Application of Stem Cells Shows Antiinflammatory Effect in an Irradiated Random Pattern Flap Model
Abstract
:1. Introduction
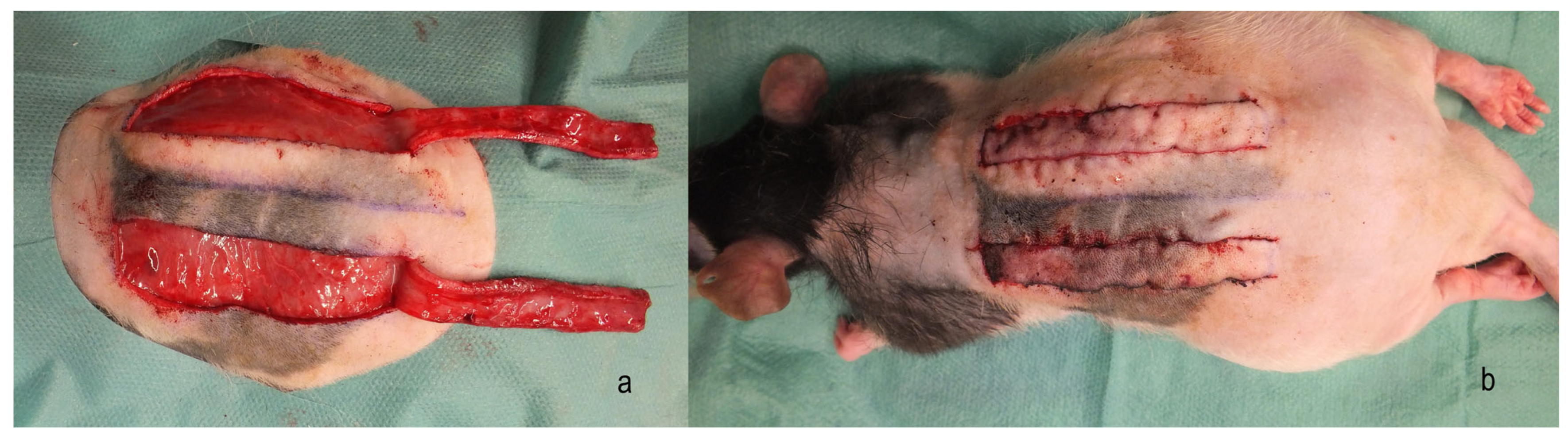
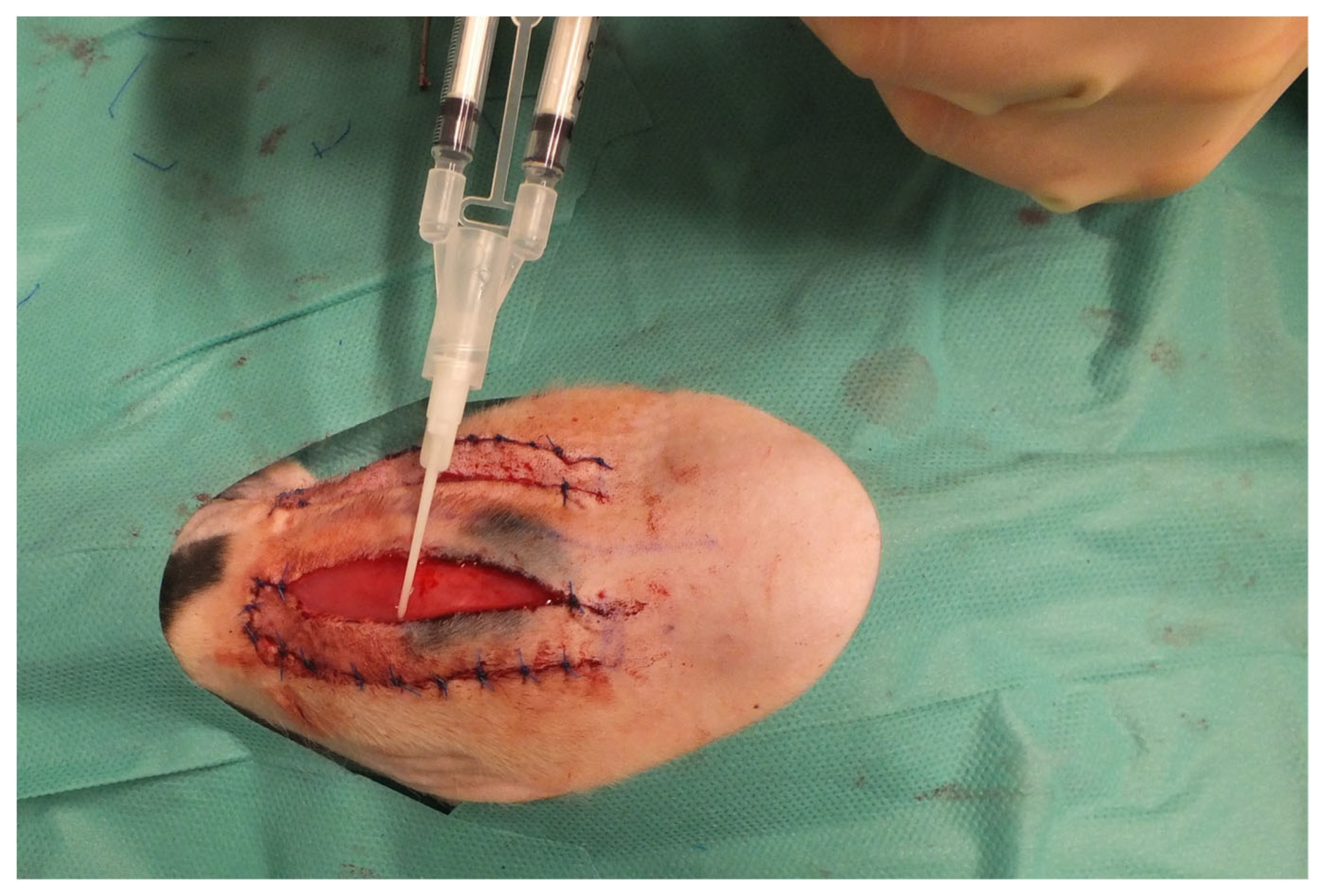
2. Materials and Methods
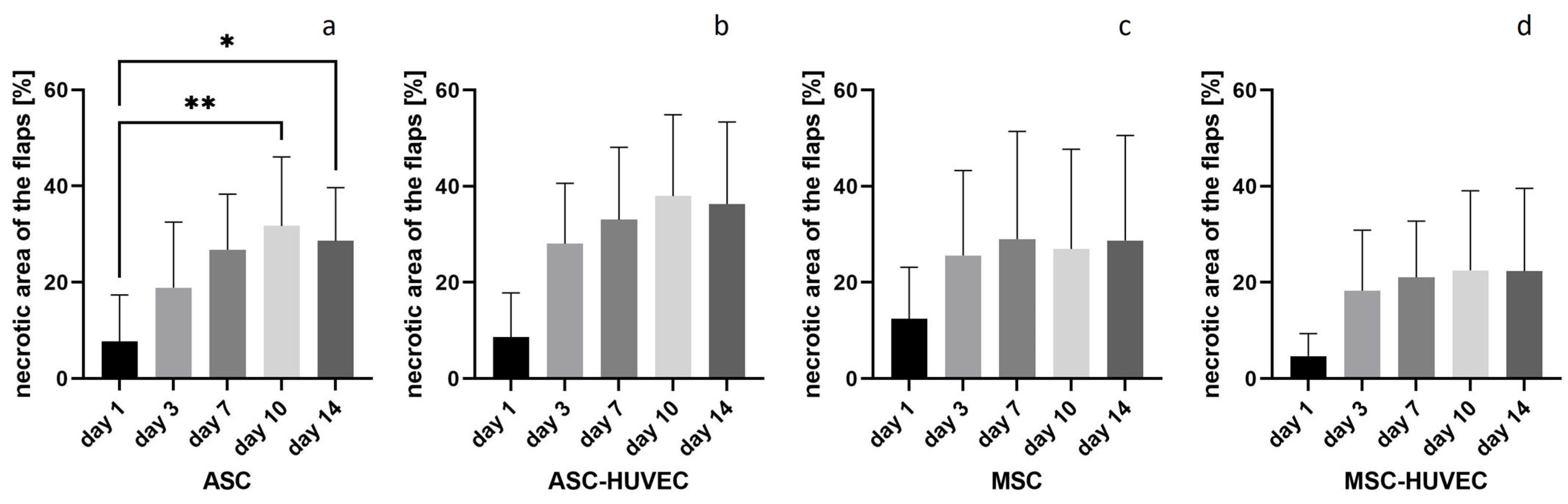
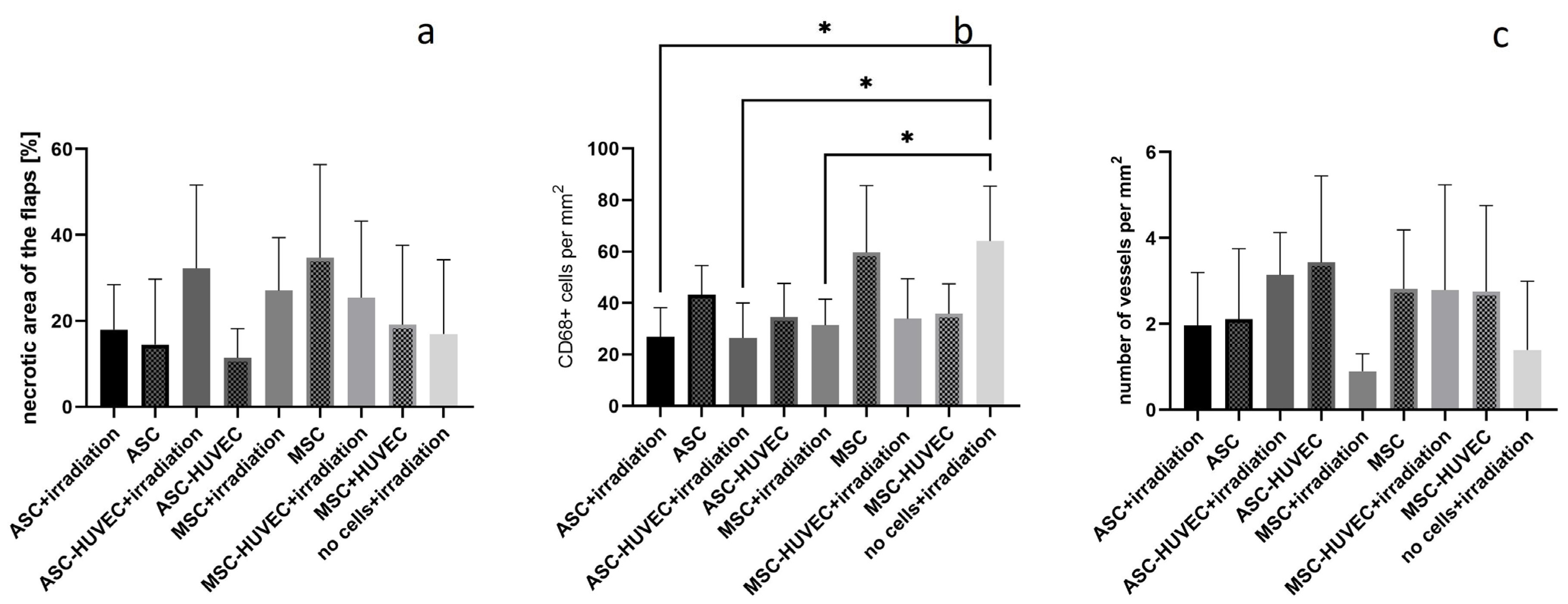
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasdemir, M.; Agir, H.; Eren, G.G.; Aksu, M.G.; Alagoz, M.S.; Duruksu, G.; Saglam, O.; Karaöz, E. Adipose-Derived Stem Cells Improve Survival of Random Pattern Cutaneous Flaps in Radiation Damaged Skin. J. Craniofac. Surg. 2015, 26, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, M.R.; Shen, A.H.; Lee, G.K.; Momeni, A.; Longaker, M.T.; Wan, D.C. Radiation-Induced Skin Fibrosis: Pathogenesis, Current Treatment Options, and Emerging Therapeutics. Ann. Plast. Surg. 2019, 83 (Suppl. 1), S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.F.; Tiwari, K.K.; Gendreau, J.L.; Burgess, P.L.; Taupin, P.; Martin, E.D.D. Mesenchymal Stem Cells and Regenerative Therapy with Bilateral Gracilis Flaps for Perineal Reconstruction of a Wound Infection in the Setting of Anal Squamous Cell Carcinoma. Adv. Skin. Wound Care 2023, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brunchukov, V.; Astrelina, T.; Usupzhanova, D.; Rastorgueva, A.; Kobzeva, I.; Nikitina, V.; Lishchuk, S.; Dubova, E.; Pavlov, K.; Brumberg, V.; et al. Evaluation of the Effectiveness of Mesenchymal Stem Cells of the Placenta and Their Conditioned Medium in Local Radiation Injuries. Cells 2020, 9, 2558. [Google Scholar] [CrossRef] [PubMed]
- Chapel, A. Stem Cells and Irradiation. Cells 2021, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wąsiatycz, G.; Antosik, P.; et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Helissey, C.; Guitard, N.; Théry, H.; Goulinet, S.; Mauduit, P.; Girleanu, M.; Favier, A.L.; Drouet, M.; Parnot, C.; Chargari, C.; et al. Two New Potential Therapeutic Approaches in Radiation Cystitis Derived from Mesenchymal Stem Cells: Extracellular Vesicles and Conditioned Medium. Biology 2022, 11, 980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beltrán-Camacho, L.; Rojas-Torres, M.; Durán-Ruiz, M.C. