Personalized Approach in Transcatheter Palliation of Congenital Heart Disease with Duct-Dependent Pulmonary Circulation: Right Ventricular Outflow Tract Stenting vs. Arterial Duct Stenting
Abstract
:1. Introduction
1.1. Historical Perspective
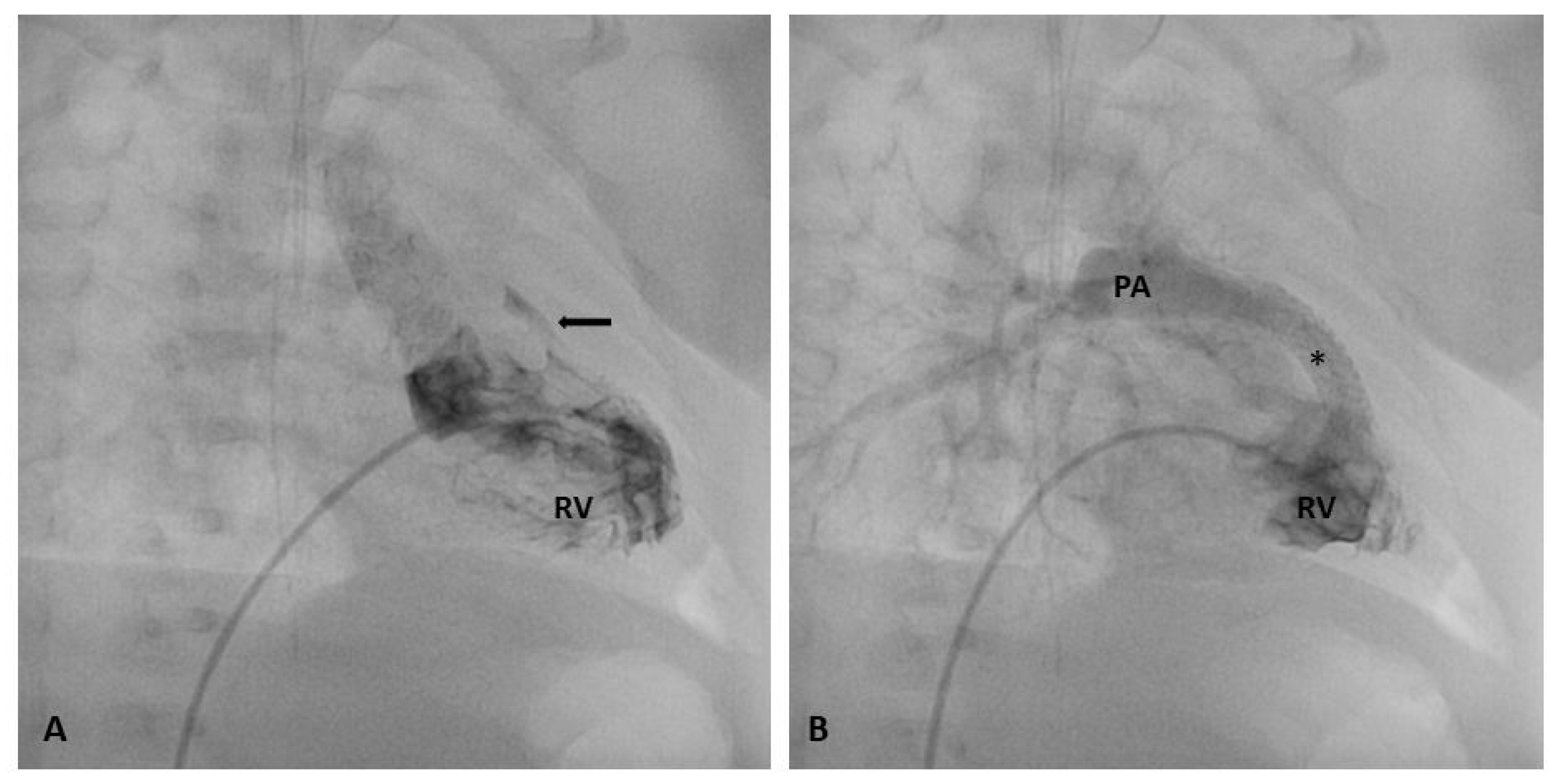
1.2. Indication and Contraindications for RVOT Stenting
- Patients with a high-risk profile for conventional surgical palliation: low-birth-weight neonate with or without any significant comorbidity or with unfavorable anatomic arrangement of pulmonary arteries, such as pulmonary artery discontinuity or hypoplasia.
