Ultrasonographic Synovitis Is Associated with the Development of Joint Destruction in Patients with Psoriatic Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Measures
2.3. US and Clinical Assessments of Joints and Enthesitis
2.4. Evaluation of the Relationships between US/Clinical Arthritis and Enthesitis and Progression in Joint Destruction
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Prevalence of US/Clinical Arthritis and Enthesitis
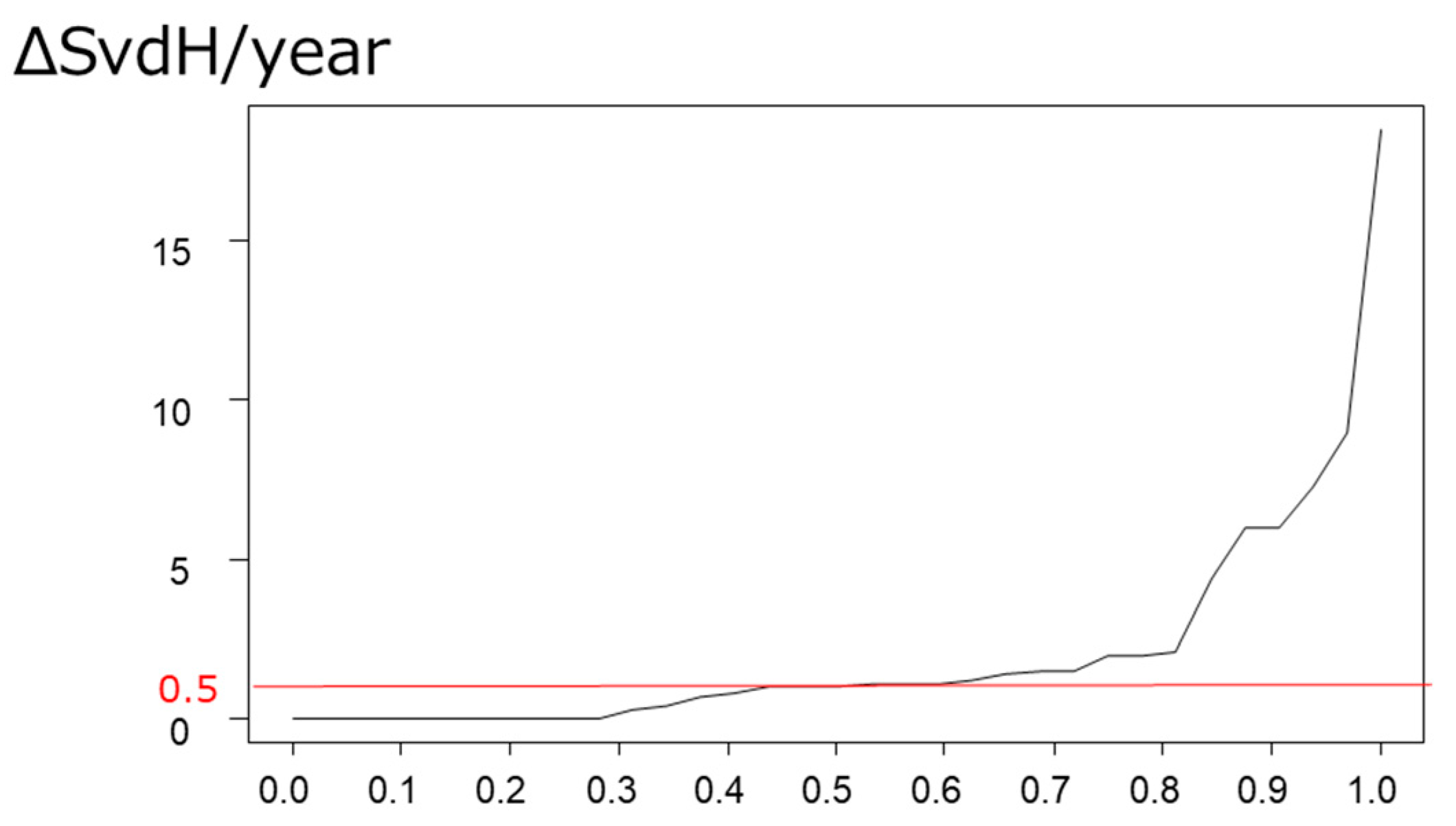
3.3. Prevalence and Predictive Factor for Progression in Joint Destruction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohara, Y.; Kishimoto, M.; Takizawa, N.; Yoshida, K.; Okada, M.; Eto, H.; Deshpande, G.A.; Ritchlin, C.T.; Tanaka, A.; Higashiyama, M.; et al. Prevalence and Clinical Characteristics of Psoriatic Arthritis in Japan. J. Rheumatol. 2015, 42, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ohtsuki, M.; Sano, S.; Igarashi, A.; Morita, A.; Okuyama, R.; Kawada, A.; Working Group of the Epidemiological Survey in the Japanese Society for Psoriasis Research. Epidemiological analysis of psoriatic arthritis patients in Japan. J. Dermatol. 2016, 43, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Chandran, V. Observational cohort studies: Lessons learnt from the University of Toronto Psoriatic Arthritis Program. Rheumatology 2011, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; Smolen, J.S.; Ramiro, S.; de Wit, M.; Cutolo, M.; Dougados, M.; Emery, P.; Landewe, R.; Oliver, S.; Aletaha, D.; et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Kavanaugh, A.; Mease, P.J.; Soriano, E.R.; Laura Acosta-Felquer, M.; Armstrong, A.W.; Bautista-Molano, W.; Boehncke, W.H.; Campbell, W.; Cauli, A.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016, 68, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res. 2011, 63 (Suppl. S11), S64–S85. [Google Scholar] [CrossRef] [PubMed]
- Gandjbakhch, F.; Terslev, L.; Joshua, F.; Wakefield, R.J.; Naredo, E.; D’Agostino, M.A. Ultrasound in the evaluation of enthesitis: Status and perspectives. Arthritis Res. Ther. 2011, 13, R188. [Google Scholar] [CrossRef]
- Healy, P.J.; Helliwell, P.S. Measuring clinical enthesitis in psoriatic arthritis: Assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheumatol. 2008, 59, 686–691. [Google Scholar] [CrossRef]
- Yamada, Y.; Inui, K.; Okano, T.; Mandai, K.; Mamoto, K.; Koike, T.; Takeda, S.; Yamashita, E.; Yoshida, Y.; Tateishi, C.; et al. Ultrasound assessment, unlike clinical assessment, reflects enthesitis in patients with psoriatic arthritis. Clin. Exp. Rheumatol. 2021, 39, 139–145. [Google Scholar] [CrossRef]
