, S. Precision nutrition—Review of methods for point-of-care assessment of nutritional status. Curr. Opin. Biotechnol. 2017, 44, 103–108. [Google Scholar] [CrossRef]
- Shrestha, B.; Tang, L.; Hood, R.L. Nanotechnology for Personalized Medicine. In Nanomedicine; Springer Nature: Singapore, 2023; pp. 555–603. [Google Scholar]
- Khalil, Y.; Mahmoud, A.E.D. Nanomaterial-based Sensors for Wearable Health Monitoring in Bioelectronics Nano Engineering. J. Contemp. Healthc. Anal. 2023, 7, 126–144. [Google Scholar]
- Safaee, M.M.; Gravely, M.; Roxbury, D. A wearable optical microfibrous biomaterial with encapsulated nanosensors enables wireless monitoring of oxidative stress. Adv. Funct. Mater. 2021, 31, 2006254. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A. Advanced nanoscale build-up sensors for daily life monitoring of diabetics. Adv. Mater. Interfaces 2020, 7, 2000153. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Nanotechnology approaches for increasing nutrient bioavailability. Adv. Food Nutr. Res. 2017, 81, 1–30. [Google Scholar] [PubMed]
- Zečević, M.; Mijatović, D.; Koklič, M.K.; Žabkar, V.; Gidaković, P. User perspectives of diet-tracking apps: Reviews content analysis and topic modeling. J. Med. Internet Res. 2021, 23, e25160. [Google Scholar] [CrossRef]
- Li, L. Measuring and Supporting Adherence to Dietary Reporting and Dietary Intervention Using a Smartphone Application in the PREDITION Trial (PRotEin DIet SatisfacTION). Ph.D. Thesis, ResearchSpace@, Auckland, New Zealand, 2022. [Google Scholar]
- Chen, J.; Lieffers, J.; Bauman, A.; Hanning, R.; Allman-Farinelli, M. Designing health apps to support dietetic professional practice and their patients: Qualitative results from an international survey. JMIR mHealth uHealth 2017, 5, e6945. [Google Scholar] [CrossRef]
- Ueland, Ø.; Altintzoglou, T.; Kirkhus, B.; Lindberg, D.; Rognså, G.H.; Rosnes, J.T.; Rud, I.; Varela, P. Perspectives on personalised food. Trends Food Sci. Technol. 2020, 102, 169–177. [Google Scholar] [CrossRef]
- Gibney, M.J.; Walsh, M.C. The future direction of personalised nutrition: My diet, my phenotype, my genes. Proc. Nutr. Soc. 2013, 72, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hedin, B.; Katzeff, C.; Eriksson, E.; Pargman, D. A systematic review of digital behaviour change interventions for more sustainable food consumption. Sustainability 2019, 11, 2638. [Google Scholar] [CrossRef]
- Chen, Y.; Perez-Cueto, F.J.A.; Giboreau, A.; Mavridis, I.; Hartwell, H. The promotion of eating behaviour change through digital interventions. Int. J. Environ. Res. Public Health 2020, 17, 7488. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Whitehead, M.; Sheats, J.Q.; Mastromonico, J.; Hardy, D.; Smith, S.A. Smartphone applications for promoting healthy diet and nutrition: A literature review. Jacobs J. Food Nutr. 2015, 2, 021. [Google Scholar]
- Xu, F.; Cohen, S.A.; Lofgren, I.E.; Greene, G.W.; Delmonico, M.J.; Greaney, M.L. Relationship between diet quality, physical activity and health-related quality of life in older adults: Findings from 2007–2014 national health and nutrition examination survey. J. Nutr. Health Aging 2018, 22, 1072–1079. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Grenkowitz, S.; Schlickeiser, S.; Sandforth, A.; Friedrich, M.; Kunkel, D.; Glauben, R.; Brachs, S.; Mai, K.; Thürmer, A.; et al. Effects of caloric restriction on the gut microbiome are linked with immune senescence. Microbiome 2022, 10, 57. [Google Scholar] [CrossRef]
- Food for Healthy Life. Available online: https://priorityapp.shinyapps.io/Food/ (accessed on 26 December 2023).