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. Int. J. Mol. Sci. 2021, 22, 2335. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.J.; Li, Y.; Gao, Y. Mesenchymal Stem Cells Decrease M1/M2 Ratio and Alleviate Inflammation to Improve Limb Ischemia in Mice. Med. Sci. Monit. 2020, 26, e923287. [Google Scholar] [CrossRef]
- Sokolova, I.B.; Gorshkova, O.P. Cell Therapy: A New Technology for Cerebral Circulation Restoration after Ischemia/Reperfusion. Acta Naturae 2023, 15, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Kraskiewicz, H.; Paprocka, M.; Bielawska-Pohl, A.; Krawczenko, A.; Panek, K.; Kaczyńska, J.; Szyposzyńska, A.; Psurski, M.; Kuropka, P.; Klimczak, A. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Res. Ther. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, C.W.; Kim, K.-S.; Bae, S.; Son, H.K.; Myung, P.-K.; Hong, H.J.; Kim, H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Najman, S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. Int. J. Mol. Sci. 2019, 20, 1671. [Google Scholar] [CrossRef] [PubMed]
- Bezenah, J.R.; Rioja, A.Y.; Juliar, B.; Friend, N.; Putnam, A.J. Assessing the ability of human endothelial cells derived from induced-pluripotent stem cells to form functional microvasculature in vivo. Biotechnol. Bioeng. 2019, 116, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Bezenah, J.R.; Kong, Y.P.; Putnam, A.J. Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Sci. Rep. 2018, 8, 2671. [Google Scholar] [CrossRef] [PubMed]
- Friend, N.E.; Beamish, J.A.; Margolis, E.A.; Schott, N.G.; Stegemann, J.P.; Putnam, A.J. Pre-cultured, cell-encapsulating fibrin microbeads for the vascularization of ischemic tissues. J. Biomed. Mater. Res. A 2023, 112, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Kniazeva, E.; Kachgal, S.; Putnam, A.J. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng. Part A 2011, 17, 905–914. [Google Scholar] [CrossRef]
