Medical–Surgical Implications of Branching Variation of Human Aortic Arch Known as Bovine Aortic Arch (BAA)
Abstract
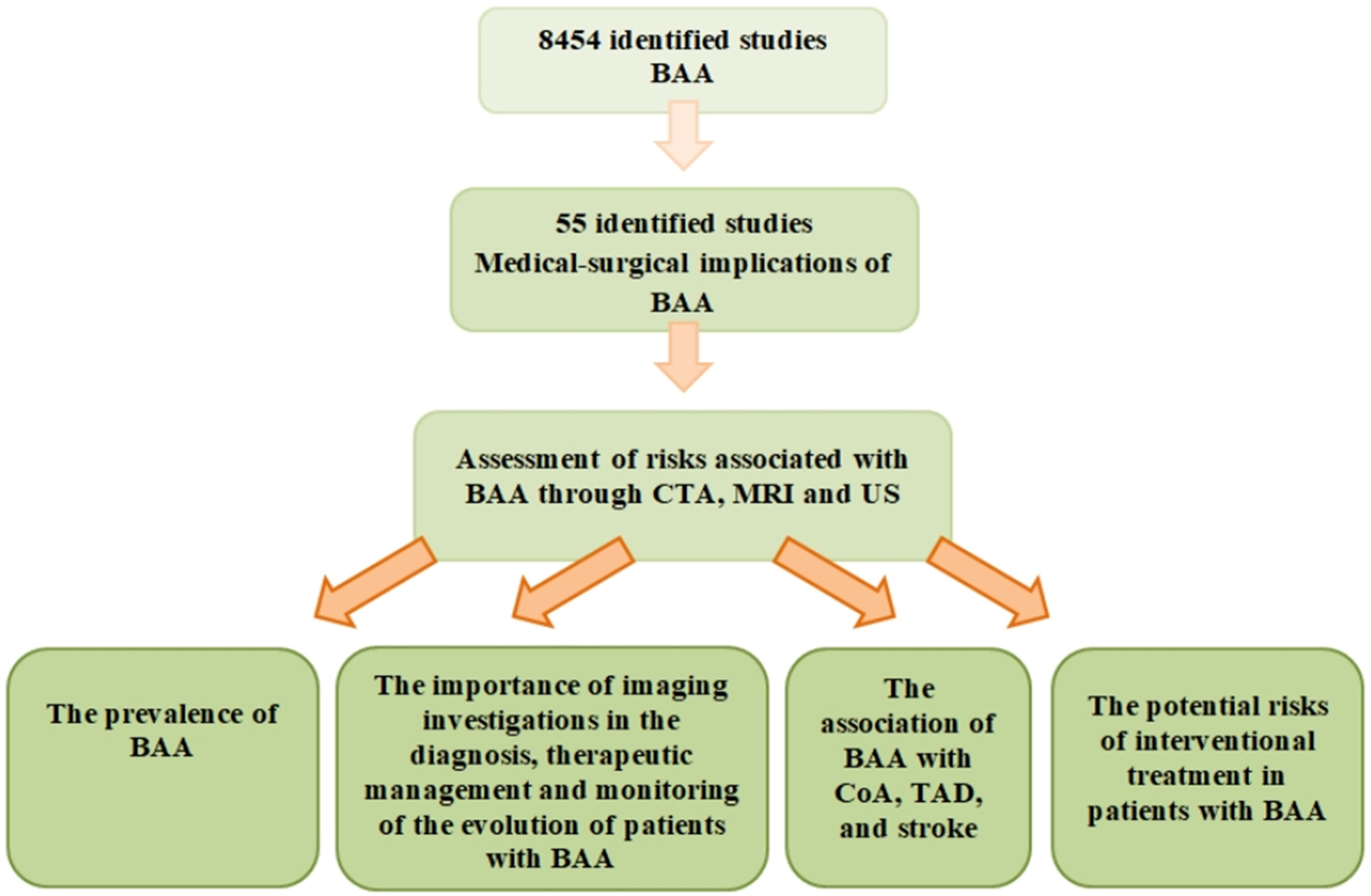
:1. Introduction
2. Materials and Methods
3. Results
3.1. The Prevalence of the Bovine Aortic Arch in Populations from Different Geographical Regions
3.2. The Importance of Imaging Investigations in the Diagnosis, Treatment, and Follow-Up of Patients with This Anatomical Variant
3.3. The Association of the Bovine Aortic Arch with Aortic Coarctation, Thoracic Aortic Disease, and Stroke
3.3.1. Coarctation of the Aorta (CoA)
3.3.2. Thoracic Aortic Disease (TAD)
3.3.3. Stroke
3.4. Potential Risks Associated with Endovascular Interventional Treatment in Patients with Bovine Aortic Arch
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Stiru, O.; Robu, M.; Platon, P.; Bubenek-Turconi, S.I.; Iliescu, V.A.; Parasca, C. Hybrid Management of Dysphagia Lusoria with Tevar Implantation and Bilateral Subclavian Arteries Debranching: A Review of the Literature and a Case Report. J. Pers. Med. 2024, 14, 547. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Sotiriadis, A.; Girardelli, S.; Spinillo, S.; Candiani, M.; Amodeo, S.; Farina, A.; Fesslova, V. Postnatal Outcome and Associated Anomalies of Prenatally Diagnosed Right Aortic Arch with Concomitant Right Ductal Arch: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 831. [Google Scholar] [CrossRef]
- D’Antonio, F.; Khalil, A.; Zidere, V.; Carvalho, J.S. Fetuses with right aortic arch: A multicenter cohort study and meta-analysis. Ultrasound Obstet. Gynecol. 2016, 47, 423–432. [Google Scholar] [CrossRef]
- Villavicencio-Guzmán, L.; Sánchez-Gómez, C.; Jaime-Cruz, R.; Ramírez-Fuentes, T.C.; Patiño-Morales, C.C.; Salazar-García, M. Human Heart Morphogenesis: A New Vision Based on In Vivo Labeling and Cell Tracking. Life 2023, 13, 165. [Google Scholar] [CrossRef]
- Bae, S.B.; Kang, E.J.; Choo, K.S.; Lee, J.; Kim, S.H.; Lim, K.J.; Kwon, H. Aortic Arch Variants and Anomalies: Embryology, Imaging Findings, and Clinical Considerations. J. Cardiovasc. Imaging 2022, 30, 231–262. [Google Scholar] [CrossRef]
- Goel, A.; Viswamitra, S. Congenital Anomalies of Aortic Arch: A Pictorial Essay. Indian J. Radiol. Imaging 2022, 32, 372–380. [Google Scholar] [CrossRef]
- Nedelcu, A.H.; Lupu, A.; Moraru, M.C.; Tarniceriu, C.C.; Stan, C.I.; Partene Vicoleanu, S.A.; Haliciu, A.M.; Statescu, G.; Ursaru, M.; Danielescu, C.; et al. Morphological Aspects of the Aberrant Right Subclavian Artery Systematic Review of the Literature. J. Pers. Med. 2024, 14, 335. [Google Scholar] [CrossRef]
- Hanneman, K.; Newman, B.; Chan, F. Congenital Variants and Anomalies of the Aortic Arch. Radiographics. 2017, 37, 32–51. [Google Scholar] [CrossRef]
- Priya, S.; Thomas, R.; Nagpal, P.; Sharma, A.; Steigner, M. Congenital anomalies of the aortic arch. Cardiovasc. Diagn. Ther. 2018, 8, S26–S44. [Google Scholar] [CrossRef] [PubMed]
- Moorehead, P.A.; Kim, A.H.; Miller, C.P.; Kashyap, T.V.; Kendrick, D.E.; Kashyap, V.S. Prevalence of Bovine Aortic Arch Configuration in Adult Patients with and without Thoracic Aortic Pathology. Ann. Vasc. Surg. 2016, 30, 132–137. [Google Scholar] [CrossRef]