- Patients at low risk for surgical palliation but with an anticipated need for short-term support for pulmonary circulation due to early surgical repair.
1.3. Indication and Contraindications for AD Stenting
- Patients with a high-risk profile for surgery, having an anatomically suitable ductus arteriosus and more than one source of pulmonary blood flow.
- Patients at low risk for surgical palliation but with an anticipated need for short-term support for pulmonary circulation due to early surgical repair or while waiting for the evolution of clinical scenarios.
1.4. Pathophysiologic Differences in Transcatheter Palliation Approaches
1.5. Procedural Technique: RVOT Stenting
1.6. Procedural Technique: AD Stenting
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Habib, H.F.; Jacobs, J.P.; Mavroudis, C.; Tchervenkov, C.I.; O’Brien, S.M.; Mohammadi, S.; Jacobs, M.L. Contemporary patterns of management of tetralogy of Fallot: Data from the society of thoracic surgeons database. Ann. Thorac. Surg. 2010, 90, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R.E.; Glatz, A.C.; Gaynor, J.W.; Nicolson, S.C.; Spray, T.L.; Wernovsky, G.; Bird, G.L. Results of elective repair at 6 months or younger in 277 patients with tetralogy of Fallot: A 14-year experience at a single center. J. Thorac. Cardiovasc. Surg. 2014, 147, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Kanter, K.R.; Kogon, B.E.; Kirshbom, P.M.; Carlock, P.R. Symptomatic Neonatal Tetralogy of Fallot: Repair or Shunt? Ann. Thorac. Surg. 2010, 89, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Fermanis, F.G.; Ekangaki, A.K.; Salmon, A.P.; Keeton, B.R.; Shore, D.F.; Lamb, R.K.; Monro, J.L. Twelve year experience with the modified Blalock-Taussig shunt in neonates. Eur. J. Cardio-Thorac. Surg. 1992, 6, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Dirks, V.; Prêtre, R.; Knirsch, W.; Valsangiacomo Buechel, E.R.; Seifert, B.; Schweiger, M.; Hübler, M.; Dave, H. Modified Blalock Taussig shunt: A not-so-simple palliative procedure. Eur. J. Cardio-Thorac. Surg. 2013, 44, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Ades, A.M.; Dominguez, T.E.; Nicolson, S.C.; Gaynor, J.W.; Spray, T.L.; Wernovsky, G.; Tabbutt, S. Morbidity and mortality after surgery for congenital cardiac disease in the infant born with low weight. Cardiol. Young 2010, 20, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Dohlen, G.; Chaturvedi, R.R.; Benson, L.N.; Ozawa, A.; Van Arsdell, G.S.; Fruitman, D.S.; Lee, K.J. Stenting of the right ventricular outflow tract in the symptomatic infant with tetralogy of Fallot. Heart 2009, 95, 142–147. [Google Scholar] [CrossRef]
- Gibbs, J.L.; Uzun, O.; Blackburn, M.E.C.; Parsons, J.M.; Dickinson, D.F. Right ventricular outflow stent implantation: An alternative to palliative surgical relief of infundibular pulmonary stenosis. Heart 1997, 77, 176–179. [Google Scholar] [CrossRef]
- Gewillig, M.; Boshoff, D.E.; Dens, J.; Mertens, L.; Benson, L.N. Stenting the Neonatal Arterial Duct in Duct-Dependent Pulmonary Circulation: New Techniques, Better Results. J. Am. Coll. Cardiol. 2004, 43, 107–112. [Google Scholar] [CrossRef]