- Müller-Seubert, W.; Ostermaier, P.; Horch, R.E.; Distel, L.; Frey, B.; Cai, A.; Arkudas, A. Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography. J. Pers. Med. 2022, 12, 237. [Google Scholar] [CrossRef]
- Müller-Seubert, W.; Ostermaier, P.; Horch, R.E.; Distel, L.; Frey, B.; Erber, R.; Arkudas, A. The Influence of Different Irradiation Regimens on Inflammation and Vascularization in a Random-Pattern Flap Model. J. Pers. Med. 2023, 13, 1514. [Google Scholar] [CrossRef] [PubMed]
- Thanik, V.D.; Chang, C.C.; Zoumalan, R.A.; Lerman, O.Z.; Allen, R.J.; Nguyen, P.D.; Warren, S.M.; Coleman, S.R.; Hazen, A. A novel mouse model of cutaneous radiation injury. Plast. Reconstr. Surg. 2011, 127, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Myers, B. Understanding flap necrosis. Plast. Reconstr. Surg. 1986, 78, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Han, T.T.Y.; Flynn, L.E. Perfusion bioreactor culture of human adipose-derived stromal cells on decellularized adipose tissue scaffolds enhances in vivo adipose tissue regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1827–1840. [Google Scholar] [CrossRef]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Wan Safwani, W.K. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Monaci, S.; Coppola, F.; Filippi, I.; Falsini, A.; Carraro, F.; Naldini, A. Targeting hypoxia signaling pathways in angiogenesis. Front. Physiol. 2024, 15, 1408750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reichenberger, M.A.; Mueller, W.; Schäfer, A.; Heimer, S.; Leimer, U.; Lass, U.; Germann, G.; Köllensperger, E. Fibrin-embedded adipose derived stem cells enhance skin flap survival. Stem Cell Rev. Rep. 2012, 8, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, J.; Putnam, A.J. Sculpting the blank slate: How fibrin’s support of vascularization can inspire biomaterial design. Acta Biomater. 