- Dubash, S.R.; De Marco, G.; Wakefield, R.J.; Tan, A.L.; McGonagle, D.; Marzo-Ortega, H. Ultrasound Imaging in Psoriatic Arthritis: What Have We Learnt in the Last Five Years? Front. Med. 2020, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.A. Enthesitis detection by ultrasound: Where are we now? Clin. Exp. Rheumatol. 2018, 36 (Suppl. S114), 127–130. [Google Scholar] [PubMed]
- Bandinelli, F.; Prignano, F.; Bonciani, D.; Bartoli, F.; Collaku, L.; Candelieri, A.; Lotti, T.; Matucci-Cerinic, M. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clin. Exp. Rheumatol. 2013, 31, 219–224. [Google Scholar] [PubMed]
- Balint, P.V.; Terslev, L.; Aegerter, P.; Bruyn, G.A.W.; Chary-Valckenaere, I.; Gandjbakhch, F.; Iagnocco, A.; Jousse-Joulin, S.; Moller, I.; Naredo, E.; et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann. Rheum. Dis. 2018, 77, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Gladman, D.D.; Kavanaugh, A.; Mease, P.J. Assessing structural damage progression in psoriatic arthritis and its role as an outcome in research. Arthritis Res. Ther. 2020, 22, 18. [Google Scholar] [CrossRef]
- de Miguel, E.; Cobo, T.; Munoz-Fernandez, S.; Naredo, E.; Uson, J.; Acebes, J.C.; Andreu, J.L.; Martin-Mola, E. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann. Rheum. Dis. 2009, 68, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Conaghan, P.G.; Karim, Z.; Quinn, M.A.; Ikeda, K.; Peterfy, C.G.; Hensor, E.; Wakefield, R.J.; O’Connor, P.J.; Emery, P. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheumatol. 2008, 58, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- El Miedany, Y.; El Gaafary, M.; Youssef, S.; Ahmed, I.; Nasr, A. Tailored approach to early psoriatic arthritis patients: Clinical and ultrasonographic predictors for structural joint damage. Clin. Rheumatol. 2015, 34, 307–313. [Google Scholar] [CrossRef]
- Elalouf, O.; Bakirci Ureyen, S.; Touma, Z.; Anderson, M.; Kaeley, G.S.; Aydin, S.Z.; Eder, L. Psoriatic Arthritis Sonographic Enthesitis Instruments: A Systematic Review of the Literature. J. Rheumatol. 2019, 46, 43–56. [Google Scholar] [CrossRef]
- Agache, M.; Popescu, C.C.; Popa, L.; Codreanu, C. Ultrasound Enthesitis in Psoriasis Patients with or without Psoriatic Arthritis, a Cross-Sectional Analysis. Medicina 2022, 58, 1557. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglioli, K.R.; Lopes, F.O.d.A.; Figueiredo, L.Q.d.; Ferrari, L.F.F.; Guedes, L. Ultrasonographic Insights into Peripheral Psoriatic Arthritis: Updates in Diagnosis and Monitoring. J. Pers. Med. 2024, 14, 550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Patients with PsA (n = 47) | |
