Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment Due to Alzheimer’s Disease (The BDSC-MCI Project): Ecological Validity of the Corsi Learning Suvra-Span Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
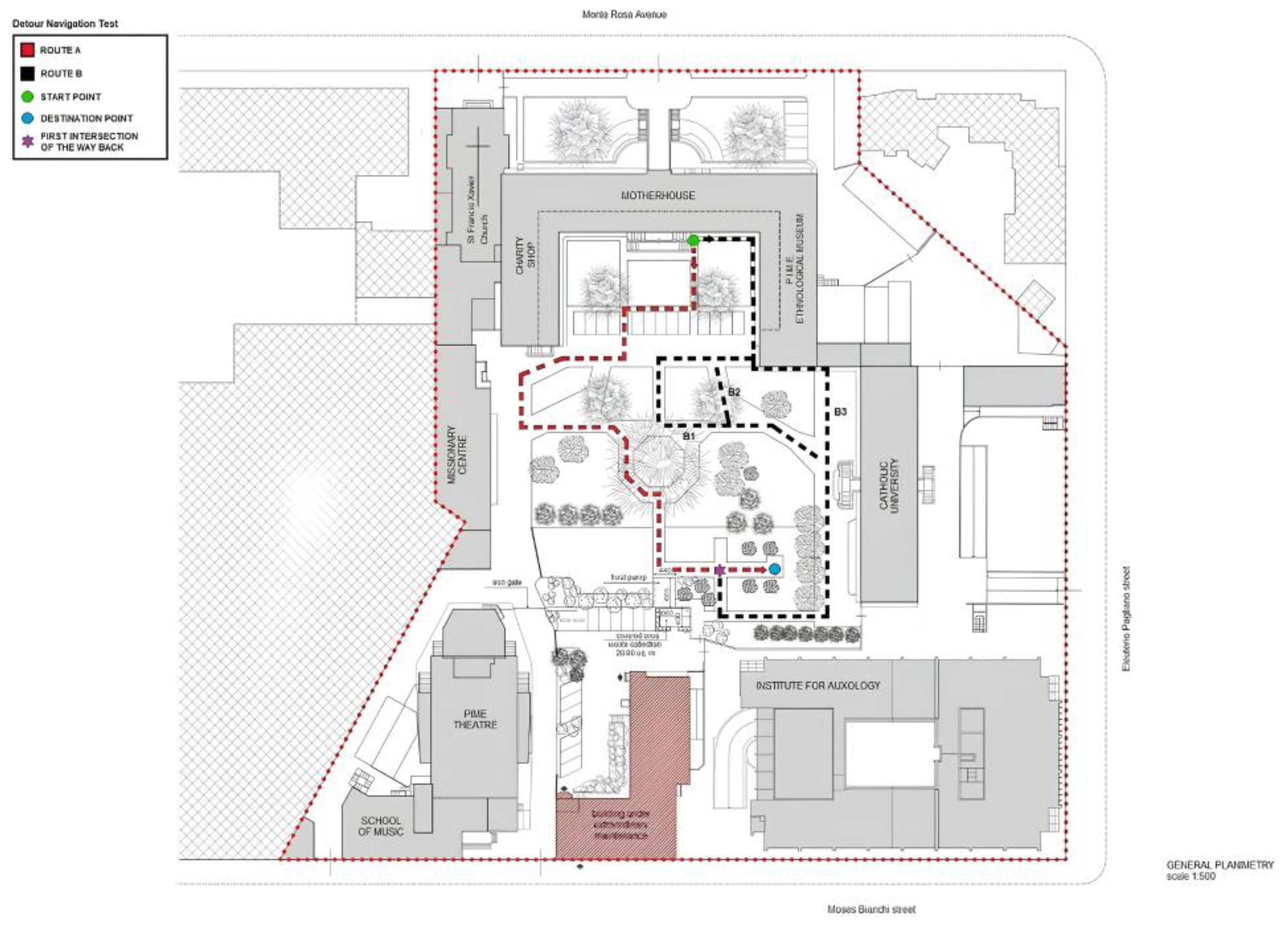
2.3. Real-World Navigation Paradigm
2.4. Neuropsychological Evaluation
2.5. Statistics
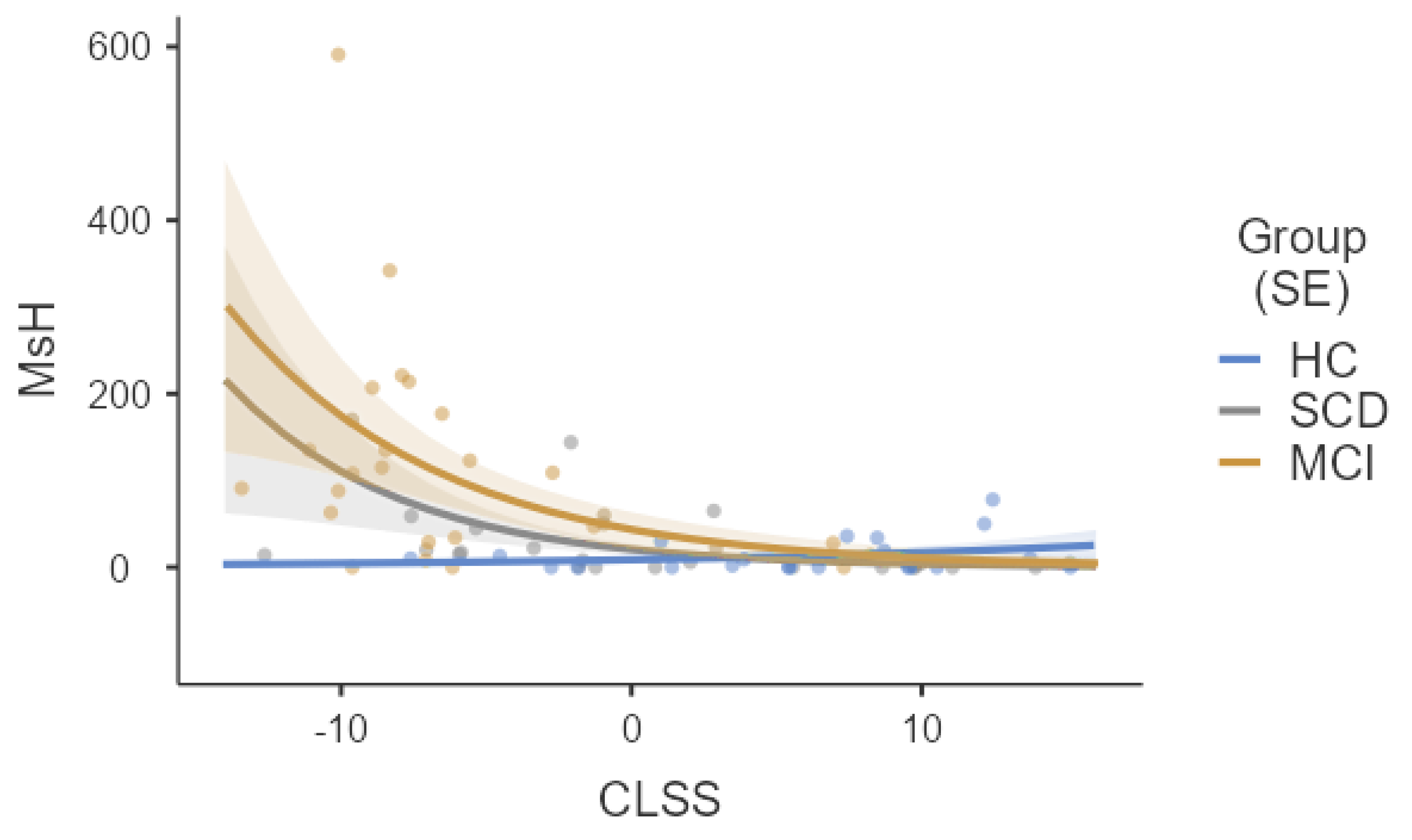
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ávila-Villanueva, M.; Marcos Dolado, A.; Gómez-Ramírez, J.; Fernández-Blázquez, M. Brain structural and functional changes in cognitive impairment due to Alzheimer’s disease. Front. Psychol. 2022, 13, 886619. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.M.; Hornberger, M. Spatial navigation deficits—Overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.F.; Allison, S.L.; Stojanovic, M.; Fagan, A.M.; Morris, J.C.; Head, D. Spatial navigation ability predicts progression of dementia symptomatology. Alzheimer’s Dement. 2020, 16, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Tuena, C.; Serino, S.; Dutriaux, L.; Riva, G.; Piolino, P. Virtual enactment effect on memory in young and aged populations: A systematic review. J. Clin. Med. 2019, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Kunz, L.; Schröder, T.N.; Lee, H.; Montag, C.; Lachmann, B.; Sariyska, R.; Reuter, M.; Stirnberg, R.; Stöcker, T.; Messing-Floeter, P.C.; et al. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science 2015, 350, 430–433. [Google Scholar] [CrossRef]
- Allison, S.L.; Fagan, A.M.; Morris, J.C.; Head, D. Spatial Navigation in Preclinical Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 52, 77–90. [Google Scholar] [CrossRef]
- Bierbrauer, A.; Kunz, L.; Gomes, C.A.; Luhmann, M.; Deuker, L.; Getzmann, S.; Wascher, E.; Gajewski, P.D.; Hengstler, J.G.; Fernandez-Alvarez, M.; et al. Unmasking selective path integration deficits in Alzheimer’s disease risk carriers. Sci. Adv. 2020, 6, eaba1394. [Google Scholar] [CrossRef]
- Montero-Odasso, M. Gait as a biomarker of cognitive impairment and dementia syndromes. Quo vadis? Eur. J. Neurol. 2016, 23, 437–438. [Google Scholar] [CrossRef]
- Skillbäck, T.; Blennow, K.; Zetterberg, H.; Skoog, J.; Rydén, L.; Wetterberg, H.; Guo, X.; Sacuiu, S.; Mielke, M.M.; Zettergren, A.; et al. Slowing gait speed precedes cognitive decline by several years. Alzheimer’s Dement. 2022, 18, 1667–1676. [Google Scholar] [CrossRef]