2014, 10, 1515–1523. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Casado, J.G.; Abellán, E.; Vela, F.J.; Álvarez, V.; Usón, A.; Blázquez, R.; Sánchez-Margallo, F.M.; Ballestín, A. Adipose-Derived Stem Cells Ameliorate Ischemia-Reperfusion Injury in a Rat Skin Free Flap Model. J. Reconstr. Microsurg. 2018, 34, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, C.; Li, Y.; Sun, Q.; Li, G.; Wang, D. Effects of human adipose-derived stem cells on the viability of rabbit random pattern flaps. Cytotherapy 2014, 16, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Foroglou, P.; Demiri, E.; Koliakos, G.; Karathanasis, V. Autologous administration of adipose stromal cells improves skin flap survival through neovascularization: An experimental study. Int. Wound J. 2019, 16, 1471–1476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, W.; Qiao, X.; Ma, S.; Cui, L. Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1α. J. Cell. Mol. Med. 2011, 15, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sasaki, M.M.; Kataoka-Sasaki, Y.M.; Yotsuyanagi, T.M.; Radtke, C.M.; Kocsis, J.D.; Honmou, O.M. Intravenous Infusion of Mesenchymal Stem Cells Promotes the Survival of Random Pattern Flaps in Rats. Plast. Reconstr. Surg. 2021, 148, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Steingen, C.; Brenig, F.; Baumgartner, L.; Schmidt, J.; Schmidt, A.; Bloch, W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell. Cardiol. 2008, 44, 1072–1084. [Google Scholar] [CrossRef]
- Lee, D.W.; Jeon, Y.R.; Cho, E.J.; Kang, J.H.; Lew, D.H. Optimal administration routes for adipose-derived stem cells therapy in ischaemic flaps. J. Tissue Eng. Regen. Med. 2014, 8, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Rückert, M.; Deloch, L.; Frey, B.; Schlücker, E.; Fietkau, R.; Gaipl, U.S. Combinations of Radiotherapy with Vaccination and Immune Checkpoint Inhibition Differently Affect Primary and Abscopal Tumor Growth and the Tumor Microenvironment. Cancers 2021, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Rzeszowska-Wolny, J.; Przybyszewski, W.M.; Widel, M. Ionizing radiation-induced bystander effects, potential targets for modulation of radiotherapy. Eur. J. Pharmacol. 