- Prada, G.; Granados, A.M.; Calle, J.S.; Rodríguez, S.Y.; Baena, G.P. Anatomic variations of the aortic arch are depicted on 444 CT angiographies. Eur. J. Anat. 2016, 20, 137–141. [Google Scholar]
- Boyacı, N.; Dokumacı Şen, D.; Karakaş, E.; Yıldız, S.; Cece, H.; Kocarslan, A.; Aydın, M.S. Multidetector computed tomography evaluation of aortic arch and branching variants. Turk. Gogus Kalp Dama. 2015, 23, 051–057. [Google Scholar] [CrossRef]
- Karacan, A.; Türkvatan, A.; Karacan, K. Anatomical variations of aortic arch branching: Evaluation with computed tomographic angiography. Cardiol. Young 2014, 24, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Meguid, E.A. Anatomical variation in the branching pattern of the aortic arch: A literature review. Ir. J. Med. Sci. 2023, 192, 1807–1817. [Google Scholar] [CrossRef]
- Abraham, V.; Mathew, A.; Cherian, V.; Chandran, S.; Mathew, G. Aberrant subclavian artery: Anatomical curiosity or clinical entity. Int. J. Surg. 2009, 7, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Koizumi, J.; Tanno, K.; Okochi, T.; Nomura, T.; Shimura, S.; Imai, Y. Kommerell Diverticulum in Adults: Evaluation of Routine CT Examinations. Tokai J. Exp. Clin. Med. 2016, 41, 65–69. [Google Scholar]
- Ahn, S.S.; Chen, S.W.; Miller, T.J.; Chen, J.F. What is the true incidence of anomalous bovine left common carotid artery configuration? Ann. Vasc. Surg. 2014, 28, 381–385. [Google Scholar] [CrossRef]
- Terzioğlu, E.; Damar, Ç. Evaluation of aortic arch morphologies by computed tomographic angiography in Turkish population. Turk. Gogus Kalp Damar Cerrahisi Derg. 2022, 30, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Shalhub, S.; Schäfer, M.; Hatsukami, T.S.; Sweet, M.P.; Reynolds, J.J.; Bolster, F.A.; Shin, S.H.; Reece, T.B.; Singh, N.; Starnes, B.W.; et al. Association of variant arch anatomy with type B aortic dissection and hemodynamic mechanisms. J. Vasc. Surg. 2018, 68, 1640–1648. [Google Scholar] [CrossRef]
- Clerici, G.; Giulietti, E.; Babucci, G.; Chaoui, R. Bovine aortic arch: Clinical significance and hemodynamic evaluation. J. Matern. Fetal Neonatal Med. 2018, 31, 2381–2387. [Google Scholar] [CrossRef]
- Turek, J.W.; Conway, B.D.; Cavanaugh, N.B.; Meyer, A.M.; Aldoss, O.; Reinking, B.E.; El-Hattab, A.; Rossi, N.P. Bovine arch anatomy influences coarctation rates in the era of extended end-to-end anastomosis. J. Thorac. Cardiovasc. Surg. 2018, 155, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Alotay, A.; Alkashlan, E.; Ghazy, M.; Abdelkader, A. Computed tomography study of bovine arch in patients with coarctation of aorta: A retrospective report analysis. Medicine 2022, 101, e29852. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Turek, J.W.; Froud, J.; Endelman, L.A.; Cavanaugh, N.B.; Torres, J.E.; Ashwath, R. Insights into Arch Vessel Development in the Bovine Aortic Arch. Pediatr. Cardiol. 2019, 40, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Froud, J.R.; Meyer, A.M.; Endelman, L.A.; Cavanaugh, N.B.; Maldonado, J.R.; Turek, J.W.; Ashwath, R. Significance of clamping distance in bovine and normal aortic arch patients. Prog. Pediatr. Cardiol. 2020, 57, 101204. [Google Scholar] [CrossRef]
