Symptom Clusters in Acute SARS-CoV-2 Infection and Long COVID Fatigue in Male and Female Outpatients
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.1.1. Data Collection
2.1.2. Fatigue Assessment
2.2. Statistical Analysis
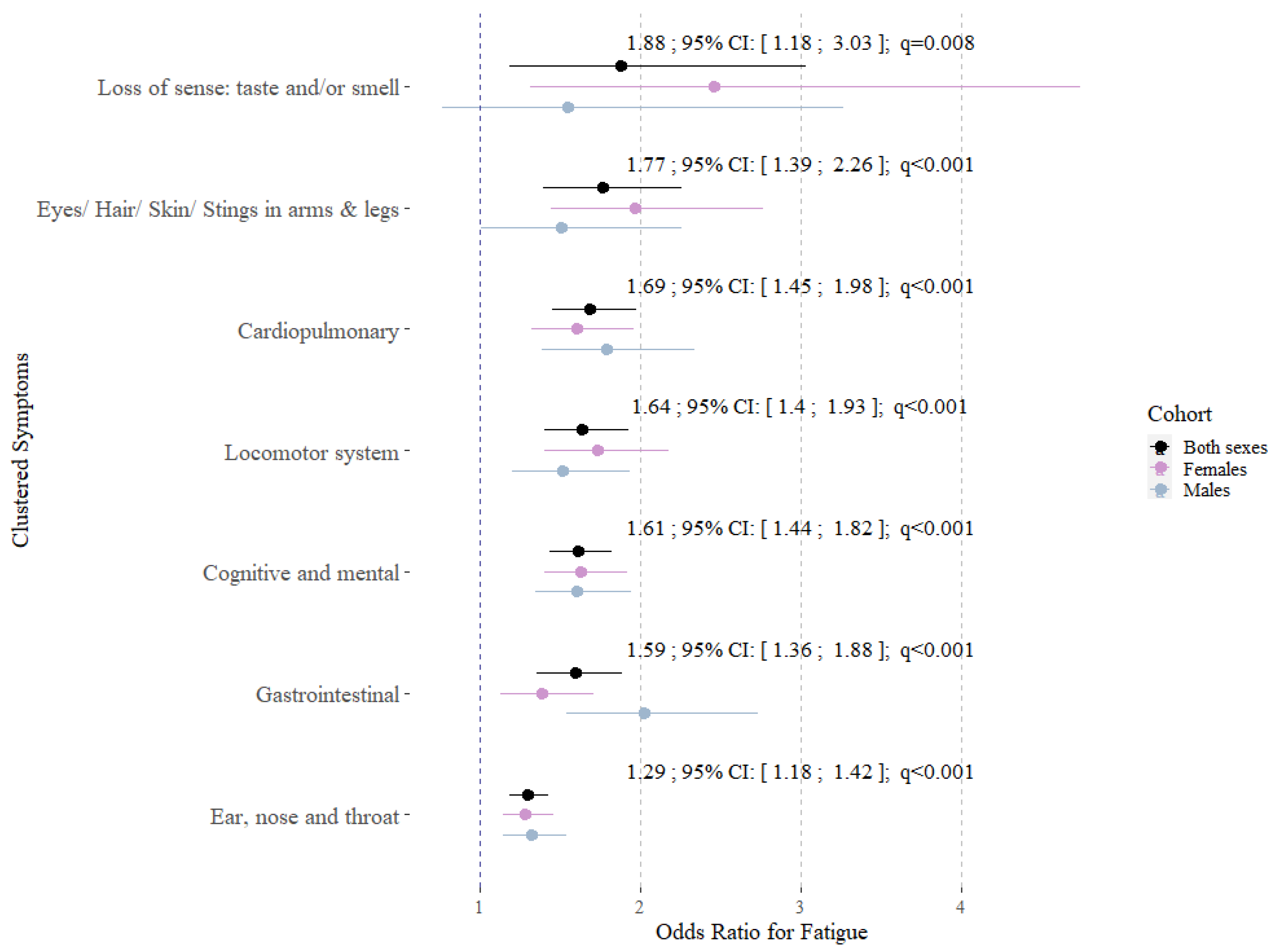
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| CFS | Chronic Fatigue Syndrome |
| ME | Myalgic encephalomyelitis |
| COVID-T | COVID thrombosis study |
| BMI | Body mass index |
| FAS | Fatigue Assessment Scale |
| VR-12 | Validated Veterans RAND 12-Item Health Survey |
| MCS-12 | Mental Component Summary Scale |
| PCS-12 | Physical Component Summary Scale |
| IQR | Interquartile range |
| OR | Odds ratio |
| CI | Confidence interval |
| FDR | False discovery rate |
References
- Koczulla, A.; Ankermann, T.; Behrends, U.; Berlit, P.; Brinkmann, F.; Frank, U.; Glöckl, R.; Gogoll, C.; Häuser, W.; Hohberger, B.; et al. S1-Leitlinie Long/Post-COVID [S1 Guideline]. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) e. V.; 2023. Available online: https://register.awmf.org/de/leitlinien/detail/020-027 (accessed on 3 May 2023).