- Claessen, M.H.; Visser-Meily, J.M.; de Rooij, N.K.; Postma, A.; van der Ham, I.J. A Direct Comparison of Real-World and Virtual Navigation Performance in Chronic Stroke Patients. J. Int. Neuropsychol. Soc. 2016, 22, 467–477. [Google Scholar] [CrossRef]
- Tam, J.; Wyble, B. Location has a privilege, but it is limited: Evidence from probing task-irrelevant location. J. Exp. Psychol. Learn. Mem. Cogn. 2022, 49, 1051–1067. [Google Scholar] [CrossRef]
- Bicanski, A.; Burgess, N. A neural-level model of spatial memory and imagery. elife 2018, 7, e33752. [Google Scholar] [CrossRef] [PubMed]
- Yousif, S.R.; Forrence, A.D.; McDougle, S.D. A common format for representing spatial location in visual and motor working memory. Psychon. Bull. Rev. 2024, 31, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Iachini, I.; Iavarone, A.; Senese, V.P.; Ruotolo, F.; Ruggiero, G. Visuospatial memory in healthy elderly, AD and MCI: A review. Curr. Aging Sci. 2009, 2, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Caffò, A.O.; Bosco, A. Topographical disorientation in aging. Familiarity with the environment does matter. Neurol. Sci. 2018, 39, 1519–1528. [Google Scholar] [CrossRef]
- Laczó, M.; Wiener, J.M.; Kalinova, J.; Matuskova, V.; Vyhnalek, M.; Hort, J.; Laczó, J. Spatial navigation and visuospatial strategies in typical and atypical aging. Brain Sci. 2021, 11, 1421. [Google Scholar] [CrossRef]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef]
- Baddeley, A. The Concept of Episodic Memory. Philos. Trans. R Soc. Lond. Biol. Sci. 2001, 356, 1345–1350. [Google Scholar] [CrossRef]
- Logie, R.H. Visuo-Spatial Working Memory; Erlbaum: Hove, UK, 1995. [Google Scholar]
- Rhodes, S.; Parra, M.A.; Cowan, N.; Logie, R.H. Healthy aging and visual working memory: The effect of mixing feature and conjunction changes. Psychol. Aging 2017, 32, 354. [Google Scholar] [CrossRef]
- Tulving, E. What Is Episodic Memory? Curr. Dir. Psychol. Sci. 1993, 2, 67–70. [Google Scholar] [CrossRef]
- Tulving, E. Episodic memory: From mind to brain. Ann. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Asperholm, M.; Högman, N.; Rafi, J.; Herlitz, A. What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychol. Bull. 2019, 145, 785. [Google Scholar] [CrossRef]
- Tascón, L.; Di Cicco, C.; Piccardi, L.; Palmiero, M.; Bocchi, A.; Cimadevilla, J.M. Sex differences in spatial memory: Comparison of three tasks using the same virtual context. Brain Sci. 2021, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, H.; Tognoni, G. Standardizzazione italiana e taratura di test neuropsicologici. Ital. J. Neurol. Sci. 1987, 6, 25–27. [Google Scholar]