- Dumfarth, J.; Chou, A.S.; Ziganshin, B.A.; Bhandari, R.; Peterss, S.; Tranquilli, M.; Mojibian, H.; Fang, H.; Rizzo, J.A.; Elefteriades, J.A. Atypical aortic arch branching variants: A novel marker for thoracic aortic disease. J. Thorac. Cardiovasc. Surg. 2015, 149, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, S.; Qi, H.; Sun, C.; Hou, Z.; Wang, X.; Qian, X. Association of the bovine aortic arch and bicuspid aortic valve with thoracic aortic disease. BMC Cardiovasc. Disord. 2023, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Singh, S.; Alkukhun, A.; Alturkmani, B.; Mori, M.; Chen, J.; Mullan, C.W.; Brooks, C.W.; Assi, R.; Gruber, P.J.; et al. Variants of the aortic arch in adult general population and their association with thoracic aortic aneurysm disease. J. Card. Surg. 2021, 36, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Vinholo, T.F.; Buntin, J.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Bovine Aortic Arch: A Result of Chance or Mandate of Inheritance? Am. J. Cardiol. 2022, 172, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, Y.; Koide, Y.; Matsueda, T.; Yamanaka, K.; Inoue, T.; Ishihara, S.; Nakayama, S.; Tanaka, H.; Sugimoto, K.; Okita, Y. Anatomical variations of aortic arch vessels in Japanese patients with the aortic arch disease. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 219–226. [Google Scholar] [CrossRef]
- Dumfarth, J.; Peterss, S.; Kofler, M.; Plaikner, M.; Ziganshin, B.A.; Schachner, T.; Tranquilli, M.; Grimm, M.; Elefteriades, J.A. In DeBakey Type I Aortic Dissection, Bovine Aortic Arch Is Associated with Arch Tears and Stroke. Ann. Thorac. Surg. 2017, 104, 2001–2008. [Google Scholar] [CrossRef]
- Dumfarth, J.; Plaikner, M.; Krapf, C.; Bonaros, N.; Semsroth, S.; Rizzo, J.A.; Fang, H.; Grimm, M.; Elefteriades, J.A.; Schachner, T. Bovine aortic arch: Predictor of entry site and risk factor for neurologic injury in acute type a dissection. Ann. Thorac. Surg. 2014, 98, 1339–1346. [Google Scholar] [CrossRef]
- Syperek, A.; Angermaier, A.; Kromrey, M.L.; Hosten, N.; Kirsch, M. The so-called “bovine aortic arch”: A possible biomarker for embolic strokes? Neuroradiology. 2019, 61, 1165–1172. [Google Scholar] [CrossRef]
- Gold, M.; Khamesi, M.; Sivakumar, M.; Natarajan, V.; Motahari, H.; Caputo, N. Right-left propensity of cardiogenic cerebral embolism in standard versus bovine aortic arch variant. Clin. Anat. 2018, 31, 310–313. [Google Scholar] [CrossRef]
- Matakas, J.D.; Gold, M.M.; Sterman, J.; Haramati, L.B.; Allen, M.T.; Labovitz, D.; Slasky, S.E. Bovine Arch and Stroke Laterality. J. Am. Heart Assoc. 2020, 9, e015390. [Google Scholar] [CrossRef]
- Samadhiya, S.; Sardana, V.; Bhushan, B.; Maheshwari, D.; Yadav, S.R.; Goyal, R. Propensity of Stroke in Standard versus Various Aortic Arch Variants: A 200 Patients Study. Ann. Indian Acad. Neurol. 2022, 25, 634–639. [Google Scholar] [CrossRef]
