Improving Quality Assurance in a Radiation Oncology Using ARIA Visual Care Path
Abstract
:1. Introduction
2. Materials and Methods
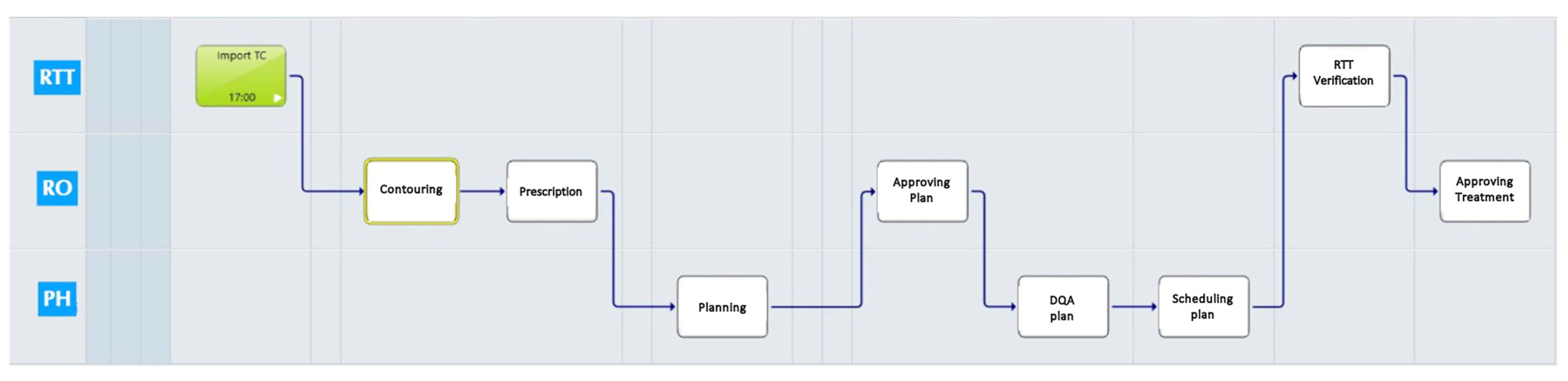
2.1. VPC Customization
- Simulation task: It includes a simulation report with patients’ face photos for recognition and a clinical summary; immobilization procedure details and an immobilization system photo; and simulation computed tomography (CT) import.
- Treatment planning task: Contouring activities, dose prescription, treatment planning, plan approval, and dosimetric quality assurance (DQA) assessment are included.
- Treatment start verification task: This includes dose prescription and monitor unit review; verification of patients’ RT documents (plan document, simulation document, plan check DQA, DVH, SGRT data procedures, correct immobilization system); and photo review.
- Treatment completion task: This includes the verification of RT dose and fractions (delivered versus planned).
2.2. Study Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RT | Radiotherapy |
| QA | Quality Assurance |
| VCP | Visual Care Path |
| RO | Radiation Oncologists |
| MP | Medical Physicists |
| RTT | Radiotherapists |
| DQA | Dosimetric Quality Assurance |
| SGRT | Surface-Guided RT |
| VMAT | Volumetric Modulated Arc Therapy |
| IAEA | International Atomic Energy Agency |
References
- Hanna, T.P.; Shafiq, J.; Delaney, G.P.; Vinod, S.K.; Thompson, S.R.; Barton, M.B. The population benefit of evidence-based radiotherapy: 5-Year local control and overall survival benefits. Radiother. Oncol. 2018, 126, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, N.; Russo, G.A.; Shin, J.Y.; Kachnic, L.A. Optimizing efficiency and safety in a radiation oncology department through the use of ARIA 11 Visual Care Path. Pract. Radiat. Oncol. 2015, 5, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Gershan, V.; Holmberg, O. Safety in radiation oncology (SAFRON): Learning about incident causes and safety barriers in external beam radiotherapy. Phys. Med. 2023, 111, 102618. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.C.; Gaudette, R.; Myers, L.; Vanderver, B.; Engineer, L.; Zellars, R.; Song, D.Y.; Wong, J.; DeWeese, T.L. Evaluation of safety in a radiation oncology setting using failure mode and effects analysis. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Faiella, G.; Parand, A.; Franklin, B.D.; Chana, P.; Cesarelli, M.; Stanton, N.A.; Sevdalis, N. Expanding healthcare failure mode and effect analysis: A composite proactive risk analysis approach. Reliab. Eng. Syst. Saf. 2018, 169, 117–126. [Google Scholar] [CrossRef]
