Role of Autologous Micro-Fragmented Adipose Tissue in Osteoarthritis Treatment
Abstract
1. Introduction
2. Materials and Methods
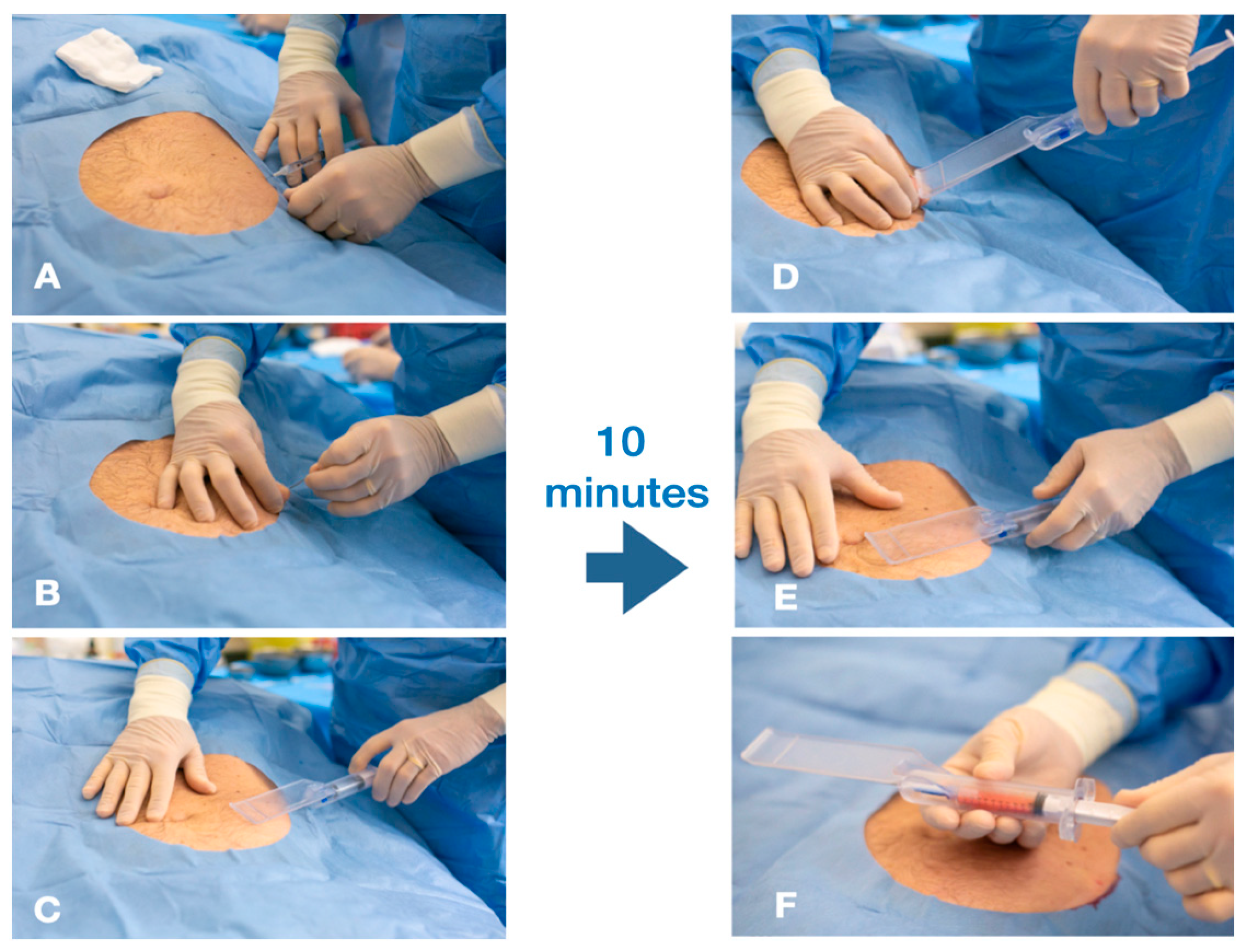
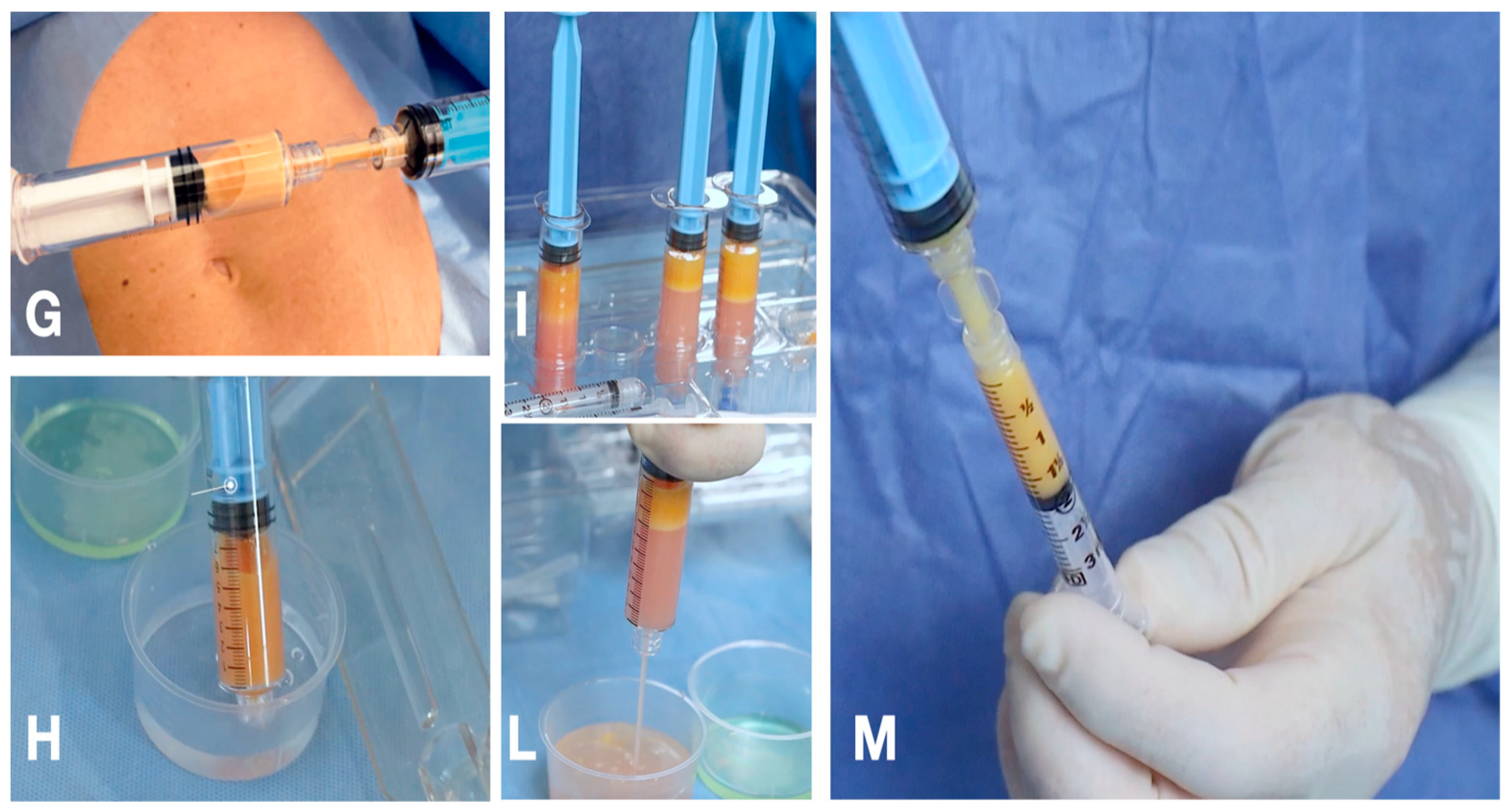
2.1. Technique
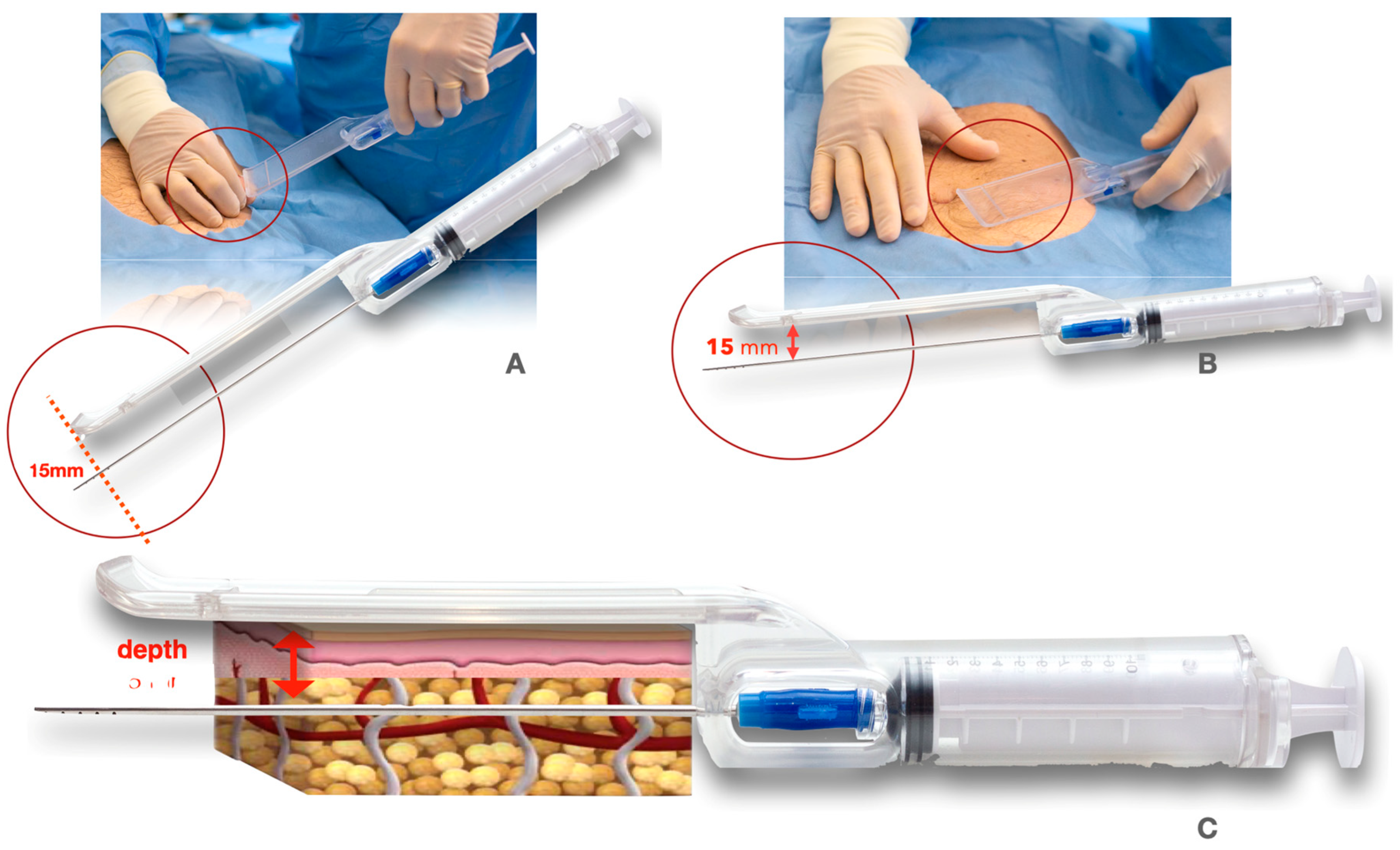
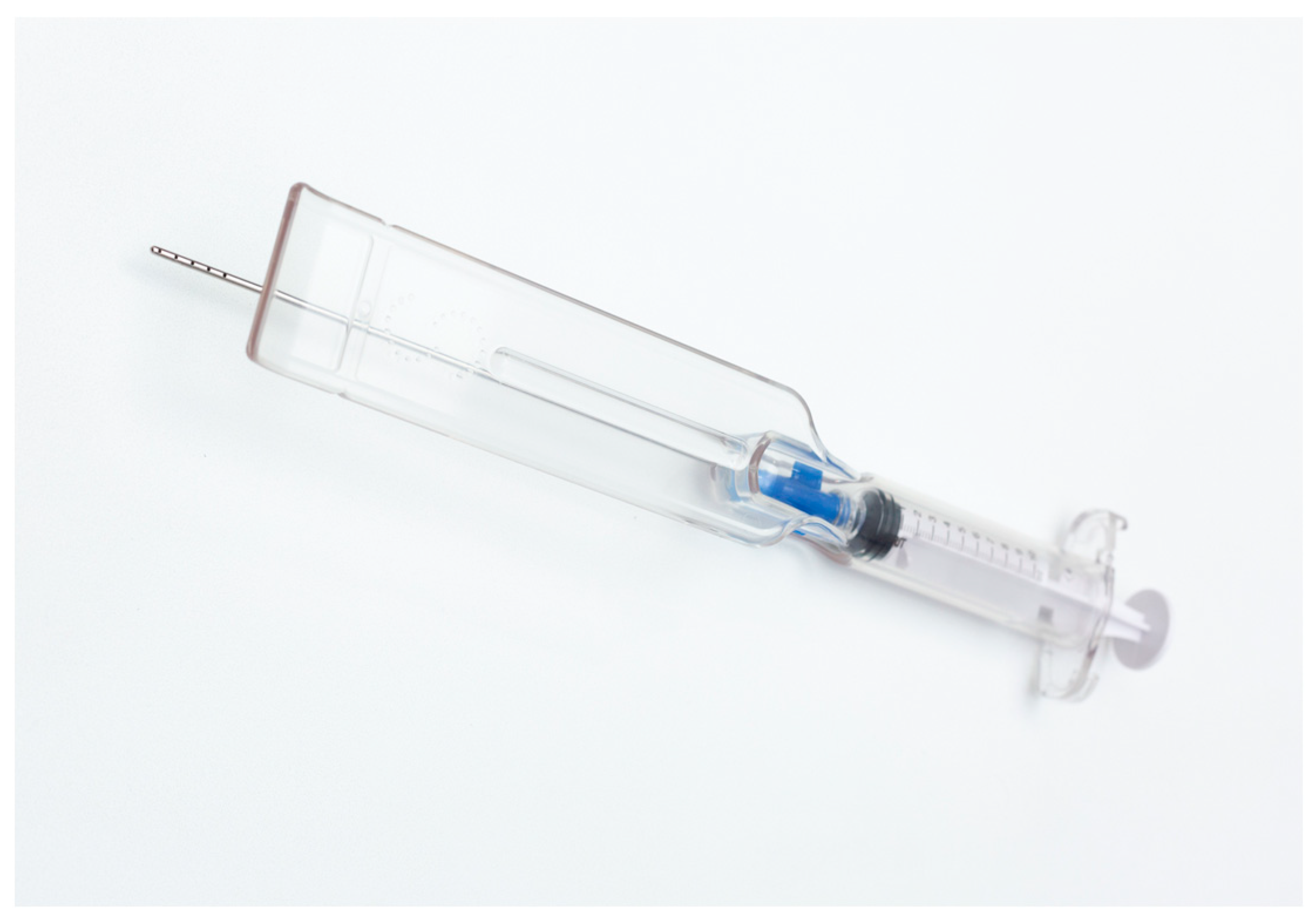
2.1.1. The Guided SEFFI Device and Procedure
2.1.2. Postoperative Indications and Evaluation
2.2. Statistical Analysis
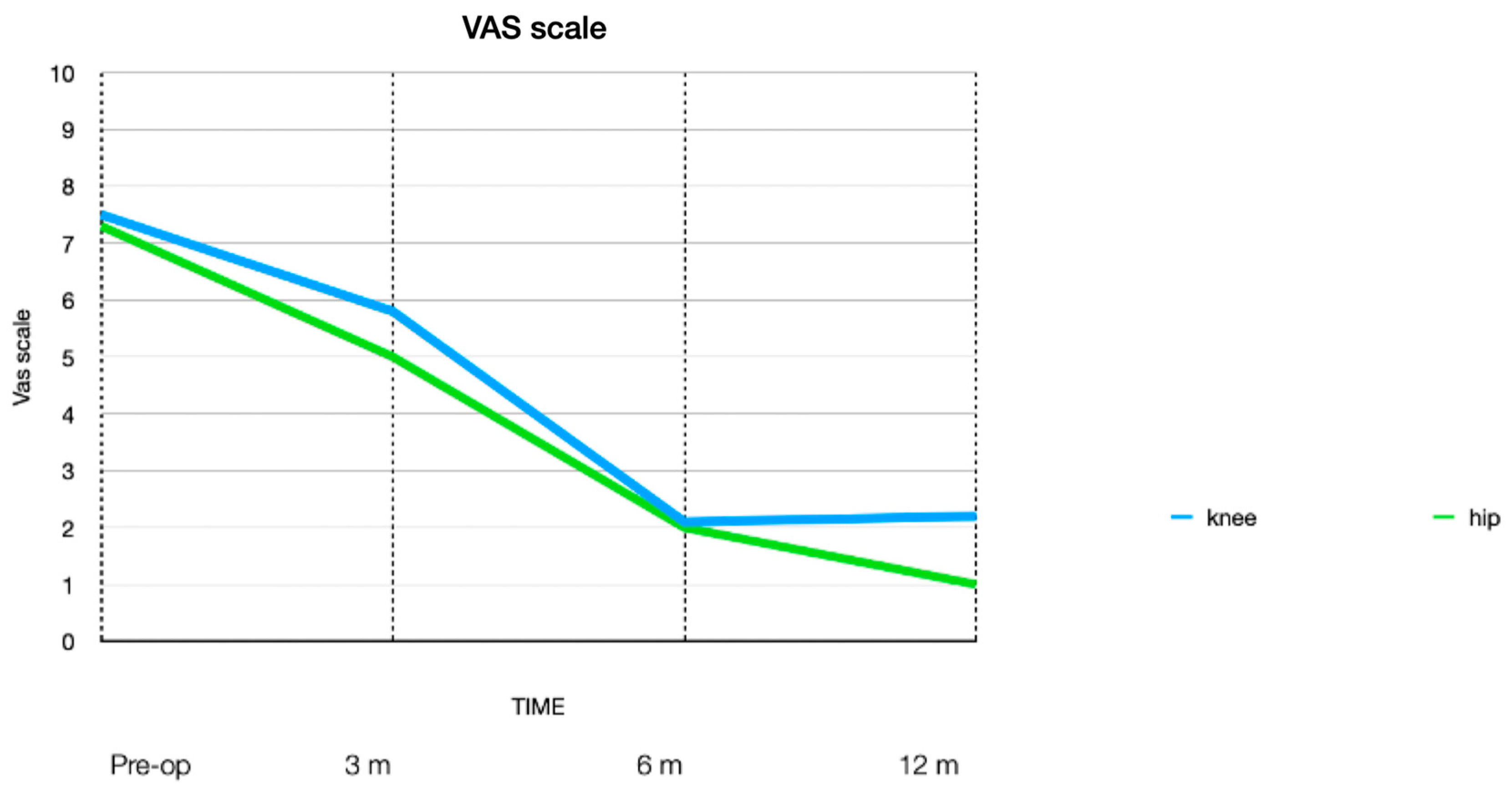
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buckwalter, J.A.; Martin, J.A. Osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 150–167. [Google Scholar] [CrossRef]
- Dunlop, D.D.; Manheim, L.M.; Yelin, E.H.; Song, J.; Chang, R.W. The costs of arthritis. Arthritis Rheum. 2003, 49, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Moshref, S.S.; Jamal, Y.S.; Al-Hibshi, A.M.; Kaki, A.M. The Regenerative Effect of Intra-Articular Injection of Autologous Fat Micro-Graft in Treatment of Chronic Knee Osteoarthritis. In Tibia Pathology and Fractures; IntechOpen: London, UK, 2019. [Google Scholar]
- Mordin, M.; Parrish, W.; Masaquel, C.; Bisson, B.; Copley-Merriman, C. Intra-articular Hyaluronic Acid for Osteoarthritis of the Knee in the United States: A Systematic Review of Economic Evaluations. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2021, 14, 11795441211047284. [Google Scholar] [CrossRef]