- Zhu, J.; Tong, G.; Zhuang, D.; Yang, Y.; Liang, Z.; Liu, Y.; Yu, C.; Zhang, Z.; Chen, Z.; Liu, J.; et al. Surgical treatment strategies for patients with type A aortic dissection involving arch anomalies. Front. Cardiovasc. Med. 2022, 13, 979431. [Google Scholar] [CrossRef]
- Montorsi, P.; Galli, S.; Ravagnani, P.M.; Trabattoni, D.; Fabbiocchi, F.; Lualdi, A.; Ballerini, G.; Andreini, D.; Pontone, G.; Caputi, L.; et al. Carotid artery stenting in patients with left ICA stenosis and bovine aortic arch: A single-center experience in 60 consecutive patients treated via the right radial or brachial approach. J. Endovasc. Ther. 2014, 21, 127–136. [Google Scholar] [CrossRef]
- Burzotta, F.; Nerla, R.; Pirozzolo, G.; Aurigemma, C.; Niccoli, G.; Leone, A.M.; Saffioti, S.; Crea, F.; Trani, C. Clinical and procedural impact of aortic arch anatomic variants in carotid stenting procedures. Catheter. Cardiovasc. Interv. 2015, 86, 480–489. [Google Scholar] [CrossRef]
- Tarniceriu, C.C.; Hurjui, L.L.; Tanase, D.M.; Nedelcu, A.H.; Gradinaru, I.; Ursaru, M.; Stefan Rudeanu, A.; Delianu, C.; Lozneanu, L. The Pulmonary Venous Return from Normal to Pathological-Clinical Correlations and Review of Literature. Medicina 2021, 57, 293. [Google Scholar] [CrossRef]
- Tapia-Nañez, M.; Landeros-Garcia, G.A.; Sada-Treviño, M.A.; Pinales-Razo, R.; Quiroga-Garza, A.; Fernandez-Rodarte, B.A.; Elizondo-Omaña, R.E.; Guzman-Lopez, S. Morphometry of the aortic arch and its branches. A computed tomography angiography-based study. Folia. Morphol. 2021, 80, 575–582. [Google Scholar] [CrossRef]
- Malone, C.D.; Urbania, T.H.; Crook, S.E.; Hope, M.D. Bovine aortic arch: A novel association with thoracic aortic dilation. Clin. Radiol. 2012, 67, 28–31. [Google Scholar] [CrossRef]
- Pham, T.; Martin, C.; Elefteriades, J.; Sun, W. Biomechanical characterization of ascending aortic aneurysm with concomitant bicuspid aortic valve and bovine aortic arch. Acta. Biomater. 2013, 9, 7927–7936. [Google Scholar] [CrossRef]
- Hohri, Y.; Numata, S.; Itatani, K.; Inoue, T.; Yaku, H. Determination of the dominant arch by computational fluid dynamics analysis using computed tomography images in double aortic arch. Int. J. Cardiovasc. Imaging 2021, 37, 2573–2575. [Google Scholar] [CrossRef]
- Miyazaki, S.; Itatani, K.; Furusawa, T.; Nishino, T.; Sugiyama, M.; Takehara, Y.; Yasukochi, S. Validation of numerical simulation methods in aortic arch using 4D Flow MRI. Heart Vessels. 2017, 32, 1032–1044. [Google Scholar] [CrossRef]
- Hedna, V.S.; Bodhit, A.N.; Ansari, S.; Falchook, A.D.; Stead, L.; Heilman, K.M.; Waters, M.F. Hemispheric differences in ischemic stroke: Is left-hemisphere stroke more common? J. Clin. Neurol. 2013, 9, 97–102. [Google Scholar] [CrossRef]
- Baccaro, A.; Wang, Y.P.; Brunoni, A.R.; Candido, M.; Conforto, A.B.; da Costa Leite, C.; Lotufo, P.A.; Benseñor, I.M.; Goulart, A.C. Does stroke laterality predict major depression and cognitive impairment after stroke? Two-year prospective evaluation in the EMMA study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109639. [Google Scholar] [CrossRef]
