Genome Sequencing of Multiple Primary Lung Cancers Harbouring Mixed Histology and Spontaneously Regressing Small-Cell Lung Cancer
Abstract
:1. Introduction
2. Case Description
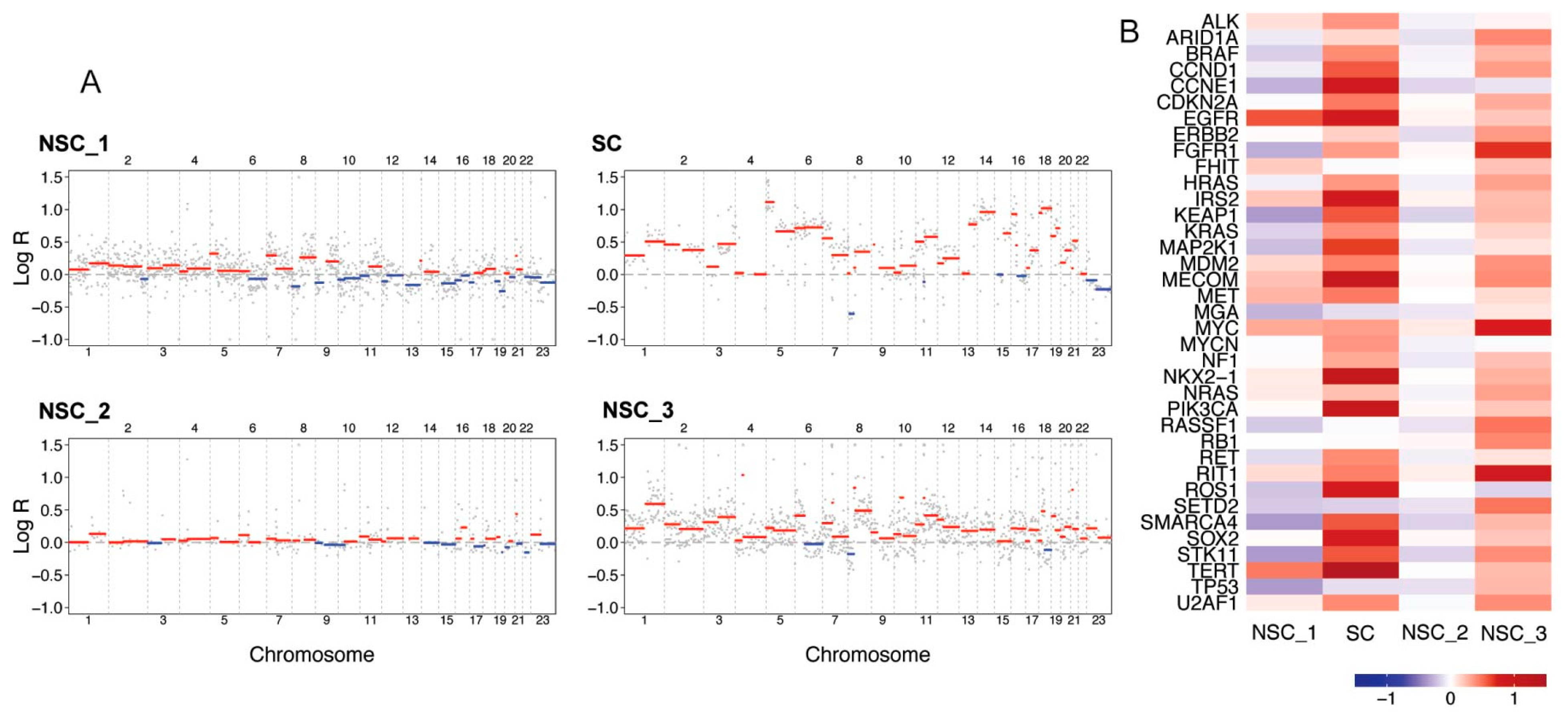
2.1. Mutational Load, Mutational Overlap and Driver Analysis of SNVs, InDels and SVs
2.2. Mutational Signatures
2.3. Copy Number Alterations and CNA Driver Analysis
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Zito Marino, F.; Bianco, R.; Accardo, M.; Ronchi, A.; Cozzolino, I.; Morgillo, F.; Rossi, G.; Franco, R. Molecular Heterogeneity in Lung Cancer: From Mechanisms of Origin to Clinical Implications. Int. J. Med. Sci. 2019, 16, 981–989. [Google Scholar] [CrossRef]
- van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, P.W., II. Lung Cancer Biomarkers. Hematol. Oncol. Clin. N. Am. 2017, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2018, 30, 44–56. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Beasley, M.B.; Chitale, D.A.; Dacic, S.; Giaccone, G.; Jenkins, R.B.; Kwiatkowski, D.J.; Saldivar, J.S.; Squire, J.; et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J. Thorac. Oncol. 2013, 8, 823–859. [Google Scholar] [PubMed]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef]
- Kwak, E.L.; Bang, Y.-J.; Camidge, D.R.; Shaw, A.T.; Solomon, B.; Maki, R.G.; Ou, S.-H.I.; Dezube, B.J.; Jänne, P.A.; Costa, D.B.; et al. Anaplastic Lymphoma Kinase Inhibition in Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Bergethon, K.; Shaw, A.T.; Ou, S.-H.I.; Katayama, R.; Lovly, C.M.; McDonald, N.T.; Massion, P.P.; Siwak-Tapp, C.; Gonzalez, A.; Fang, R.; et al. ROS1 Rearrangements Define a Unique Molecular Class of Lung Cancers. J. Clin. Oncol. 2012, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Li, L.; Yin, G.; Zhang, J.; Zheng, S.; Cheung, H.; Wu, N.; Lu, N.; Mao, X.; et al. Genomic heterogeneity of multiple synchronous lung cancer. Nat. Commun. 2016, 7, 13200. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, C.; Xie, G.; Wu, F.; Hu, C. Multiple Primary Lung Cancers: A New Challenge in the Era of Precision Medicine. Cancer Manag. Res. 2020, 12, 10361–10375. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Wang, X.; Liu, Y.; Sun, C.; Guo, Y.; Cai, Y.; Shao, G.; Yang, Z.; Qiu, S.; et al. Whole exome sequencing of lung adenocarcinoma and lung squamous cell carcinoma in one individual: A case report. Thorac. Cancer 2020, 11, 2361–2364. [Google Scholar] [CrossRef]
- Pei, G.; Li, M.; Min, X.; Liu, Q.; Li, D.; Yang, Y.; Wang, S.; Wang, X.; Wang, H.; Cheng, H.; et al. Molecular Identification and Genetic Characterization of Early-Stage Multiple Primary Lung Cancer by Large-Panel Next-Generation Sequencing Analysis. Front. Oncol. 2021, 11, 653988. [Google Scholar] [CrossRef]
- Song, Y.; Jia, Z.; Wu, P.; Wang, W.; Ou, Q.; Bao, H.; Yu, M.; Wu, X.; Liu, P.; Liang, N.; et al. Comprehensive genomic profiling aids in understanding the lesion origins of a patient with six synchronous invasive lung adenocarcinomas: A case study. BMC Pulm. Med. 2020, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.; Rathi, V.; Conron, M.; Wright, G.M. Genomic and Clinical Significance of Multiple Primary Lung Cancers as Determined by Next-Generation Sequencing. J. Thorac. Oncol. 2021, 16, 1166–1175. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Gazdar, A.F.; Minna, J.D. Molecular genetics of small cell lung carcinoma. Semin. Oncol. 2001, 28 (Suppl. S4), 3–13. [Google Scholar] [CrossRef]
- Menis, J.; Giaj Levra, M.; Novello, S. MET inhibition in lung cancer. Transl. Lung Cancer Res. 2013, 2, 23–39. [Google Scholar]
- De Mello, R.A.; Neves, N.M.; Amaral, G.A.; Lippo, E.G.; Castelo-Branco, P.; Pozza, D.H.; Tajima, C.C.; Antoniou, G. The Role of MET Inhibitor Therapies in the Treatment of Advanced Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 1918. [Google Scholar] [CrossRef]
- Shi, J.; Hua, X.; Zhu, B.; Ravichandran, S.; Wang, M.; Nguyen, C.; Brodie, S.A.; Palleschi, A.; Alloisio, M.; Pariscenti, G.; et al. Somatic Genomics and Clinical Features of Lung Adenocarcinoma: A Retrospective Study. PLoS Med. 2016, 13, e1002162. [Google Scholar] [CrossRef]