- Boussat, B.; Seigneurin, A.; Giai, J.; Kamalanavin, K.; Labarère, J.; François, P. Involvement in root cause analysis and patient safety culture among hospital care providers. J. Patient Saf. 2021, 17, e1194–e1201. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Jackson, M.; Xie, L.; Chang, S.X.; Burkhardt, K.D.; Mazur, L.; Jones, E.L.; Saponaro, P.; LaChapelle, D.; Baynes, D.C.; et al. The challenge of maximizing safety in radiation oncology. Pract. Radiat. Oncol. 2011, 1, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Stump, K. Improving radiation oncology workflow and efficiency with RT Workspace. In Standard Imaging White Paper; Standard Imaging: Middleton, WI, USA, 2009. [Google Scholar]
- Yeung, T.K.; Bortolotto, K.; Cosby, S.; Hoar, M.; Lederer, E. Quality assurance in radiotherapy: Evaluation of errors and incidents recorded over a 10 year period. Radiother. Oncol. 2005, 74, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gregucci, F.; Surgo, A.; Bonaparte, I.; Laera, L.; Ciliberti, M.P.; Carbonara, R.; Gentile, M.A.; Giraldi, D.; Calbi, R.; Caliandro, M.; et al. Poor-Prognosis Patients Affected by Glioblastoma: Retrospective Study of Hypofractionated Radiotherapy with Simultaneous Integrated Boost and Concurrent/Adjuvant Temozolomide. J. Pers. Med. 2021, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Fiorentino, A.; Gaasch, A.; Schönecker, S.; Reitz, D.; Heinz, C.; Niyazi, M.; Duma, M.N.; Alongi, F.; Belka, C.; et al. Dose variability in different lymph node levels during locoregional breast cancer irradiation: The impact of deep-inspiration breath hold. Strahlenther. Onkol. 2019, 195, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gregucci, F.; Carbonara, R.; Surgo, A.; Ciliberti, M.P.; Curci, D.; Ciocia, A.; Branà, L.; Ludovico, G.M.; Scarcia, M.; Portoghese, F.; et al. Extreme hypofractionated stereotactic radiotherapy for elderly prostate cancer patients: Side effects preliminary analysis of a phase II trial. Radiol. Med. 2023, 128, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, A.; Gregucci, F.; Bonaparte, I.; Romanazzi, I.; Troisi, F.; Surgo, A.; Vitulano, N.; Quadrini, F.; Valenti, N.; Carbonara, R.; et al. Linear accelerator-based stereotactic arrhythmia radioablation for paroxysmal atrial fibrillation in elderly: A prospective phase II trial. Europace 2023, 25, euad344. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gregucci, F.; Bonaparte, I.; Vitulano, N.; Surgo, A.; Mazzola, R.; Di Monaco, A.; Carbonara, R.; Alongi, F.; Langialonga, T.; et al. Stereotactic Ablative radiation therapy (SABR) for cardiac arrhythmia: A new therapeutic option? Radiol. Med. 2021, 126, 155–162. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. IAEA Safety Glossary, 2018th ed.; IAEA: Vienna, Austria, 2019. [Google Scholar]