- Fadnes, L.T.; Økland, J.-M.; Haaland, A.; Johansson, K.A. Correction: Estimating impact of food choices on life expectancy: A modeling study. PLoS Med. 2022, 19, e1003962. [Google Scholar] [CrossRef]
- Arora, S.; Yttri, J.; Nilsen, W. Privacy and security in mobile health (mHealth) research. Alcohol Res. Curr. Rev. 2014, 36, 143. [Google Scholar]
- Armand, T.P.T.; Mozumder, M.A.I.; Carole, K.S.; Joo, M.I.; Kim, H.C. Enhancing Patient’s Confidence and Trust in Remote Monitoring Systems Using Natural Language Processing in the Medical Metaverse. In Proceedings of the 2023 International Conference on Intelligent Metaverse Technologies & Applications (iMETA), Tartu, Estonia, 18–20 September 2023; pp. 1–6. [Google Scholar]
- Aich, S.; Sinai, N.K.; Kumar, S.; Ali, M.; Choi, Y.R.; Joo, M.I.; Kim, H.C. Protecting personal healthcare record using blockchain & federated learning technologies. In Proceedings of the 2022 24th International Conference on Advanced Communication Technology (ICACT), Pyeongchang, Republic of Korea, 13–16 February 2022; pp. 109–112. [Google Scholar]
- Kim, J.; Lee, H.Y.; Christensen, M.C.; Merighi, J.R. Technology access and use, and their associations with social engagement among older adults: Do women and men differ? J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2017, 72, 836–845. [Google Scholar] [CrossRef]
- Nabot, A.; Omar, F.; Almousa, M. Perceptions of Smartphone Users Acceptance and Adoption of Mobile Commerce (MC) The Case of Jordan. arXiv 2021, arXiv:2101.01401. [Google Scholar] [CrossRef]
- Fuller-Tyszkiewicz, M.; Richardson, B.; Klein, B.; Skouteris, H.; Christensen, H.; Austin, D.; Castle, D.; Mihalopoulos, C.; O’Donnell, R.; Arulkadacham, L.; et al. A mobile app–based intervention for depression: End-user and expert usability testing study. JMIR Ment. Health 2018, 5, e9445. [Google Scholar] [CrossRef]
- Sen, K.; Prybutok, G.; Prybutok, V. The use of digital technology for social wellbeing reduces social isolation in older adults: A systematic review. SSM-Popul. Health 2022, 17, 101020. [Google Scholar] [CrossRef]
| No. | App Name | Description | Country | Year | Cost |
|---|---|---|---|---|---|
| 1 | MyFitnessPal | This app tracks food and calorie intake with a large database, barcode scanner, recipe importer, restaurant logger, and food insights. | United States | 2005 | Basic: Free Premium: (USD 9.99/month) |
| 2 | Lose It | Lose It simplifies your weight loss process with tools like a recipe library, nutrition log, and calorie counter. The app collects information about your height, weight, age, and specific goals to create a personalized plan tailored to your needs. | United States | 2008 | Basic: Free Premium: (USD 4.17/month) |
| 3 | Noom | Noom offers customized meal plans, weekly challenges, and a team of virtual coaches. It provides educational information, tools for tracking your progress, and training plans to incorporate more activities into your daily life. | United States | 2008 | Basic: Free Premium: (USD 60/month) |
| 4 | HealthifyMe | The app provides a comprehensive list of recipes and meal plans. Lifesum offers a barcode scanner and macro tracking to view your daily meals and calories. | India | 2012 | Basic: Free Premium: (USD 12/month) |
| 5 | YAZIO Fasting & Food Tracker | A popular and effective tool for nutrition tracking that allows users to monitor their food intake, track nutrients, and manage their fitness goals. | Germany | 2013 | Basic: Free Premium: (USD 100/year) |
| 6 | FatSecret | It offers a food diary, a large food database, a training diary, a weight chart and diary, and tons of healthy recipes to support your efforts. The app also features food image recognition, making logging and tracking calories easier than ever, and a unique meal calendar that visually shows when you are eating and burning the most calories. | Australia | 2007 | Basic: Free Premium: (USD 9.49/month) |
| 7 | Fastic | The app is designed to help users with intermittent fasting and offers features like tracking fasting times, providing meal plans, and providing educational content about intermittent fasting and healthy living. | Germany | 2019 | Basic: Free Premium: (USD 11.99/month) |