- Bonuzzi, G.M.G.; de Freitas, T.B.; Palma, G.C.D.S.; Soares, M.A.A.; Lange, B.; Pompeu, J.E.; Torriani-Pasin, C. Effects of the brain-damaged side after stroke on the learning of a balance task in a non-immersive virtual reality environment. Physiother. Theory. Pract. 2022, 38, 28–35. [Google Scholar] [CrossRef]
- Faggioli, G.L.; Ferri, M.; Freyrie, A.; Gargiulo, M.; Fratesi, F.; Rossi, C.; Manzoli, L.; Stella, A. Aortic arch anomalies are associated with increased risk of neurological events in carotid stent procedures. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 436–441. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart. J. 2018, 39, 763–816. [Google Scholar]
- Vasile, C.M.; Laforest, G.; Bulescu, C.; Jalal, Z.; Thambo, J.-B.; Iriart, X. From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions. J. Clin. Med. 2023, 12, 7350. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Backer, C.L.; Patel, J.N.; Patel, S.K.; Walker, B.L.; Weigel, T.J.; Randolph, G.; Wax, D.; Mavroudis, C. Coarctation of the aorta: Midterm outcomes of resection with extended end-to-end anastomosis. Ann. Thorac. Surg. 2009, 88, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.E.; Nowak, C.A.; Goldberg, C.S.; Ohye, R.G.; Bove, E.L.; Rocchini, A.P. Extended resection and end-to-end anastomosis for aortic coarctation in infants: Results of a tailored surgical approach. Ann. Thorac. Surg. 2005, 80, 1453–1459. [Google Scholar] [CrossRef]
- Ma, W.G.; Zhu, J.M.; Zheng, J.; Liu, Y.M.; Ziganshin, B.A.; Elefteriades, J.A.; Sun, L.Z. Sun’s procedure for complex aortic arch repair: Total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann. Cardiothorac. Surg. 2013, 2, 642–648. [Google Scholar] [CrossRef]
- Waterford, S.D.; Di Eusanio, M.; Ehrlich, M.P.; Reece, T.B.; Desai, N.D.; Sundt, T.M.; Myrmel, T.; Gleason, T.G.; Forteza, A.; de Vincentiis, C.; et al. Postoperative myocardial infarction in acute type A aortic dissection: A report from the International Registry of Acute Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2017, 153, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.H. Carotid Artery Stenting. Korean Circ. J. 2018, 48, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Stickley, J.; Stümper, O.; Khan, N.; Jones, T.J.; Barron, D.J.; Brawn, W.J. Repair of isolated aortic coarctation over two decades: Impact of surgical approach and associated arch hypoplasia. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 865–870. [Google Scholar] [CrossRef]
- Costopoulos, K.; Philip, J.; Lopez-Colon, D.; Kaliki, G.; Chandran, A.; Bleiweis, M. A single centre experience with an evolving approach for the repair of coarctation of the aorta. Cardiol. Young 2019, 29, 885–887. [Google Scholar] [CrossRef]



| Study | Geographic Region | Imaging Modality | Number of Patients | Outcome |
|---|---|---|---|---|