- International Atomic Energy Agency. Lessons Learned from Accidental Exposures in Radiotherapy; IAEA: Vienna, Austria, 2000. [Google Scholar]
- Derreumaux, S.; Etard, C.; Huet, C.; Trompier, F.; Clairand, I.; Bottollier-Depois, J.-F.; Aubert, B.; Gourmelon, P. Lessons from recent accidents in radiation therapy in France. Radiat. Prot. Dosim. 2008, 131, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; de Los, F.; Santos, L.; Pawlicki, T.; Sutlief, S.; Dunscombe, P. Consensus recommendations for incident learning database structures in radiation oncology. Med. Phys. 2012, 39, 7272–7290. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, D.J.; Dicker, A.P.; Eads, N.L.; Ezzell, G.A.; Fraass, B.A.; Kwiatkowski, T.M.; Lash, K.; Patton, G.A.; Piotrowski, T.; Tomlinson, C.; et al. RO-ILS: Radiation oncology incident learning system: A report from the first year of experience. Pract. Radiat. Oncol. 2015, 5, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, D.J.; Ford, E.C.; Eads, N.L.; Kapetanovic, K.; Tomlinson, C. RO-ILS: Radiation Oncology Incident Learning System Data Trends 2014–2015. Am. Soc. Clin. Oncol. 2016, 34, 59. [Google Scholar] [CrossRef]
- Oelofse, I.; van Staden, J.; Coetzee, N.; Steyn, J. Quality management in radiotherapy: A 9-year review of incident reporting within a multifacility organisation. South. Afr. J. Oncol. 2021, 5, 170. [Google Scholar] [CrossRef]
- Cunningham, J.; Coffey, M.; Knöös, T.; Holmberg, O. Radiation oncology safety information system (ROSIS)–Profiles of participants and the first 1074 incident reports. Radiother. Oncol. 2010, 97, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Trusko, B.E.; Pexton, C.; Harrington, J.; Gupta, P.K. Improving Healthcare Quality and Cost With Six Sigma, 1st ed.; FT Press: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Nyflot, M.J.; Thammasorn, P.; Wootton, L.S.; Ford, E.C.; Chaovalitwongse, W.A. Deep learning for patient-specific quality assurance: Identifying errors in radiotherapy delivery by radiomic analysis of gamma images with convolutional neural networks. Med. Phys. 2019, 46, 456–464. [Google Scholar] [CrossRef] [PubMed]


| Period A | Period B | Period C | ||
|---|---|---|---|---|
| Number of Patients | ||||
| 36 | 34 | 35 | ||
| Gender | ||||
| Male | 24 (66.7%) | 14 (41.2%) | 20 (57.1%) | |
| Female | 12 (33.3%) | 20 (58.8%) | 15 (42.9%) | |
| Age | ||||
| Median (IQR) (years) | 67 (28–82) | 63 (40–83) | 64 (38–92) | |