2009, 625, 156–164. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects: Past history and future directions. Radiat. Res. 2001, 155, 759–767. [Google Scholar] [CrossRef]


| Group | Right Flap | Left Flap |
|---|---|---|
| 1 | 20 Gy IR + ASC | ASC |
| 2 | 20 Gy IR + ASC + HUVEC | ASC + HUVEC |
| 3 | 20 Gy IR + MSC | MSC |
| 4 | 20 Gy IR + MSC + HUVEC | MSC + HUVEC |
| Staining | PCR | |
|---|---|---|
| Right/left flap | Cranial lateral third | Cranial medial third |
| Gene | 5′-3′ Primer Sequence |
|---|---|
| GAPDH | For: GAAGGTCGGTGTGAACGGAT Rev: TGAACTTGCCGTGGGTAGAG |
| Interleukin 6 | For: GACTTCCAGCCAGTTGCCTT Rev: GCAGTGGCTGTCAACAACAT |
| HIF-1α | For: GCAACTGCCACCACTGATGA Rev: GCTGTCCGACTGTGAGTACC |
| VEGF | For: AATGATGAAGCCCTGGAGTG Rev: ATGCTGCAGGAAGCTCATCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Seubert, W.; Fuchs, L.; Horch, R.E.; Distel, L.; Frey, B.; Renno, I.; Erber, R.; Arkudas, A. Application of Stem Cells Shows Antiinflammatory Effect in an Irradiated Random Pattern Flap Model. J. Pers. Med. 2024, 14, 554. https://doi.org/10.3390/jpm14060554
Müller-Seubert W, Fuchs L, Horch RE, Distel L, Frey B, Renno I, Erber R, Arkudas A. Application of Stem Cells Shows Antiinflammatory Effect in an Irradiated Random Pattern Flap Model. Journal of Personalized Medicine. 2024; 14(6):554. https://doi.org/10.3390/jpm14060554
Chicago/Turabian StyleMüller-Seubert, Wibke, Lena Fuchs, Raymund E. Horch, Luitpold Distel, Benjamin Frey, Isabell Renno, Ramona Erber, and Andreas Arkudas. 2024. "Application of Stem Cells Shows Antiinflammatory Effect in an Irradiated Random Pattern Flap Model" Journal of Personalized Medicine 14, no. 6: 554. https://doi.org/10.3390/jpm14060554