- Ghaderian, M.; Ahmadi, A.; Sabri, M.R.; Behdad, S.; Dehghan, B.; Mahdavi, C.; Mansourian, M.; Shahsanaei, F. Clinical Outcome of Right Ventricular Outflow Tract Stenting Versus Blalock-Taussig Shunt in Tetralogy of Fallot: A systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2020, 46, 100643. [Google Scholar] [CrossRef]
- Qureshi, A.M.; Goldstein, B.H.; Glatz, A.C.; Agrawal, H.; Aggarwal, V.; Ligon, R.A.; McCracken, C.; McDonnell, A.; Buckey, T.M.; Whiteside, W.; et al. Classification scheme for ductal morphology in cyanotic patients with ductal dependent pulmonary blood flow and association with outcomes of patent ductus arteriosus stenting. Catheter. Cardiovasc. Interv. 2019, 93, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Quandt, D.; Ramchandani, B.; Stickley, J.; Mehta, C.; Bhole, V.; Barron, D.J.; Stumper, O. Stenting of the Right Ventricular Outflow Tract Promotes Better Pulmonary Arterial Growth Compared with Modified Blalock-Taussig Shunt Palliation in Tetralogy of Fallot–Type Lesions. JACC Cardiovasc. Interv. 2017, 10, 1774–1784. [Google Scholar] [CrossRef]
- Boucek, D.M.; Qureshi, A.M.; Goldstein, B.H.; Petit, C.J.; Glatz, A.C. Blalock-Taussig shunt versus patent ductus arteriosus stent as first palliation for ductal-dependent pulmonary circulation lesions: A review of the literature. Congenit. Heart Dis. 2019, 14, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; Gaio, G.; Castaldi, B.; Palladino, M.T.; Iacono, C.; Russo, M.G.; Calabrò, R. Arterial duct stenting in low-weight newborns with duct-dependent pulmonary circulation. Catheter. Cardiovasc. Interv. 2011, 78, 677–685. [Google Scholar] [CrossRef]
- Pizzuto, A.; Cuman, M.; Assanta, N.; Franchi, E.; Marrone, C.; Pak, V.; Santoro, G. Right Ventricular Outflow Tract Stenting as Palliation of Critical Tetralogy of Fallot: Techniques and Results. Hearts 2021, 2, 278–287. [Google Scholar] [CrossRef]
- Santoro, G.; Capozzi, G.; Capogrosso, C.; Mahmoud, H.T.; Gaio, G.; Palladino, M.T.; Russo, M.G. Pulmonary artery growth after arterial duct stenting in completely duct-dependent pulmonary circulation. Heart 2016, 102, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; Gaio, G.; Capozzi, G.; Giugno, L.; Palladino, M.T.; Capogrosso, C.; D’aiello, A.F.; Caianiello, G.; Russo, M.G. Fate of Hypoplastic Pulmonary Arteries after Arterial Duct Stenting in Congenital Heart Disease with Duct-Dependent Pulmonary Circulation. JACC Cardiovasc. Interv. 2015, 8, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Celebi, A.; Yucel, I.K.; Bulut, M.O.; Kucuk, M.; Balli, S. Stenting of the ductus arteriosus in infants with functionally univentricular heart disease and ductal-dependent pulmonary blood flow: A single-center experience. Catheter. Cardiovasc. Interv. 2017, 89, 699–708. [Google Scholar] [CrossRef]
- Alwi, M.; Choo, K.K.; Latiff, H.A.; Kandavello, G.; Samion, H.; Mulyadi, M.D. Initial results and medium-term follow-up of stent implantation of patent ductus arteriosus in duct-dependent pulmonary circulation. J. Am. Coll. Cardiol. 2004, 44, 438–445. [Google Scholar] [CrossRef]