- RKI Hinweise zur Testung von Patientinnen und Patienten auf SARS-CoV-2: Robert Koch-Institut; 2023. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Vorl_Testung_nCoV.html?nn=13490888#doc13490982bodyText1 (accessed on 20 December 2023).
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2023, 55, 101959. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- The National Academies Collection: Reports funded by National Institutes of Health. In Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Board on the Health of Select Populations, Institute of Medicine, National Academies Press (US): Washington, DC, USA, 2015.
- Norheim, K.B.; Jonsson, G.; Omdal, R. Biological mechanisms of chronic fatigue. Rheumatology 2011, 50, 1009–1018. [Google Scholar] [CrossRef]
- Gottschalk, C.G.; Peterson, D.; Armstrong, J.; Knox, K.; Roy, A. Potential molecular mechanisms of chronic fatigue in long haul COVID and other viral diseases. Infect. Agents Cancer 2023, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- González-Hermosillo, J.A.; Martínez-López, J.P.; Carrillo-Lampón, S.A.; Ruiz-Ojeda, D.; Herrera-Ramírez, S.; Amezcua-Guerra, L.M.; Martínez-Alvarado, M.d.R. Post-Acute COVID-19 Symptoms, a Potential Link with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A 6-Month Survey in a Mexican Cohort. Brain Sci. 2021, 11, 760. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Myalgic Encephalomyelitis (or Encephalopathy)/Chronic Fatigue Syndrome: Diagnosis and Management. In National Institute for Health and Care Excellence: Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2021. [Google Scholar]
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Prevalence and Determinants of Fatigue after COVID-19 in Non-Hospitalized Subjects: A Population-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 2030. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- WHO. COVID-19 Weekly Epidemiological Update: World Health Organization. 2021. Available online: https://iris.who.int/bitstream/handle/10665/341329/CoV-weekly-sitrep11May21-eng.pdf?sequence=1 (accessed on 11 May 2021).
- Sabes-Figuera, R.; McCrone, P.; Hurley, M.; King, M.; Donaldson, A.N.; Ridsdale, L. The hidden cost of chronic fatigue to patients and their families. BMC Health Serv. Res. 2010, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Schmidbauer, L.; Kirchberger, I.; Goßlau, Y.; Warm, T.D.; Hyhlik-Dürr, A.; Linseisen, J.; Meisinger, C. The association between the number of symptoms and the severity of Post-COVID-Fatigue after SARS-CoV-2 infection treated in an outpatient setting. J. Neurol. 2023, 270, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Michielsen, H.J.; Van Heck, G.L. Assessment of fatigue among working people: A comparison of six questionnaires. Occup. Environ. Med. 2003, 60, i10–i15. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, H.J.; De Vries, J.; Van Heck, G.L. Psychometric qualities of a brief self-rated fatigue measure. J. Psychosom. Res. 2003, 54, 345–352. [Google Scholar] [CrossRef] [PubMed]
- How to Use the Fatigue Assessment Scale (FAS)?: Interstitial Lung Disease Care Foundation. 2015. Available online: https://www.ildcare.nl/index.php/how-to-use-the-fas-fatigue-assessment-scale/ (accessed on 9 September 2022).