| RT Sites | ||||
| Brain | 6 (16.7%) | 4 (11.8%) | 2 (5.7%) | |
| Bone metastases | 7 (19.4%) | 3 (8.8%) | 8 (22.9%) | |
| Breats | 6 (16.7%) | 9 (26.5%) | 7 (20%) | |
| Prostate | 6 (16.7%) | 2 (5.9%) | 6 (17.1%) | |
| Lung | 3 (8.3%) | 3 (8.8%) | 3 (8.6%) | |
| Pelvis (rectum/gynecological cancer) | 1 (2.8%) | 1 (2.9%) | 2 (5.7%) | |
| Others | 7 (19.4%) | 12 (35.3%) | 7 (20%) | |
| RT Doses/Techniques | ||||
| SBRT (1–3 fx) | 6 (16.7%) | 1 (2.9%) | 4 (11.4%) | |
| SBRT (5–8 fr) | 8 (22.2%) | 9 (26.5%) | 8 (22.8%) | |
| RT ≤ 15 fr | 11 (30.55%) | 12 (35.3%) | 13 (37.2%) | |
| RT > 15 fr | 11 (30.55%) | 12 (35.3%) | 10 (28.6%) | |
| All Patients | Period A | Period B | Period C | ||
|---|---|---|---|---|---|
| n = 105 | n = 36 | n = 34 | n = 35 | p | |
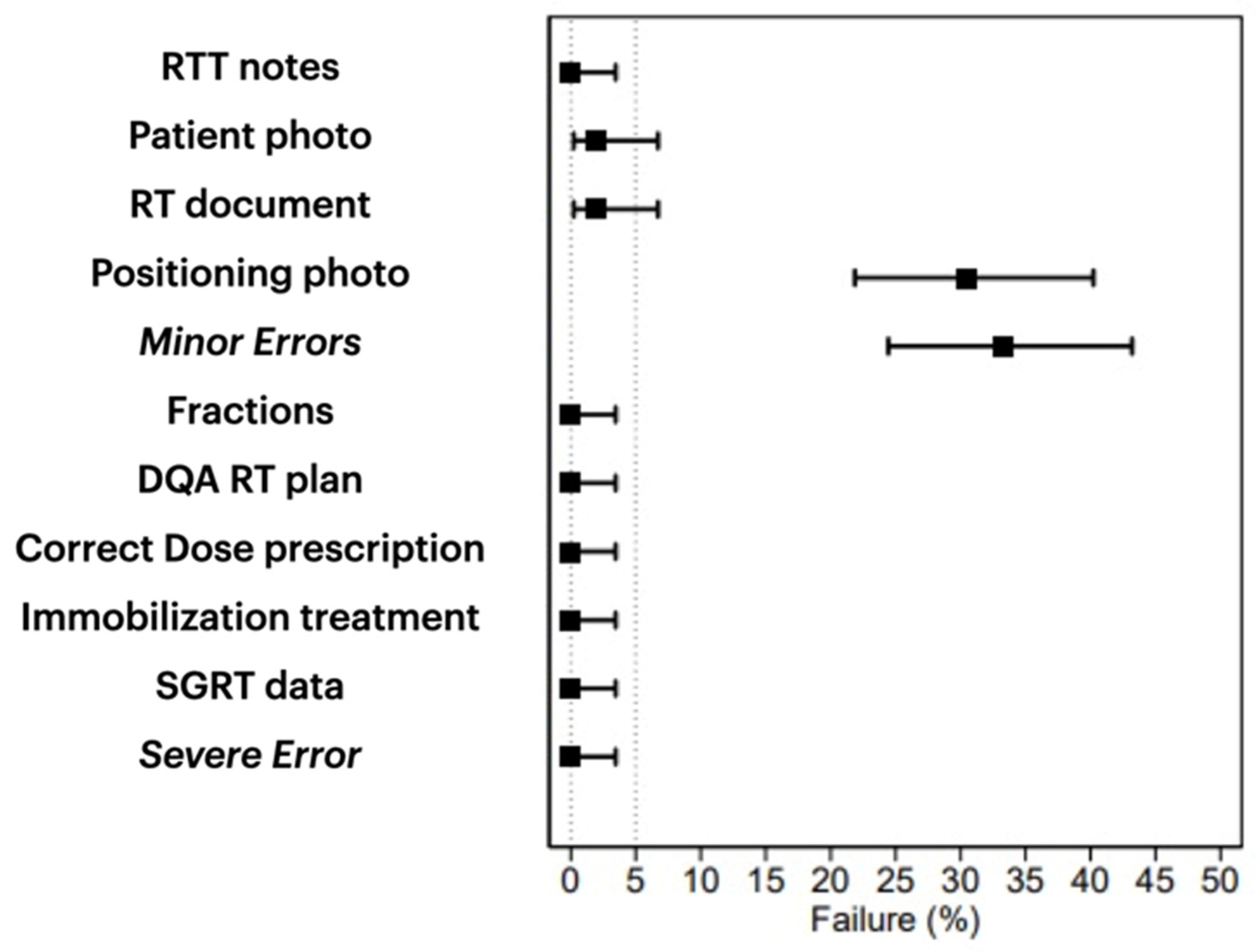
| Dose Prescription | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Patients photo | 2 (1.9%) | 1 (2.8%) | 0 (0.0%) | 1 (2.9%) | 1.000 |
| Treatments note | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Positioning photo | 32 (30.5%) | 17 (47.2%) | 10 (29.4%) | 5 (14.3%) | 0.010 |
| Immobilization system | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| SGRT data | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Treatment documents | 2 (1.9%) | 1 (2.8%) | 0 (0.0%) | 1 (2.9%) | 1.000 |
| DQA RT plan | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Number of fractions | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Minor incidents | 35 (33.3%) | 18 (50.0%) | 10 (29.4%) | 7 (20.0%) | 0.023 |
| Severe incidents | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaparte, I.; Fragnoli, F.; Gregucci, F.; Carbonara, R.; Di Guglielmo, F.C.; Surgo, A.; Davì, V.; Caliandro, M.; Sanfrancesco, G.; De Pascali, C.; et al. Improving Quality Assurance in a Radiation Oncology Using ARIA Visual Care Path. J. Pers. Med. 2024, 14, 416. https://doi.org/10.3390/jpm14040416
Bonaparte I, Fragnoli F, Gregucci F, Carbonara R, Di Guglielmo FC, Surgo A, Davì V, Caliandro M, Sanfrancesco G, De Pascali C, et al. Improving Quality Assurance in a Radiation Oncology Using ARIA Visual Care Path. Journal of Personalized Medicine. 2024; 14(4):416. https://doi.org/10.3390/jpm14040416
Chicago/Turabian StyleBonaparte, Ilaria, Federica Fragnoli, Fabiana Gregucci, Roberta Carbonara, Fiorella Cristina Di Guglielmo, Alessia Surgo, Valerio Davì, Morena Caliandro, Giuseppe Sanfrancesco, Christian De Pascali, and et al. 2024. "Improving Quality Assurance in a Radiation Oncology Using ARIA Visual Care Path" Journal of Personalized Medicine 14, no. 4: 416. https://doi.org/10.3390/jpm14040416