- Santoro, G.; Gaio, G.; Palladino, M.T.; Iacono, C.; Carrozza, M.; Esposito, R.; Russo, M.G.; Caianiello, G.; Calabrò, R. Stenting of the arterial duct in newborns with duct-dependent pulmonary circulation. Heart 2008, 94, 925–929. [Google Scholar] [CrossRef]
- Santoro, G.; Capozzi, G.; Giordano, M.; Gaio, G.; Palladino, M.T.; Iacono, C.; Mahmoud, H.T.; Russo, M.G. Fate of Duct-Dependent, Discontinuous Pulmonary Arteries After Arterial Duct Stenting. Pediatr. Cardiol. 2017, 38, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Glatz, A.C.; Petit, C.J.; Goldstein, B.H.; Kelleman, M.S.; McCracken, C.E.; McDonnell, A.; Buckey, T.; Mascio, C.E.; Shashidharan, S.; Ligon, R.A.; et al. Comparison between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants with Ductal-Dependent Pulmonary Blood Flow: Insights from the Congenital Catheterization Research Collaborative. Circulation 2018, 137, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.H.; O’Byrne, M.L.; Petit, C.J.; Qureshi, A.M.; Dai, D.; Griffis, H.M.; France, A.; Kelleman, M.S.; McCracken, C.E.; Mascio, C.E.; et al. Differences in Cost of Care by Palliation Strategy for Infants with Ductal-Dependent Pulmonary Blood Flow. Circ. Cardiovasc. Interv. 2019, 12, e007232. [Google Scholar] [CrossRef] [PubMed]
- Lemley, B.A.; Wu, L.; Roberts, A.L.; Shinohara, R.T.; Quarshie, W.O.; Qureshi, A.M.; Smith, C.L.; Dori, Y.; Gillespie, M.J.; Rome, J.J.; et al. Trends in Ductus Arteriosus Stent Versus Blalock-Taussig-Thomas Shunt Use and Comparison of Cost, Length of Stay, and Short-Term Outcomes in Neonates With Ductal-Dependent Pulmonary Blood Flow: An Observational Study Using the Pediatric Health Information Systems Database. J. Am. Heart Assoc. 2023, 12, e030575. [Google Scholar] [CrossRef] [PubMed]
- Feltes, T.F.; Bacha, E.; Beekman, R.H.; Cheatham, J.P.; Feinstein, J.A.; Gomes, A.S.; Hijazi, Z.M.; Ing, F.F.; De Moor, M.; Morrow, W.R.; et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2607–2652. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.L.; Uzun, O.; Blackburn, M.E.C.; Wren, C.; Hamilton, J.R.L.; Watterson, K.G. Fate of the stented arterial duct. Circulation 1999, 99, 2621–2625. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.; Youssef, T.; Cinteza, E.; Voicu, C.; Balan, A.; Margarint, I.; Filip, C.; Nicolae, G.; Duica, G.; Nicolescu, A.; et al. Ductus Arteriosus Stenting in Newborns—Transcatheter Approach as a Bridge Therapy for Corrective Surgery. Maedica 2022, 17, 205–210. [Google Scholar]
- Chamberlain, R.C.; Ezekian, J.E.; Sturgeon, G.M.; Barker, P.C.A.; Hill, K.D.; Fleming, G.A. Preprocedural three-dimensional planning aids in transcatheter ductal stent placement: A single-center experience. Catheter. Cardiovasc. Interv. 2020, 95, 1141–1148. [Google Scholar] [CrossRef]
- Stumper, O.; Ramchandani, B.; Noonan, P.; Mehta, C.; Bhole, V.; Reinhardt, Z.; Dhillon, R.; Miller, P.A.; De Giovanni, J.V. Stenting of the right ventricular outflow tract. Heart 2013, 99, 1603–1608. [Google Scholar] [CrossRef]
- Quandt, D.; Ramchandani, B.; Penford, G.; Stickley, J.; Bhole, V.; Mehta, C.; Jones, T.; Barron, D.J.; Stumper, O. Right ventricular outflow tract stent versus BT shunt palliation in Tetralogy of Fallot. Heart 2017, 103, 1985–1991. [Google Scholar] [CrossRef]
