Outcomes of Endoscopic Endonasal Dacryocystorhinostomy in Glaucoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preoperative Evaluation
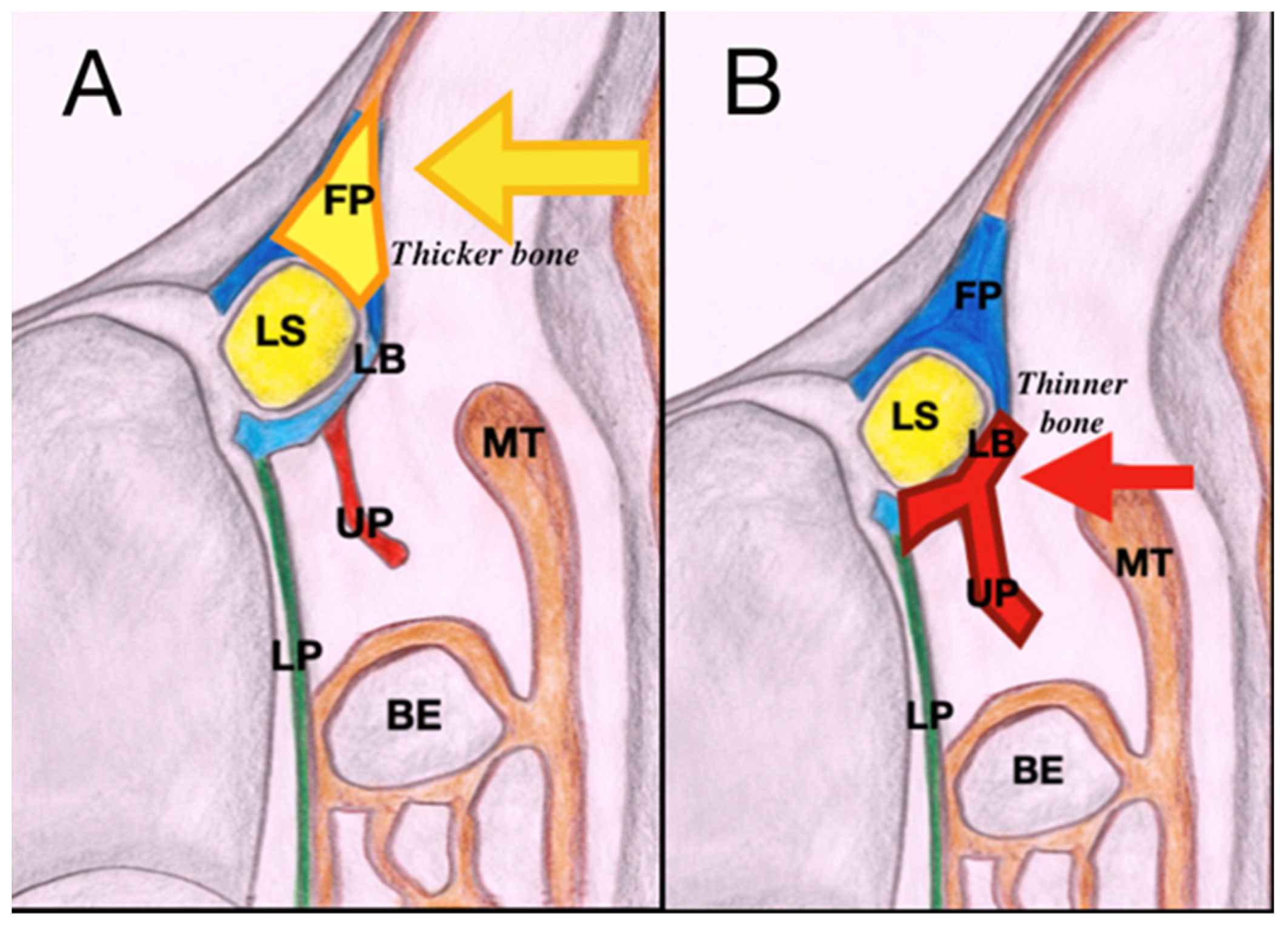
2.3. Surgical Technique
2.4. Post-Operative Care
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makselis, A.; Petroska, D.; Kadziauskiene, A.; Jaruseviciene, R.; Ruzgys, A.; Cimbalas, A.; Besusparis, J.; Asoklis, R.S. Acquired nasolacrimal duct obstruction: Clinical and histological findings of 275 cases. BMC Ophthalmol. 2022, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Gupta, H.; Honavar, S.G.; Naik, M.N. Acquired nasolacrimal duct obstructions secondary to naso-orbito-ethmoidal fractures: Patterns and outcomes. Ophthalmic Plast. Reconstr. Surg. 2012, 28, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J. Etiopathogenesis of primary acquired nasolacrimal duct obstruction (PANDO). Prog. Retin. Eye Res. 2023, 96, 101193. [Google Scholar] [CrossRef]
- Amadi, A.J. Endoscopic DCR vs External DCR: What’s Best in the Acute Setting? J. Ophthalmic Vis. Res. 2017, 12, 251–253. [Google Scholar] [CrossRef] [PubMed]
- McDonogh, M.; Meiring, J.H. Endoscopic transnasal dacryocystorhinostomy. J. Laryngol. Otol. 1989, 103, 585–587. [Google Scholar] [CrossRef]
- Rajabi, M.T.; Shahraki, K.; Nozare, A.; Moravej, Z.; Tavakolizadeh, S.; Salim, R.E.; Hosseinzadeh, F.; Mohammadi, S.; Farahi, A.; Shahraki, K. External versus Endoscopic Dacryocystorhinostomy for Primary Acquired Nasolacrimal Duct Obstruction. Middle East. Afr. J. Ophthalmol. 2022, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, L.; Zhang, L.; Ding, Y.; Liu, X.; Ali, M.J.; Xiao, C. Evaluation of primary acquired nasolacrimal duct obstruction: Comparison of CT-DCG and dacryoendoscopy in accurately localizing the lacrimal drainage obstructions. Eur. J. Ophthalmol. 2024, 7, 11206721241230581. [Google Scholar] [CrossRef] [PubMed]
- Demorest, B.H.; Midler, B. Dacryocystography: II. The Pathologic Lacrimal Apparatus. AMA Arch. Ophthalmol. 1955, 54, 410–421. [Google Scholar] [CrossRef]
- McNab, A.A. Lacrimal canalicular obstruction associated with topical ocular medication. Aust. N. Z. J. Ophthalmol. 1998, 26, 219–223. [Google Scholar] [CrossRef]
- Mandal, P.; Ahluwalia, H. Do topical ocular antihypertensives affect Dacryocystorhinostomy outcomes: The Coventry experience. Eye 2022, 36, 135–139. [Google Scholar] [CrossRef]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L. American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef] [PubMed]