|---|---|
| Age, years | 56.4 ± 15.2 |
| Female, % | 53.2 |
| BMI, kg/m2 | 23.6 ± 4.1 |
| Disease duration for psoriasis, months | 169.9 ± 164.1 |
| Disease duration for PsA, months | 90.8 ± 123.6 |
| NSAIDs use rate, % | 14.9 |
| MTX use rate, % | 19.1 |
| bDMARDs use rate, % | 14.9 |
| PASE | 45.9 ± 15.2 |
| PASI | 7.2 ± 10.1 |
| DAPSA | 20.4 ± 18.2 |
| DAS28-CRP | 3.23 ± 1.37 |
| mHAQ | 0.47 ± 0.48 |
| SvdH | 12.6 ± 18.6 |
| CRP, mg/dL | 0.90 ± 2.46 |
| MMP-3, ng/mL | 84.35 ± 53.91 |
| Tender entheses count | 1.72 ± 2.62 |
| US active enthesitis count | 3.09 ± 2.55 |
| Tender joint count | 5.93 ± 5.92 |
| Swollen joint count | 2.56 ± 3.92 |
| US arthritis assessment—GS score US arthritis assessment—PD score | 5.00 ± 4.55 2.74 ± 3.74 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| R-Value | p Value | β Value | p Value | |
| Age | 0.44 | 0.01 | 0.04 | 0.13 |
| PASE | 0.12 | 0.52 | - | - |
| PASI | −0.01 | 0.96 | - | - |
| DAS28CRP | 0.07 | 0.71 | - | - |
| DAPSA | −0.01 | 0.97 | - | - |
| HAQ | −0.07 | 0.73 | - | - |
| CRP | 0.23 | 0.20 | - | - |
| MMP-3 | 0.29 | 0.12 | - | - |
| MTX dose | 0.38 | 0.03 | - | - |
| bDMARDs use | −0.11 | 0.54 | - | - |
| Tender entheses count | −0.19 | 0.30 | - | - |
| US active enthesitis counts | −0.13 | 0.48 | - | - |
| Tender joint count | −0.10 | 0.58 | - | - |
| Swollen joint count | 0.13 | 0.48 | - | - |
| Joint GS score | 0.44 | 0.01 | 0.45 | <0.001 |
| Joint PD score | 0.38 | 0.03 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Inui, K.; Mandai, K.; Mamoto, K.; Koike, T.; Tateishi, C.; Tsuruta, D.; Okano, T. Ultrasonographic Synovitis Is Associated with the Development of Joint Destruction in Patients with Psoriatic Arthritis. J. Pers. Med. 2024, 14, 630. https://doi.org/10.3390/jpm14060630
Yamada Y, Inui K, Mandai K, Mamoto K, Koike T, Tateishi C, Tsuruta D, Okano T. Ultrasonographic Synovitis Is Associated with the Development of Joint Destruction in Patients with Psoriatic Arthritis. Journal of Personalized Medicine. 2024; 14(6):630. https://doi.org/10.3390/jpm14060630
Chicago/Turabian StyleYamada, Yutaro, Kentaro Inui, Koji Mandai, Kenji Mamoto, Tatsuya Koike, Chiharu Tateishi, Daisuke Tsuruta, and Tadashi Okano. 2024. "Ultrasonographic Synovitis Is Associated with the Development of Joint Destruction in Patients with Psoriatic Arthritis" Journal of Personalized Medicine 14, no. 6: 630. https://doi.org/10.3390/jpm14060630