- Capitani, E.; Laiacona, M.; Ciceri, E.; Gruppo Italiano per lo Studio Neuropsicologico, dell’Invecchiamento. Sex differences in spatial memory: A reanalysis of block tapping long-term memory according to the short-term memory level. Ital. J. Neurol. Sci. 1991, 12, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.I.; Chrastil, E.R. Spatial navigation: Memory mechanisms and executive function interactions. Front. Hum. Neurosci. 2019, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Puthusseryppady, V.; Morrissey, S.; Spiers, H.; Patel, M.; Hornberger, M. Predicting real world spatial disorientation in Alzheimer’s disease patients using virtual reality navigation tests. Sci. Rep. 2022, 12, 13397. [Google Scholar] [CrossRef] [PubMed]
- Pařízková, M.; Andel, R.; Lerch, O.; Marková, H.; Gažová, I.; Vyhnálek, M.; Hort, J.; Laczó, J. Homocysteine and real-space navigation performance among non-demented older adults. J. Alzheimer’s Dis. 2017, 55, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Laczó, J.; Andel, R.; Nedelska, Z.; Vyhnalek, M.; Vlcek, K.; Crutch, S.; Harrison, J.; Hort, J. Exploring the contribution of spatial navigation to cognitive functioning in older adults. Neurobiol. Aging 2017, 51, 67–70. [Google Scholar] [CrossRef]
- Peter, J.; Sandkamp, R.; Minkova, L.; Schumacher, L.V.; Kaller, C.P.; Abdulkadir, A.; Klöppel, S. Real-world navigation in amnestic mild cognitive impairment: The relation to visuospatial memory and volume of hippocampal subregions. Neuropsychologia 2018, 109, 86–94. [Google Scholar] [CrossRef]
- Laczó, J.; Andel, R.; Vyhnalek, M.; Vlcek, K.; Magerova, H.; Varjassyova, A.; Tolar, M.; Hort, J. Human analogue of the Morris water maze for testing subjects at risk of Alzheimer’s disease. Neurodegener. Dis. 2010, 7, 148–152. [Google Scholar] [CrossRef]
- Tuena, C.; Mancuso, V.; Stramba-Badiale, C.; Pedroli, E.; Stramba-Badiale, M.; Riva, G.; Repetto, C. Egocentric and allocentric spatial memory in mild cognitive impairment with real-world and virtual navigation tasks: A systematic review. J. Alzheimer’s Dis. 2021, 79, 95–116. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; DeKosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Tuena, C.; Riva, G.; Repetto, C.; Axmacher, N.; Chandreswaran, V.; Isella, V.; Pomati, S.; Zago, S.; Difonzo, T.; et al. Exploring the Remediation of Behavioral Disturbances of Spatial Cognition in Community-Dwelling Senior Citizens with Mild Cognitive Impairment via Innovative Technological Apparatus (BDSC-MCI Project): Protocol for a Prospective, Multi-Center Observational Study. J. Pers. Med. 2024, 14, 192. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef]