- Coleman, C.M.; Curtin, C.; Barry, F.P.; O’Flatharta, C.; Murphy, J.M. Mesenchymal stem cells and osteoarthritis: Remedy or accomplice? Hum. Gene Ther. 2010, 21, 1239–1250. [Google Scholar] [CrossRef]
- Peeters, C.M.M.; Leijs, M.J.C.; Reijman, M.; van Osch, G.J.V.M.; Bos, P.K. Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: A systematic literature review. Osteoarthr. Cartil. 2013, 21, 1465–1473. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Zuk, P.A. The adipose-derived stem cell: Looking back and looking ahead. Mol. Biol. Cell 2010, 21, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Tallone, T.; Realini, C.; Bohmler, A.; Kornfeld, C.; Vassalli, G.; Moccetti, T.; Bardelli, S.; Soldati, G. Adult human adipose tissue contains several types of multipotent cells. J. Cardiovasc. Transl. Res. 2011, 4, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.I.; Beanes, S.R.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P. Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast. Reconstr. Surg. 2002, 109, 1033–1041; discussion 1042–1043. [Google Scholar] [CrossRef]
- Fraser, J.K.; Schreiber, R.; Strem, B.; Zhu, M.; Alfonso, Z.; Wulur, I.; Hedrick, M.H. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3 (Suppl. S1), S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Brückl, R.; Hepp, W.; Tönnis, D. Differentiation of normal and dysplastic juvenile hip joints by means of the summarized hip factor. Arch Orthop Unfallchir. 1971, 74, 13–32. (In German) [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, B.; Bremjit, P.; Fernando, N. Classifications in Brief: Tönnis Classification of Hip Osteoarthritis. Clin. Orthop. Relat. Res. 2018, 476, 1680–1684. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.; Kelly, D.; Goldstone, L.; Gammon, J. Examining the validity of pressure ulcer risk assessment scales: Developing and using illustrated patient simulations to collect the data INFORMATION POINT: Visual Analogue Scale. J. Clin. Nurs. 2001, 10, 697–706. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2702.2001.00525.x (accessed on 28 May 2024). [CrossRef] [PubMed]
- Trivisonno, A.; Di Rocco, G.; Cannistra, C.; Finocchi, V.; Torres Farr, S.; Monti, M.; Toietta, G. Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet.Surg. J. 2014, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgro, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardinin, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and quantitative differences of adipose- derived stromal cells from superficial and deep subcutaneous lipoaspirates: A matter of fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef]