- Freitag, S.K.; Aakalu, V.K.; Foster, J.A.; McCulley, T.J.; Tao, J.P.; Vagefi, M.R.; Yen, M.T.; Kim, S.J.; Wladis, E.J. Use of Mitomycin C in Dacryocystorhinostomy: A Report by the American Academy of Ophthalmology. Ophthalmology 2023, 130, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Munk, P.L.; Lin, D.T.; Morris, D.C. Epiphora: Treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology 1990, 177, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, R. Idiopathic acquired lacrimal drainage obstruction. Br. J. Ophthalmol. 1967, 51, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Cvenkel, B.; Ihan, A. Ocular Surface Changes Induced by Topical Antiglaucoma Monotherapy. Ophthalmologica 2002, 216, 175–179. [Google Scholar] [CrossRef]
- Kashkouli, M.B.; Rezaee, R.; Nilforoushan, N.; Salimi, S.; Foroutan, A.; Naseripour, M. Topical Antiglaucoma Medications and Lacrimal Drainage System Obstruction. Ophthalmic Plast. Reconstr. Surg. 2008, 24, 172. [Google Scholar] [CrossRef] [PubMed]
- Seider, N.; Miller, B.; Beiran, I. Topical glaucoma therapy as a risk factor for nasolacrimal duct obstruction. Am. J. Ophthalmol. 2008, 145, 120–123. [Google Scholar] [CrossRef]
- Di Maria, A.; Tredici, C.; Cozzupoli, G.M.; Vinciguerra, P.; Confalonieri, F. Effects of prostaglandin analogues on epiphora persistence after EN-DCR: A hypothesis-generating study. Eur. J. Ophthalmol. 2023, 33, 182–187. [Google Scholar] [CrossRef]
- Penttilä, E.; Smirnov, G.; Tuomilehto, H.; Kaarniranta, K.; Seppä, J. Endoscopic dacryocystorhinostomy as treatment for lower lacrimal pathway obstructions in adults: Review article. Allergy Rhinol. 2015, 6, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pirola, F.; Spriano, G.; Ferreli, F.; Russo, E.; Di Bari, M.; Giannitto, C.; De Virgilio, A.; Mercante, G.; Vinciguerra, P.; Di Maria, A.; et al. Clinical outcome and quality of life of lacrimal sac mucocele treated via endoscopic posterior approach. Am. J. Otolaryngol. 2022, 43, 103244. [Google Scholar] [CrossRef] [PubMed]
- Coumou, A.D.; Genders, S.W.; Smid, T.M.; Saeed, P. Endoscopic dacryocystorhinostomy: Long-term experience and outcomes. Acta Ophthalmol. 2017, 95, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Yigit, O.; Samancioglu, M.; Taskin, U.; Ceylan, S.; Eltutar, K.; Yener, M. External and endoscopic dacryocystorhinostomy in chronic dacryocystitis: Comparison of results. Eur. Arch. Otorhinolaryngol. 2007, 264, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Psaltis, A.J.; Murphy, J.; Wormald, P.J. Outcomes in primary powered endoscopic dacryocystorhinostomy: Comparison between experienced versus less experienced surgeons. Am. J. Rhinol. Allergy 2014, 28, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, M.; Giordano Resti, A.; Bellini, C.; Forti, M.; Bussi, M. Anastomosis of nasal mucosal and lacrimal sac flaps in endoscopic dacryocystorhinostomy. Eur. Arch. Otorhinolaryngol. 