| Moorehead PA et al., 2016 [10] | USA | CTA | 817 study group: 156 patients with TAD; control group: 757 patients without TAD; | General prevalence of BAA: 31.1%; Prevalence of T1BA: 14.9%; Prevalence of T2BA: 16.2%; Statistically significantly higher prevalence of T2BA in the TAD group (23.7%) compared to controls (15.9%); Non-statistically significantly higher prevalence of T1BA in the TAD group (11.5%) compared to controls (14.9%); The prevalence of T2BA in the TAA group (24.6%) was statistically significantly higher compared to controls (15.9%); Statistically significantly higher prevalence of T2BA in the AD group (42.3%) compared to controls (30.8%). Higher statistically significant prevalence of BAA in patients with AD compared to controls (42.3% vs. 30.8%). Patients with TAD were older and had an increased prevalence of hypertension, hyperlipidemia, and aortic calcification compared with those without TAD. |
| Prada G et al., 2016 [11] | South America | CTA | 444 | Prevalence of anatomic variations of AA: 40.1%; Prevalence of different branching types of AA: Type 1 (normal branching): 59.9%; Type 2 (T1BA) “Bovine arcade” 27.9%; Type 3 (T2BA): 9.9%; Type 4 (left vertebral artery arising from AA): 2.2%; Prevalence of anatomical variations of AA by gender: women: 42.3%, men: 35.9%; The prevalence of TAD among people with AA branching variations was 14%, and it was distributed according to branching type as follows: Type 2 14.5%, Type 3 11.4%, Type 4.20%. |
| Karacan A et al., 2014 [13] | Turky | CTA | 1000 | Prevalence of normal branching types of AA: Type 1 (normal branching): 79.2%; Prevalence of anatomic variations of AA: 20.8%; Prevalence of anatomic variations of AA by gender: women:22.1%, men: 20%; General prevalence of BAA: 14.1%. |
| Ahn SS et al., 2014 [17] | Los Angeles, California | Angiography | 90 | General prevalence of BAA: 35.16%; Prevalence of T1BA: 26.88%; Prevalence of T2BA: 7.53%; Prevalence of BAA in different ethnic groups: Caucasians: 27.78%, Hispanics: 50%; Prevalence of BAA by gender: women: 40%, men: 26.67%. |
| Terzioğlu E et al., 2022 [18] | Turky | CTA | 2037 | General prevalence of BAA: 15.5%; Prevalence of BAA by gender: women: 18.2%, men: 12.8%. |
| Shalhub S et al., 2018 [19] | USA | 4D flow MRI | 552 study group: 185 patients with TBAD; control group: 367 patients without TBAD; | The prevalence of anatomical variations of AA was statistically significantly higher in patients with TBAD (40.5%) compared to controls (24.5%); The most common aortic arch branching variant was BAA (37.3% in patients with TBAD vs. 22.3% in controls), followed by aberrant SRA (2.7% in patients with TBAD vs. 0.3% in controls); Higher systolic wall shear stress along the inner curve of BAA compared with the normal AA and aberrant RSA. |
| Clerici G et al., 2018 [20] | Italy | US | 742 including 39 patients eligible for hemodynamic evaluation: 6 patients with BAA and 33 patients with normal AA pattern | General prevalence of BAA: 6.06%; Prevalence of BAA by gender: female fetuses: 33.3%, male fetuses: 66.7%; Blood flow characteristics were similar between the BAA group and the normal AA group; There were statistically significant hemodynamic differences between the BAA group and the normal AA group. |