- Fernández-de-las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, Ó.J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Castro, A.W.; Ferretti, M.T.; Martinkova, J.; Vasilevskaya, A.; Santuccione Chadha, A.; Tartaglia, M.C. Sex and gender differences in the neurological and neuropsychiatric symptoms of long COVID: A narrative review. Ital. J. Gend. -Specif. Med. 2022, 8, 18–28. [Google Scholar]
- Sylvester, S.V.; Rusu, R.; Chan, B.; Bellows, M.; O’Keefe, C.; Nicholson, S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: A review. Curr. Med. Res. Opin. 2022, 38, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Catalfamo, C.J.; Farland, L.V.; Ernst, K.C.; Jacobs, E.T.; Klimentidis, Y.C.; Jehn, M.; Pogreba-Brown, K. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PLoS ONE 2021, 16, e0254347. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.D.M.; Foppiani, A.; Peretti, G.M.; Mangiavini, L.; Battezzati, A.; Bertoli, S.; Martinelli Boneschi, F.; Zuccotti, G.V. Long-Term Coronavirus Disease 2019 Complications in Inpatients and Outpatients: A One-Year Follow-up Cohort Study. Open Forum Infect. Dis. 2021, 8, ofab384. [Google Scholar] [CrossRef]
- Huang, Y.; Pinto, M.D.; Borelli, J.L.; Asgari Mehrabadi, M.; Abrahim, H.L.; Dutt, N.; Lambert, N.; Nurmi, E.L.; Chakraborty, R.; Rahmani, A.M.; et al. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler Looking for Clarity in the Haze of the Pandemic. Clin. Nurs. Res. 2022, 31, 1390–1398. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Martín-Guerrero, J.D.; Florencio, L.L.; Navarro-Pardo, E.; Rodríguez-Jiménez, J.; Torres-Macho, J.; Pellicer-Valero, O.J. Clustering analysis reveals different profiles associating long-term post-COVID symptoms, COVID-19 symptoms at hospital admission and previous medical co-morbidities in previously hospitalized COVID-19 survivors. Infection 2023, 51, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.; Elliott, J.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Cooke, G.; Ward, H.; Elliott, P. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people. medRxiv 2021. medRxiv:2021.06.28.21259452. [Google Scholar]
- Dixon, B.E.; Wools-Kaloustian, K.K.; Fadel, W.F.; Duszynski, T.J.; Yiannoutsos, C.; Halverson, P.K.; Menachemi, N. Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: Results from a statewide epidemiological study. PLoS ONE 2021, 16, e0241875. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Lee, K.A.; Ni Lochlainn, M.; Varsavsky, T.; Murray, B.; Graham, M.S.; Menni, C.; Modat, M.; Bowyer, R.C.E.; Nguyen, L.H.; et al. Symptom clusters in COVID-19: A potential clinical prediction tool from the COVID Symptom Study app. Sci. Adv. 2021, 7, eabd4177. [Google Scholar] [CrossRef]
- Molina-Mora, J.A.; González, A.; Jiménez-Morgan, S.; Cordero-Laurent, E.; Brenes, H.; Soto-Garita, C.; Sequeira-Soto, J.; Duarte-Martínez, F. Clinical Profiles at the Time of Diagnosis of SARS-CoV-2 Infection in Costa Rica During the Pre-vaccination Period Using a Machine Learning Approach. Phenomics 2022, 2, 312–322. [Google Scholar] [CrossRef]
- RKI. SARS-CoV-2: Virologische Basisdaten Sowie Virusvarianten im Zeitraum von 2020–2022 Robert Koch-Institut (RKI). 2023. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Virologische_Basisdaten.html (accessed on 21 September 2023).