- Gennai, A.; Bernardini, F.P. Superficial enhanced fluid fat injection (SEFFI and MicroSEFFI) in facial rejuvenation. CellR4 2017, 5, e2239. [Google Scholar]
- Bernardini, F.P.; Gennai, A.; Izzo, L.; Zambelli, A.; Repaci, E.; Baldelli, I.; Fratertnali-Orcioni, G.; Hartstein, M.E.; Santi, P.L.; Quarto, R. Superficial Enhanced Fluid Fat Injection (SEFFI) to correct volume defects and skin aging of the face and periocular region. Aesthet. Surg. J. 2015, 35, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, F.P.; Gennai, A. Fluid fat injection for volume restoration and skin regeneration of the periocular aesthetic unit. JAMA Facial Plast. Surg. 2016, 18, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Gennai, A.; Bovani, B.; Colli, M.; Melfa, F.; Piccolo, D.; Russo, R.; Roda, B.; Zattoni, A.; Reschiglian, P.; Zia, S. Comparison of Harvesting and Processing Technique for Adipose Tissue Graft: Evaluation of Cell Viability. Int. J. Regenr. Med. 2021, 4, 2–5. [Google Scholar] [CrossRef]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and Enzymatic Procedures to Isolate the Stromal Vascular Fraction from Adipose Tissue: Preliminary Results. Front. Cell Dev. Biol. 2019, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Cucchiani, R.; Corrales, L. The Effects of Fat Harvesting and Preparation, Air Exposure, Obesity, and