| Turek JW et al., 2018 [21] | USA | US | 49 | The prevalence of BAA was initially underestimated: 6.1% before the review of echocardiographic reports vs. 28.6% after the review of echocardiographic reports. The prevalence of ReCoA was statistically significantly higher in patients with BAA (28.6%) compared to patients with normal AA (5.7%); The mean anastomosis index was significantly lower in patients with BAA compared to those with normal AA. |
| Shaaban M et al., 2022 [22] | Saudi Arabia | CTA | 700 | General prevalence of BAA: 2.71%; General prevalence of CoA: 16.71%; The prevalence of BAA was statistically significantly higher in patients with CoA (5.98%) compared to those without CoA (2.06%). |
| Meyer AM et al., 2019 [23] | USA | CTA/Non-con-trast CT | 178 | General prevalence of BAA: 32.58%; The distances HV1 + HV2 and HV2 + HV3 are shorter in BAA than in normal AA in patients who underwent resection with extended end-to-end anastomosis through left thoracotomy for CoA correction. |
| Froud JR et al., 2020 [24] | USA | CTA/Non-con-trast CT | 169 | General prevalence of BAA: 34%; Both the mean clamping distance and the mean clamping index were significantly lower in BAA than in normal AA patients who underwent resection with extended end-to-end anastomosis through left thoracotomy for CoA correction. |
| Dumfarth J et al., 2015 [25] | USA | CT/MRI | 5173 study group: 556 patients with TAD; control group: 4617 patients without TAD; | AA abnormalities were statistically significantly more frequent in patients with TAD (33.5%) compared to those in the control group (18.2%); BAA was the most common abnormal branching pattern of AA in TAD patients (24.6%), followed by isolated left vertebral artery (6.3%) and aberrant RSA (1.8%); All 3 arch variations showed a significantly higher prevalence in TAD patients compared to controls; Patients with TAD and anatomical variations of AA compared to those with TAD with normal AA had hypertension less often (73.5% vs. 81.8%) but had a higher rate of bicuspid aortic valve (40.8% vs. 30.6%); Patients with variations of AA and TAD compared to those with normal AA and TAD were significantly younger (58.6 ± 13.7 years vs. 62.4 ± 12.9 years) and required aortic arch surgery more frequently (46% vs. 34.6%). |
| Sun J et al., 2023 [26] | China | CT and US | 449 | General prevalence of BAA: 21.2%; Prevalence of BAA by gender: women: 26.3%, men: 73.7%; BAA had the highest prevalence among AA abnormalities: 79.8%; The prevalence of aortic bicuspids was statistically significantly higher in patients with BAA compared to those with normal AA: 52.6% vs. 38.1%; The diameter of the ascending aorta was greater in the BAA group than in the normal AA group, but the difference was not statistically significant; Aortic bicuspidity and male gender were predictors of TAD, but BAA was not a risk factor for TAD. |
| Yousef S et al., 2021 [27] | USA | CT | 21,336 | The prevalence of anatomical variations of AA was 2.8%; The most common AA branching pattern was BAA, with a prevalence of 58.7% of all anatomical variations of AA; The prevalence of TAA was statistically significantly higher in the group with AA anomalies compared to the group with normal AA anatomy (10.8% vs. 4.1%); Independent factors statistically significantly associated with increased risk of TAA were AA abnormalities, aortic valve pathology, male gender, and arterial hypertension. |