| N | Sex | p-Value 1 | ||
|---|---|---|---|---|
| Male, N = 205 | Female, N = 245 | |||
| Age (years) | 450 | 0.07 | ||
| Median (IQR) | 51.0 (36.0; 60.0) | 48.0 (33.0; 57.0) | ||
| Range | 18.0; 87.0 | 18.0; 86.0 | ||
| Body mass index (kg/m2) | 449 | <0.001 | ||
| Median (IQR) | 26.2 (24.0; 29.0) | 23.8 (21.5; 28.0) | ||
| Range | 17.5; 53.7 | 15.2; 49.6 | ||
| Missing | 1 | 0 | ||
| Smoking status | 450 | 0.005 | ||
| Never smoked | 91 (44.39%) | 146 (59.59%) | ||
| Ex-smoker | 92 (44.88%) | 78 (31.84%) | ||
| Current smoker | 22 (10.73%) | 21 (8.57%) | ||
| Time since acute infection (days) | 450 | 0.84 | ||
| Median (IQR) | 244.0 (143.0; 322.0) | 238.0 (123.0; 331.0) | ||
| Range | 32.0; 436.0 | 31.0; 441.0 | ||
| History of depression | 449 | 0.07 | ||
| Yes | 12 (5.88%) | 26 (10.61%) | ||
| No | 192 (94.12%) | 219 (89.39%) | ||
| Missing | 1 | 0 | ||
| History of anxiety | 448 | 0.43 | ||
| Yes | 9 (4.43%) | 15 (6.12%) | ||
| No | 194 (95.57%) | 230 (93.88%) | ||
| Missing | 2 | 0 | ||
| Fatigue | 447 | <0.001 | ||
| No fatigue | 152 (75.25%) | 135 (55.10%) | ||
| Fatigue | 50 (24.75%) | 110 (44.90%) | ||
| Missing | 3 | 0 | ||
| Fatigue Assessment Scale (FAS)—Total Score | 447 | <0.001 | ||
| Median (IQR) | 18.0 (15.0; 21.0) | 20.0 (16.0; 26.0) | ||
| Range | 10.0; 43.0 | 10.0; 46.0 | ||
| Missing | 3 | 0 | ||
| Symptom clusters | ||||
| Loss of sense: taste and/or smell | 450 | 0.002 | ||
| Loss of sense: taste and/or smell | 124 (60.49%) | 181 (73.88%) | ||
| No loss of sense | 81 (39.51%) | 64 (26.12%) | ||
| Locomotor system | 450 | 0.13 | ||
| Median (IQR) | 1 (0.0; 2.0) | 2 (1.0; 3.0) | ||
| Gastrointestinal | 450 | <0.001 | ||
| Median (IQR) | 1 (0.0; 2.0) | 1 (1.0; 2.0) | ||
| Ear, nose and throat | 450 | 0.22 | ||
| Median (IQR) | 4 (3.0; 6.0) | 5 (3.0; 6.0) | ||
| Cardiopulmonary | 450 | <0.001 | ||
| Median (IQR) | 1 (0.0; 2.0) | 2 (0.0; 3.0) | ||
| Cognitive and mental | 450 | <0.001 | ||
| Median (IQR) | 3 (1.0; 4.0) | 3 (2.0; 5.0) | ||
| Eyes/Hair/Skin/Stings in arms and legs | 450 | 0.021 | ||
| Median (IQR) | 0 (0.0; 1.0) | 0 (0.0; 1.0) | ||
| Symptom Clusters of Acute SARS-CoV-2 Infection | |||||||
|---|---|---|---|---|---|---|---|
| Loss of Sense: Taste and/or Smell | Ear, Nose and Throat | Cardiopulmonary | Cognitive and Mental | Locomotor System | Gastrointestinal | Eyes/Hair/Skin/Stings in Arms and Legs | |
| Characteristic | Binary | Continuous | Continuous | Continuous | Continuous | Continuous | Continuous |
| Range | 0;1 a | 0–10 | 0–5 | 0–8 | 0–5 | 0–6 | 0–6 |
| Symptoms | Loss of sense of taste | Stuffy Nose | Shortness of breath at rest | Tiredness or exhaustion | Dizziness | Loss of appetite | Watering eyes |
| Loss of sense of smell | Rhinitis or runny nose | Shortness of breath under exertion | Sleepiness | Problems with the coordination of body movements | Nausea or vomiting | Impaired vision | |