Stem Cell Enrichment on Adipocyte Viability Prior to Graft Transplantation. Aesthetic Surg. J. 2016, 35, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Ware, J., Jr.; Kosinski, M.; Keller, S. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, K.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Layte, R.; Jenkinson, D.; Lawrence, K.; Peterson, S.; Paice, C.; Stradling, J. A shorter form health survey: Can the SF-12 replicate results from the SF-36 in longitudinal studies? J. Public Health 1997, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Keller, S.D.; Kosinski, M. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales; Health Institute, New England Medical Center: Boston, MA, USA, 1995. [Google Scholar]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Thoene, M.; Bejer-Olenska, E.; Wojtkiewicz, J. The Current State of Osteoarthritis Treatment Options Using Stem Cells for Regenerative Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 8925. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Yang, L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 2022, 80, 101684. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Entessari, M.; Oliveira, L.P. Current evidence on mesenchymal stem cells for hip osteoarthritis: A narrative review. Regen. Med. 2023, 18, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Bao, R. Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. Int. J. Mol. Sci. 2022, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
| Grade | Radiographic Features |
|---|---|
| 0 | - no signs of osteoarthritis |
| 1 | - slight narrowing of joint space |
| - slight lipping at joint margin | |
| - slight sclerosis of the femoral head or acetabulum | |
| 2 | - small cysts in the femoral head or acetabulum |
| - increasing narrowing of joint space | |
| - moderate loss of sphericity of the femoral head | |
| 3 | - large cysts |
| - severe narrowing of joint space | |
| - severe deformity of the femoral head | |
| - avascular necrosis |
| Grade | Radiologic Findings |
|---|---|
| 0 | no radiological findings of osteoarthritis |
| I | doubtful narrowing of joint space and possible osteophytic lipping |
| II | definite osteophytes and possible narrowing of joint space |
| III | moderate multiple osteophytes, definite narrowing of joint space, small pseudocystic area with sclerotic walls and possible deformity of bone contour |
| IV | large osteophytes, marked narrowing of the joint space, severe sclerosis and definite deformity of bone contour |
| N | Improvement ROM (or Stiffness) Post Operative (n) and VAS | |||||
|---|---|---|---|---|---|---|
| >ROM 3-Month | <Stiffness 3-Month | VAS 3-Month | VAS 6-Month | VAS-pre | ||