- Vignot, S.; Frampton, G.M.; Soria, J.C.; Yelensky, R.; Commo, F.; Brambilla, C.; Palmer, G.; Moro-Sibilot, D.; Ross, J.S.; Cronin, M.T.; et al. Next-Generation Sequencing Reveals High Concordance of Recurrent Somatic Alterations Between Primary Tumor and Metastases From Patients With Non–Small-Cell Lung Cancer. JCO 2013, 31, 2167–2172. [Google Scholar] [CrossRef]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar]
- Adelstein, D.J.; Tomashefski, J.F.; Snow, N.J.; Horrigan, T.P.; Hines, J.D. Mixed small cell and non-small cell lung cancer. Chest 1986, 89, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Li, C.; Qian, H. Chance to rein in a cancer--Spontaneous regression of lung carcinoma (1988–2018): A 30-year perspective. Int. J. Clin. Exp. Pathol. 2020, 13, 1190–1196. [Google Scholar] [PubMed]
- Everson, T.C.; Cole, W.H. Spontaneous Regression of Cancer: Preliminary Report. Ann. Surg. 1956, 144, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Patel, N.; Talwar, A. Spontaneous regression of thoracic malignancies. Respir. Med. 2010, 104, 1543–1550. [Google Scholar] [CrossRef]
- Cafferata, M.A.; Chiaramondia, M.; Monetti, F.; Ardizzoni, A. Complete spontaneous remission of non-small-cell lung cancer: A case report. Lung Cancer 2004, 45, 263–266. [Google Scholar] [CrossRef]
- Hong, G.; Chung, C.; Park, D.; Lee, S.I.; Lee, J.E.; Kang, D.H. Spontaneous regression of recurrent pulmonary large cell neuroendocrine carcinoma with alteration of PD-L1 expression after surgical resection: A case report. Thorac. Cancer 2024, 15, 266–270. [Google Scholar] [CrossRef]
- Goto, M.; Fukumoto, K.; Ichikawa, Y.; Tsubouchi, H.; Uchiyama, M.; Mori, S. Pathologically confirmed spontaneous regression of small cell lung cancer after computed tomography-guided percutaneous transthoracic needle biopsy followed by surgery. Surg. Case Rep. 2023, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Otani, M.; Nishimori, M.; Iwasa, H.; Iwamura, M.; Izumi, T.; Nakaji, K.; Nitta, N.; Miyatake, K.; Yoshimatsu, R.; Yamanishi, T.; et al. Spontaneous regression of small cell lung cancer associated with Lambert-Eaton Myasthenic Syndrome: Case report. Radiol. Case Rep. 2023, 18, 4036–4041. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Cotter, M.B.; Tosetto, M.; Khaw, Y.L.; Geraghty, R.; Winter, D.C.; Ryan, E.J.; Sheahan, K.; Furney, S.J. Personalised mapping of tumour development in synchronous colorectal cancer patients. NPJ Genom. Med. 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
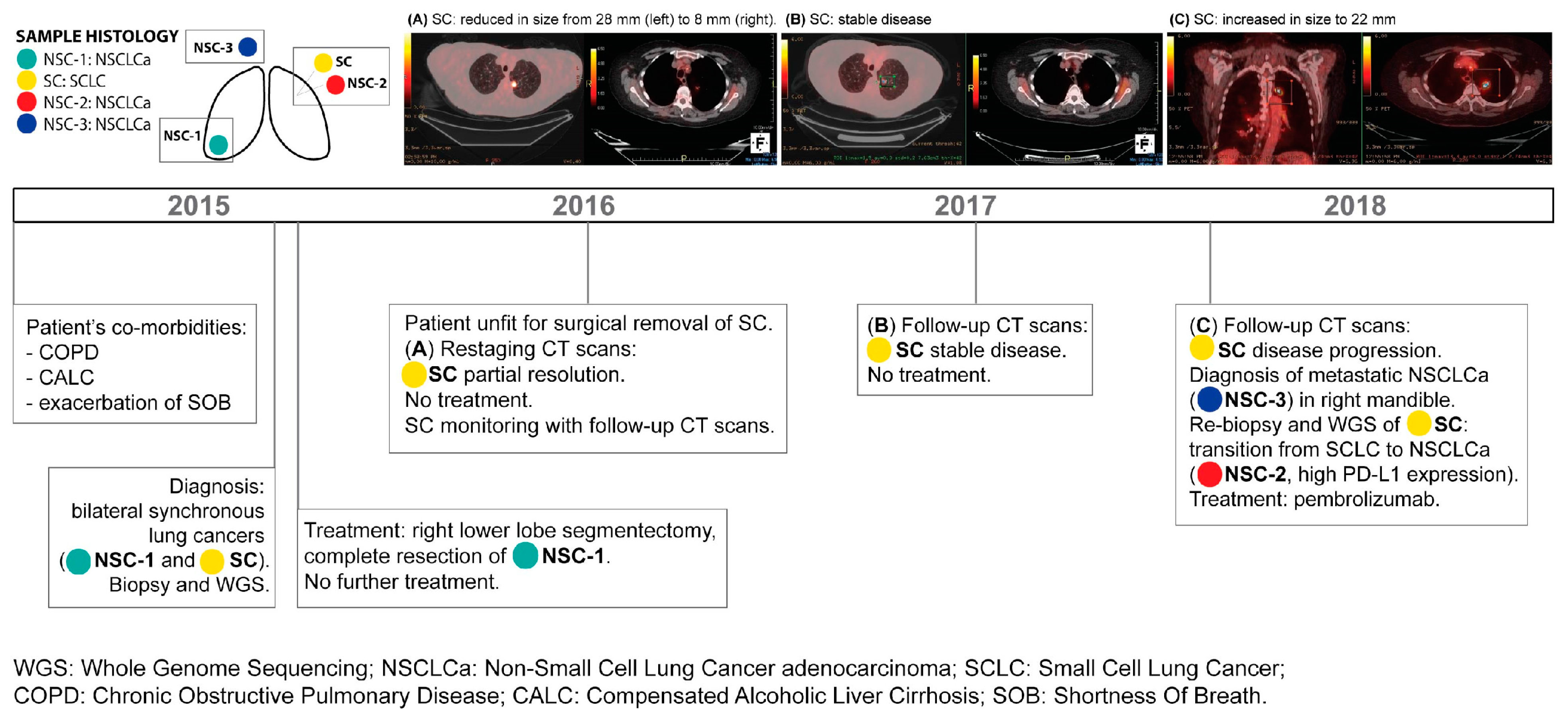

| Sample | Tumour Subtype | Tumour Location | Tumour Stage | Diagnosis and Biopsy | Sequencing Method | Treatment |
|---|---|---|---|---|---|---|
| NSC-1 | NSCLCa | Right lower lobe | pT1aN0 | 2015 | WGS | Surgical removal |
| SC | SCLC | Left upper lobe | cT1cN0 | 2015 | WGS | None |
| NSC-2 | NSCLCa | Left upper lobe | cT1cNx | 2018 | WGS | Pembrolizumab |
| NSC-3 | NSCLCa | Right mandible | cT1cNxM1b | 2018 | WGS | Pembrolizumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, V.; Rashed, A.; Faul, C.; Nicholson, S.; Young, V.; Hanson, J.; Hennessy, B.T.; Toomey, S.; Furney, S.J. Genome Sequencing of Multiple Primary Lung Cancers Harbouring Mixed Histology and Spontaneously Regressing Small-Cell Lung Cancer. J. Pers. Med. 2024, 14, 257. https://doi.org/10.3390/jpm14030257
Thomas V, Rashed A, Faul C, Nicholson S, Young V, Hanson J, Hennessy BT, Toomey S, Furney SJ. Genome Sequencing of Multiple Primary Lung Cancers Harbouring Mixed Histology and Spontaneously Regressing Small-Cell Lung Cancer. Journal of Personalized Medicine. 2024; 14(3):257. https://doi.org/10.3390/jpm14030257
Chicago/Turabian StyleThomas, Valentina, Ahmed Rashed, Clare Faul, Siobhan Nicholson, Vincent Young, John Hanson, Bryan T. Hennessy, Sinead Toomey, and Simon J. Furney. 2024. "Genome Sequencing of Multiple Primary Lung Cancers Harbouring Mixed Histology and Spontaneously Regressing Small-Cell Lung Cancer" Journal of Personalized Medicine 14, no. 3: 257. https://doi.org/10.3390/jpm14030257