- Measso, G.; Cavarzeran, F.; Zappalà, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari, D.; Grigoletto, F. The mini-mental state examination: Normative study of an Italian random sample. Dev. Neurops. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-Mental State Examination: A normative study in Italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Aiello, E.N.; Pasotti, F.; Appollonio, I.; Bolognini, N. Equating mini-mental state examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores: Conversion norms from a healthy Italian population sample. Aging Clin. Exp. Res. 2022, 34, 1721–1724. [Google Scholar] [CrossRef]
- Yesavage, J.A. Geriatric depression scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- Kim, H.Y. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013, 38, 52–54. [Google Scholar] [CrossRef]
- Green, J.A. Too many zeros and/or highly skewed? A tutorial on modelling health behaviour as count data with Poisson and negative binomial regression. Health Psychol. Behav. Med. 2021, 9, 436–455. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Isella, V.; Verde, F.; Silani, V.; Ticozzi, N.; Pomati, S.; Bellocchio, V.; Granese, V.; Vignati, B.; Marchesi, G.; et al. Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease: Preliminary Findings from the BDSC-MCI Project. J. Clin. Med. 2024, 13, 1178. [Google Scholar] [CrossRef]
- Piccardi, L.; Iaria, G.; Ricci, M.; Bianchini, F.; Zompanti, L.; Guariglia, C. Walking in the Corsi test: Which type of memory do you need? Neurosci. Lett. 2008, 432, 127–131. [Google Scholar] [CrossRef]
- Piccardi, L.; Bianchini, F.; Argento, O.; De Nigris, A.; Maialetti, A.; Palermo, L.; Guariglia, C. The Walking Corsi Test (WalCT): Standardization of the topographical memory test in an Italian population. Neurol. Sci. 2013, 34, 971–978. [Google Scholar] [CrossRef]
- Rodriguez, F.S.; Pabst, A.; Heser, K.; Kleineidam, L.; Hajek, A.; Eisele, M.; Röhr, S.; Löbner, R.; Wiese, B.; Angermeyer, M.C.; et al. Disorientation in time and place in old age: Longitudinal evidence from three old age cohorts in Germany (AgeDifferent. de platform). JAD 2021, 79, 1589–1599. [Google Scholar] [CrossRef]