| 8 | MyNetDiary | MyNetDiary is a mobile app and online platform designed to help individuals track their diet, manage their weight, and achieve their health and fitness goals. The app incorporates an easy-to-use calorie counter that facilitates digital diet assistance for weight loss. | United States | 2007 | Basic: Free Premium: (USD 18.95/month) |
| No. | Data Source | Description | Data Type | Ref. |
|---|---|---|---|---|
| 1 | USDA National Nutrient Database | Provides information on food and nutrients in various subsets: Food and Nutrient Database for Dietary Studies (FNDDS), National Nutrient Database for Standard Reference Legacy (SR Legacy), USDA Global Branded Food Products Database (Branded Foods), and Experimental Foods. | Anthropometric, demographic, biochemical, clinical, and dietary | [101] |
| 2 | Global Dietary Database (GDD) | Contains vast amount of original survey data and metadata, with vital information on dietary patterns. The metadata allow the user to select their data interest by choosing the desired diet methodology, country of interest, year range, sex, age, dietary variables, etc. | Demographic, clinical, and dietary | [102] |
| 3 | European Food Information Resource (EuroFIR) | EuroFIR contains food composition and nutritional information across Europe. It includes portion size and serving information, quality control validation, and metadata documentation. | Demographic, clinical, and dietary | [103] |
| 4 | Food Images Dataset | Food-5k contains 2500 × 2 food and non-food images for image classification. Food-11 has 16,643 food images grouped into 11 major food categories. Other datasets include Food-101, Caltech-256, RagusaDS, UEC-FOOD100, UEC-FOOD256, UEC-FoodPIX, and the Pittsburgh fast food image dataset | Images, annotations, mask images, and dietary data | [104,105,106,107,108] |
| 5 | National dietary surveys | NHANES in the United States or the National Diet and Nutrition Survey in the United Kingdom contains data on various health and nutrition indicators, including dietary intake, physical activity, and biomarkers. | Demographic, clinical, and dietary | [109] |
| 6 | National Institutes of Health (NIH) | NIH provides a dietary supplement label database of products marketed in the USA, including ingredients’ dosage information | Demographic, clinical, and dietary | [110] |
| 7 | KNHANES | Korea National Health and Nutrition Examination Survey contains health-related information such as demographics, health behaviors, healthcare utilization, physical measurements, biochemical and laboratory tests, dietary intake, and socioeconomic status. | Demographic, clinical, and dietary | [111] |
| 8 | CAN-Pro | The Computer-Aided Nutritional analysis Program is a nutrient database in Korea. It describes commonly consumed Korean food’s composition in a revised edition, with the latest nutritional composition per specific portion of food, which is useful for dietary assessment and menu planning. | Dietary, menu | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodore Armand, T.P.; Kim, H.-C.; Kim, J.-I. Digital Anti-Aging Healthcare: An Overview of the Applications of Digital Technologies in Diet Management. J. Pers. Med. 2024, 14, 254. https://doi.org/10.3390/jpm14030254
Theodore Armand TP, Kim H-C, Kim J-I. Digital Anti-Aging Healthcare: An Overview of the Applications of Digital Technologies in Diet Management. Journal of Personalized Medicine. 2024; 14(3):254. https://doi.org/10.3390/jpm14030254
Chicago/Turabian StyleTheodore Armand, Tagne Poupi, Hee-Cheol Kim, and Jung-In Kim. 2024. "Digital Anti-Aging Healthcare: An Overview of the Applications of Digital Technologies in Diet Management" Journal of Personalized Medicine 14, no. 3: 254. https://doi.org/10.3390/jpm14030254
APA StyleTheodore Armand, T. P., Kim, H.-C., & Kim, J.-I. (2024). Digital Anti-Aging Healthcare: An Overview of the Applications of Digital Technologies in Diet Management. Journal of Personalized Medicine, 14(3), 254. https://doi.org/10.3390/jpm14030254