| Sore throat or pharyngeal pain | Feeling of pressure or pain in the chest | Concentration difficulties | Muscular weakness | Acid reflux | Red eyes or conjunctivitis | ||
| Cough | Cyanotic lips | Sleep disorders | Muscular stiffness | Abdominal pain | Feeling of pinpricks in arms and legs | ||
| Headache | Strong palpitations | Memory problems | Muscle or joint pain | Diarrhea | Loss of hair | ||
| Hemoptysis | Depressed mood | Flatulence | Eczema | ||||
| Increased body temperature | Anxiety or panic | ||||||
| Fever | Mood swings | ||||||
| Chills and shivering | |||||||
| Pain when swallowing | |||||||
| Characteristics 1 | Percentage Change of the ß Estimate | ß Estimate | 95% CI 2 | p-Value | q-Value 3 |
|---|---|---|---|---|---|
| Clustered symptoms: Loss of sense: taste and/or smell | |||||
| Yes 4 | 2.02 | 0.02 | −0.04; 0.08 | 0.55 | 0.77 |
| Clustered symptoms: Ear, nose and throat | 0 | 0 | −0.01; 0.02 | 0.66 | 0.77 |
| Clustered symptoms: Cardiopulmonary | 3.05 | 0.03 | 0.01; 0.06 | 0.018 | 0.08 |
| Clustered symptoms: Cognitive and mental | 5.13 | 0.05 | 0.04; 0.07 | <0.001 | <0.001 |
| Clustered symptoms: Locomotor system | 0 | 0 | −0.03; 0.03 | 0.96 | 0.96 |
| Clustered symptoms: Gastrointestinal | 1.01 | 0.01 | −0.02; 0.03 | 0.61 | 0.77 |
| Clustered symptoms: Eyes/Hair/Skin/Stings in arms and legs | 3.05 | 0.03 | 0.00; 0.07 | 0.08 | 0.16 |
| Characteristics 1 | OR 2 | 95% CI 3 | p-Value | q-Value 4 |
|---|---|---|---|---|
| Clustered symptoms: Loss of sense: taste and/or smell | ||||
| Yes 5 | 1.43 | 0.85; 2.41 | 0.18 | 0.48 |
| Clustered symptoms: Ear, nose and throat | 1.02 | 0.90; 1.15 | 0.73 | 0.86 |
| Clustered symptoms: Cardiopulmonary | 1.22 | 0.99; 1.51 | 0.060 | 0.21 |
| Clustered symptoms: Cognitive and mental | 1.42 | 1.23; 1.66 | <0.001 | <0.001 |
| Clustered symptoms: Locomotor system | 0.99 | 0.79; 1.23 | 0.92 | 0.92 |
| Clustered symptoms: Gastrointestinal | 1.13 | 0.92; 1.40 | 0.24 | 0.48 |
| Clustered symptoms: Eyes/Hair/Skin/Stings in arms and legs | 1.10 | 0.82; 1.47 | 0.53 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, V.; Freuer, D.; Goßlau, Y.; Kirchberger, I.; Warm, T.; Hyhlik-Dürr, A.; Meisinger, C.; Linseisen, J. Symptom Clusters in Acute SARS-CoV-2 Infection and Long COVID Fatigue in Male and Female Outpatients. J. Pers. Med. 2024, 14, 602. https://doi.org/10.3390/jpm14060602
Leone V, Freuer D, Goßlau Y, Kirchberger I, Warm T, Hyhlik-Dürr A, Meisinger C, Linseisen J. Symptom Clusters in Acute SARS-CoV-2 Infection and Long COVID Fatigue in Male and Female Outpatients. Journal of Personalized Medicine. 2024; 14(6):602. https://doi.org/10.3390/jpm14060602
Chicago/Turabian StyleLeone, Vincenza, Dennis Freuer, Yvonne Goßlau, Inge Kirchberger, Tobias Warm, Alexander Hyhlik-Dürr, Christine Meisinger, and Jakob Linseisen. 2024. "Symptom Clusters in Acute SARS-CoV-2 Infection and Long COVID Fatigue in Male and Female Outpatients" Journal of Personalized Medicine 14, no. 6: 602. https://doi.org/10.3390/jpm14060602