2009, 266, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Lee, Y.H.; Kim, K.N.; Kang, T.S.; Lee, S.B. Surgical outcomes of endoscopic dacryocystorhinostomy: Analysis of age effect. Sci. Rep. 2019, 9, 19861. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, M.; Giordano Resti, A.; Vinciguerra, A.; Danè, G.; Bussi, M. Dacryocystorhinostomy: Evolution of endoscopic techniques after 498 cases. Eur. J. Ophthalmol. 2020, 30, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.C.; Macewen, C.J.; White, P.S. A systematic review of outcomes after dacryocystorhinostomy in adults. Am. J. Rhinol. Allergy 2010, 24, 81–90. [Google Scholar] [CrossRef]
- Knisely, A.; Harvey, R.; Sacks, R. Long-term outcomes in endoscopic dacryocystorhinostomy. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 53–58. [Google Scholar] [CrossRef]
- Rabina, G.; Golan, S.; Neudorfer, M.; Leibovitch, I. External Dacryocystorhinostomy: Characteristics and Surgical Outcomes in Patients with and without Previous Dacryocystitis. J. Ophthalmol. 2013, 2013, 287524. [Google Scholar] [CrossRef] [PubMed]
- Guglielminetti, E.; Barabino, S.; Monaco, M.; Mantero, S.; Rolando, M. HLA-DR expression in conjunctival cells after latanoprost. J. Ocul. Pharmacol. Ther. 2002, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Patient | Anti-Glaucoma Eye Drop | |
|---|---|---|
| Left Eye | Right Eye | |
| #1 | Latanoprost | |
| #2 | Latanoprost, timolol | Latanoprost, timolol |
| #3 | Brinzolamide, brimonidine | |
| #4 | Travoprost | |
| #5 | Latanoprost, timolol | Latanoprost, timolol |
| #6 | Brinzolamide, timolol | |
| #7 | Brinzolamide, timolol, bimatoprost | Brinzolamide, timolol, bimatoprost |
| #8 | Latanoprost, timolol | Latanoprost, timolol |
| #9 | Dorzolamide, timolol | Dorzolamide, timolol |
| #10 | Travoprost, timolol | Travoprost, timolol |
| #11 | Brinzolamide, timolol | Brinzolamide, timolol |
| #12 | Brinzolamide, timolol | Brinzolamide, timolol |
| #13 | Tafluprost, brinzolamide, timolol | |
| #14 | Tafluprost, timolol | Tafluprost, timolol |
| #15 | Dorzolamide, timolol | Dorzolamide, timolol |
| #16 | Travoprost, timololo | Travoprost, timololo |
| #17 | Dorzolamide, timolol | Dorzolamide, timolol |
| #18 | Latanoprost, timolol | |
| #19 | Latanoprost | |
| #20 | Dorzolamide, timolol | |
| Munk Score | Overall (%) | Left (%) | Right (%) | |
|---|---|---|---|---|
| T0 | 0 | 2 (6.25) | 1 (5.55) | 1 (7.14) |
| 1 | 0 (0) | 0 (0) | 0 (0) | |
| 2 | 0 (0) | 0 (0) | 0 (0) | |
| 3 | 6 (18.75) | 4 (22.22) | 2 (14.28) | |
| 4 | 24 (75.0) | 13 (72.22) | 11 (78.57) | |
| T1 | 0 | 16 (50.0) | 8 (44.44) | 8 (57.14) |