- Franzen, M.D.; Wilhelm, K.L. Conceptual foundations of ecological validity in neuropsychological assessment. In Ecological Validity of Neuropsychological Testing; Sbordone, R.J., Long, C.J., Eds.; St. Lucie Press: Boca Raton, FL, USA, 1996; pp. 91–112. [Google Scholar]
- Dawson, D.R.; Marcotte, T.D. Special issue on ecological validity and cognitive assessment. Neuropsychol. Rehabil. 2017, 27, 599–602. [Google Scholar] [CrossRef]
- Robinson, J.S.; Collins, R.L.; Miller, B.I.; Pacheco, V.H.; Wisdom, N.M. The severe impairment profile: A conceptual shift. Arch. Clin. Neuropsycol. 2018, 33, 238–246. [Google Scholar] [CrossRef]
- Kessels, R.P. Improving precision in neuropsychological assessment: Bridging the gap between classic paper-and-pencil tests and paradigms from cognitive neuroscience. TCN 2019, 33, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Alcañiz, M.; Riera-Prunera, M.C.; Solé-Auró, A. “When I retire, I’ll move out of the city”: Mental well-being of the elderly in rural vs. urban settings. Int. J. Environ. Res. Public Health 2020, 17, 2442. [Google Scholar] [CrossRef] [PubMed]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain. Doctoral Dissertation, McGill University, Montreal, QC, Canada, 1972. [Google Scholar]
- Milner, B. Interhemispheric differences in the localization of psychological processes in man. Br. Med. Bull. 1971, 27, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. L’examen psychologique dans les cas d’encéphalopathie traumatique. The psychological examination in cases of traumatic encephalopathy. Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Osterrieth, P.A. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. Test of copying a complex figure; contribution to the study of perception and memory. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Meneghetti, C.; Miola, L.; Toffalini, E.; Pastore, M.; Pazzaglia, F. Learning from navigation, and tasks assessing its accuracy: The role of visuospatial abilities and wayfinding inclinations. J. Environ. Psychol. 2021, 75, 101614. [Google Scholar] [CrossRef]



| HCs | SCD | MCI | p | |
|---|---|---|---|---|
| N | 21 | 27 | 24 | - |
| Sex (male/female) | 9/12 | 12/15 | 16/8 | 0.185 c |
| Age (yrs.) | 62.9 ± 10.7 (28–82) | 54.1 ± 10.9 (24–86) | 54.1 ± 10.9 (24–86) | n.s. d |
| Education (yrs.) | 12.7 ± 3.8 (5–18) | 13.2 ± 4.0 (5–22) | 13.2 ± 4.0 (5–22) | n.s. d |