| Knee patients Tot 190 | ||||||
| KL grade 1 | 84 | 78 | 78 | 5.7 | 1.9 | 7.5 |
| KL grade 2 | 101 | 61 | 61 | 5.9 | 2.2 | 7.5 |
| KL grade 3 | 4 | 0 | 0 | 5.9 | 2.3 | 8 |
| KL grade 4 | 1 | 0 | 0 | 6 | 2.5 | 8.5 |
| Hip patients Tot 60 | ||||||
| Tonnis grade 1 | 29 | 26 | 26 | 5.3 | 2.0 | 7.1 |
| Tonnis grade 2 | 31 | 19 | 19 | 5.3 | 2.2 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trentani, P.; Meredi, E.; Zarantonello, P.; Gennai, A. Role of Autologous Micro-Fragmented Adipose Tissue in Osteoarthritis Treatment. J. Pers. Med. 2024, 14, 604. https://doi.org/10.3390/jpm14060604
Trentani P, Meredi E, Zarantonello P, Gennai A. Role of Autologous Micro-Fragmented Adipose Tissue in Osteoarthritis Treatment. Journal of Personalized Medicine. 2024; 14(6):604. https://doi.org/10.3390/jpm14060604
Chicago/Turabian StyleTrentani, Paolo, Elena Meredi, Paola Zarantonello, and Alessandro Gennai. 2024. "Role of Autologous Micro-Fragmented Adipose Tissue in Osteoarthritis Treatment" Journal of Personalized Medicine 14, no. 6: 604. https://doi.org/10.3390/jpm14060604
APA StyleTrentani, P., Meredi, E., Zarantonello, P., & Gennai, A. (2024). Role of Autologous Micro-Fragmented Adipose Tissue in Osteoarthritis Treatment. Journal of Personalized Medicine, 14(6), 604. https://doi.org/10.3390/jpm14060604