| 1 | 8 (25.0) | 3 (16.66) | 3 (21.43) | |
| 2 | 3 (9.37) | 2 (11.11) | 1 (7.14) | |
| 3 | 2 (6.25) | 1 (5.55) | 1 (7.14) | |
| 4 | 3 (9.37) | 2 (11.11) | 1 (7.14) | |
| T2 | 0 | 22 (68.75) | 11 (61.11) | 8 (57.14) |
| 1 | 4 (12.5) | 3 (16.67) | 1 (7.14) | |
| 2 | 3 (9.38) | 1 (5.56) | 2 (14.29) | |
| 3 | 1 (3.12) | 0 (0) | 1 (7.14) | |
| 4 | 2 (6.25) | 1 (5.56) | 1 (7.14) |
| Overall (%) | Left (%) | Right (%) | ||
|---|---|---|---|---|
| T0 | No | 10 (31.25) | 6 (33.33) | 4 (28.57) |
| Occasionally (<one/month) | 10 (31.25) | 5 (27.78) | 5 (35.71) | |
| Often (>one/month) | 12 (37.5) | 7 (38.89) | 5 (35.71) | |
| T1 | No | 26 (81.25) | 15 (83.33) | 11 (78.57) |
| Occasionally (<one/month) | 3 (9.37) | 2 (11.11) | 1 (7.14) | |
| Often (>one/month) | 3 (9.37) | 1 (5.55) | 2 (14.28) | |
| T2 | No | 30 (93.75) | 17 (94.44) | 13 (92.86) |
| Occasionally (<one/month) | 0 (0) | 0 (0) | 0 (0) | |
| Often (>one/month) | 2 (6.25) | 1 (5.55) | 1 (7.14) |
| Timepoint | Epiphora | Dacryocystitis | |||||
|---|---|---|---|---|---|---|---|
| Overall | Left | Right | Overall | Left | Right | ||
| Aggregate | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| T0 vs. T1 | <0.001 | 0.001 | 0.006 | 0.001 | 0.034 | 0.008 | <0.001 |
| T0 vs. T2 | <0.001 | <0.001 | 0.002 | <0.001 | 0.017 | 0.003 | <0.001 |
| T1 vs. T2 | 0.431 | 0.596 | 0.695 | 0.694 | 0.791 | 0.769 | 0.431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, G.M.; Giombi, F.; Muci, G.; Giunta, G.; Pirola, F.; Serra, E.; Zuppardo, J.; Ferreli, F.; Vinciguerra, P.; Mercante, G.; et al. Outcomes of Endoscopic Endonasal Dacryocystorhinostomy in Glaucoma Patients. J. Pers. Med. 2024, 14, 348. https://doi.org/10.3390/jpm14040348
Pace GM, Giombi F, Muci G, Giunta G, Pirola F, Serra E, Zuppardo J, Ferreli F, Vinciguerra P, Mercante G, et al. Outcomes of Endoscopic Endonasal Dacryocystorhinostomy in Glaucoma Patients. Journal of Personalized Medicine. 2024; 14(4):348. https://doi.org/10.3390/jpm14040348
Chicago/Turabian StylePace, Gian Marco, Francesco Giombi, Giovanna Muci, Gianmarco Giunta, Francesca Pirola, Egidio Serra, Jessica Zuppardo, Fabio Ferreli, Paolo Vinciguerra, Giuseppe Mercante, and et al. 2024. "Outcomes of Endoscopic Endonasal Dacryocystorhinostomy in Glaucoma Patients" Journal of Personalized Medicine 14, no. 4: 348. https://doi.org/10.3390/jpm14040348
APA StylePace, G. M., Giombi, F., Muci, G., Giunta, G., Pirola, F., Serra, E., Zuppardo, J., Ferreli, F., Vinciguerra, P., Mercante, G., Maria, A. D., Spriano, G., & Malvezzi, L. (2024). Outcomes of Endoscopic Endonasal Dacryocystorhinostomy in Glaucoma Patients. Journal of Personalized Medicine, 14(4), 348. https://doi.org/10.3390/jpm14040348