| GDS | 12.7 ± 3.8 (5–18) | 13.2 ± 4.0 (5–22) | 13.2 ± 4.0 (5–22) | SCD > HCs (p = 0.031) d MCI > HCs (p = 0.020) d |
| MoCA (adjusted scores) a | 12.7 ± 3.8 (5–18) | 13.2 ± 4.0 (5–22) | 13.2 ± 4.0 (5–22) | SCD > HCs (p = 0.003) d MCI < HCs (p = 0.001) d MCI < SCD (p < 0.001) d |
| CLSS (adjusted scores) b | 12.7 ± 3.8 (5–18) | 13.2 ± 4.0 (5–22) | 13.2 ± 4.0 (5–22) | SCD > HCs (p = 0.069) d MCI < HCs (p < 0.001) d MCI < SCD (p < 0.001) d |
| WTs (n.) | 12.7 ± 3.8 (5–18) | 13.2 ± 4.0 (5–22) | 13.2 ± 4.0 (5–22) | MCI > HCs (p < 0.001) e MCI > SCD (p < 0.001) e |
| MsH (s) | 12.7 ± 3.8 (5–18) | 13.2 ± 4.0 (5–22) | 13.2 ± 4.0 (5–22) | MCI > HCs (p < 0.001) e MCI > SCD (p < 0.001) e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammisuli, D.M.; Marchesi, G.; Bellocchio, V.; Aiello, E.N.; Poletti, B.; Verde, F.; Silani, V.; Ticozzi, N.; Zago, S.; Difonzo, T.; et al. Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment Due to Alzheimer’s Disease (The BDSC-MCI Project): Ecological Validity of the Corsi Learning Suvra-Span Test. J. Pers. Med. 2024, 14, 539. https://doi.org/10.3390/jpm14050539
Cammisuli DM, Marchesi G, Bellocchio V, Aiello EN, Poletti B, Verde F, Silani V, Ticozzi N, Zago S, Difonzo T, et al. Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment Due to Alzheimer’s Disease (The BDSC-MCI Project): Ecological Validity of the Corsi Learning Suvra-Span Test. Journal of Personalized Medicine. 2024; 14(5):539. https://doi.org/10.3390/jpm14050539
Chicago/Turabian StyleCammisuli, Davide Maria, Gloria Marchesi, Virginia Bellocchio, Edoardo Nicolò Aiello, Barbara Poletti, Federico Verde, Vincenzo Silani, Nicola Ticozzi, Stefano Zago, Teresa Difonzo, and et al. 2024. "Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment Due to Alzheimer’s Disease (The BDSC-MCI Project): Ecological Validity of the Corsi Learning Suvra-Span Test" Journal of Personalized Medicine 14, no. 5: 539. https://doi.org/10.3390/jpm14050539
APA StyleCammisuli, D. M., Marchesi, G., Bellocchio, V., Aiello, E. N., Poletti, B., Verde, F., Silani, V., Ticozzi, N., Zago, S., Difonzo, T., Isella, V., Pomati, S., Granese, V., Vignati, B., Prete, L. A., & Castelnuovo, G. (2024). Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment Due to Alzheimer’s Disease (The BDSC-MCI Project): Ecological Validity of the Corsi Learning Suvra-Span Test. Journal of Personalized Medicine, 14(5), 539. https://doi.org/10.3390/jpm14050539