- Meadows, J.J.; Qureshi, A.M.; Goldstein, B.H.; Petit, C.J.; McCracken, C.E.; Kelleman, M.S.; Aggarwal, V.; Bauser-Heaton, H.; Combs, C.S.; Gartenberg, A.J.; et al. Comparison of Outcomes at Time of Superior Cavopulmonary Connection between Single Ventricle Patients with Ductal-Dependent Pulmonary Blood Flow Initially Palliated with Either Blalock-Taussig Shunt or Ductus Arteriosus Stent: Results from the Congenital Catheterization Research Collaborative. Circ. Cardiovasc. Interv. 2019, 12, e008110. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Petit, C.J.; Glatz, A.C.; Goldstein, B.H.; Qureshi, A.M. Stenting of the ductus arteriosus for ductal-dependent pulmonary blood flow—Current techniques and procedural considerations. Congenit. Heart Dis. 2019, 14, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bentham, J.R.; Zava, N.K.; Harrison, W.J.; Shauq, A.; Kalantre, A.; Derrick, G.; Chen, R.H.; Dhillon, R.; Taliotis, D.; Kang, S.-L.; et al. Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates with Duct-Dependent Pulmonary Blood Flow: Associations with Clinical Outcomes in a Multicenter National Study. Circulation 2018, 137, 581–588. [Google Scholar] [CrossRef] [PubMed]
- McMullan, D.M.; Permut, L.C.; Jones, T.K.; Johnston, T.A.; Rubio, A.E. Modified Blalock-Taussig shunt versus ductal stenting for palliation of cardiac lesions with inadequate pulmonary blood flow. J. Thorac. Cardiovasc. Surg. 2014, 147, 397–403. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalera, S.T.; Pizzuto, A.; Marchese, P.; Cantinotti, M.; Franchi, E.; Marrone, C.; Assanta, N.; Santoro, G. Personalized Approach in Transcatheter Palliation of Congenital Heart Disease with Duct-Dependent Pulmonary Circulation: Right Ventricular Outflow Tract Stenting vs. Arterial Duct Stenting. J. Pers. Med. 2024, 14, 302. https://doi.org/10.3390/jpm14030302
Scalera ST, Pizzuto A, Marchese P, Cantinotti M, Franchi E, Marrone C, Assanta N, Santoro G. Personalized Approach in Transcatheter Palliation of Congenital Heart Disease with Duct-Dependent Pulmonary Circulation: Right Ventricular Outflow Tract Stenting vs. Arterial Duct Stenting. Journal of Personalized Medicine. 2024; 14(3):302. https://doi.org/10.3390/jpm14030302
Chicago/Turabian StyleScalera, Silvia Teresa, Alessandra Pizzuto, Pietro Marchese, Massimiliano Cantinotti, Eliana Franchi, Chiara Marrone, Nadia Assanta, and Giuseppe Santoro. 2024. "Personalized Approach in Transcatheter Palliation of Congenital Heart Disease with Duct-Dependent Pulmonary Circulation: Right Ventricular Outflow Tract Stenting vs. Arterial Duct Stenting" Journal of Personalized Medicine 14, no. 3: 302. https://doi.org/10.3390/jpm14030302
APA StyleScalera, S. T., Pizzuto, A., Marchese, P., Cantinotti, M., Franchi, E., Marrone, C., Assanta, N., & Santoro, G. (2024). Personalized Approach in Transcatheter Palliation of Congenital Heart Disease with Duct-Dependent Pulmonary Circulation: Right Ventricular Outflow Tract Stenting vs. Arterial Duct Stenting. Journal of Personalized Medicine, 14(3), 302. https://doi.org/10.3390/jpm14030302