| Shang M et al., 2022 [28] | USA | CT/MRI with/without contrast | 24 patients with BA și TAA 43 relatives of the 24 patients had preexisting imaging investigations available for AA anatomy evaluation. | The prevalence of BAA in relatives of patients with BAA and TAA was 53%; The heritability of BAA was very high: 0.71. |
| Ikeno Y et al., 2019 [29] | Japan | CT | 2321 group A: 815 patients with TAD; group C: 1506 patients without TAD; | Branching abnormalities of AA were more frequent in group A patients (17.2%) compared to those in group C (14.7%); Statistically, significantly more TAA patients in group A had AA abnormalities compared to group C (20.2% vs. 14.7%), including BAA (12.3% vs. 9%) and aberrant RSA (2.6%, compared to 0.5%); Regarding TAA location, the proximal aneurysm was detected more frequently in patients with BAA (15.2%), and the distal one was detected more frequently in patients with aberrant RSA (3.7%); Regarding acute or chronic AD, no statistically significant difference in AA abnormality was found. |
| Dumfarth J et al., 2017 [30] | Austria, SUA | CT | 315 group BAA+: 49 patients with BAA; group BAA−: 266 patients without BAA; | General prevalence of BAA in patients with AADA: 15.6%; The location of the entry site of the dissection was more frequent in AA in patients with BAA (BAA+ 46.8%) compared to those without AA abnormalities (BAA− 14.3%); Independent predictors for AA rupture were BAA and preoperative competent aortic valve; 12.4% of all patients suffered a stroke; Patients with BAA had higher stroke rates (BAA+ 24.5%) compared to those with normal AA anatomy (BAA− 10.2%); BAA emerged as an independent risk factor for stroke in the AADA. |
| Dumfarth J et al., 2014 [31] | Austria, SUA | CT | 157 group BAA+: 22 patients with BAA; group BAA−: 135 patients without BAA; | General prevalence of BAA in patients with AADA: 14%; The location of the primary rupture was statistically significantly more frequent at the AA level in the BAA+ group (59.1%) compared to the BAA− group (13.3%); Early mortality (first 24 h after surgery) was slightly higher in the BAA+ group (9.1%) than the BAA− group (7.2%) but with no statistically significant difference between the two groups. In-hospital mortality was 9.1% in the BAA+ group and 14.8% in the BAA− group; Multivariate analysis showed that the presence of a BAA is an independent risk factor for the occurrence of primary rupture in AA and for postoperative neurologic damage but not for in-hospital mortality. |
| Syperek A el al., 2019 [32] | Germany | CTA | 474 study group: 152 patients with stroke; control group: 322 patients without stroke; | The prevalence of BAA was statistically significantly higher in the group of patients suffering from embolic stroke compared to the control group (25.7% versus 17.1%); T1BA was identified approximately equally frequently in both groups (15.1% vs. 12.1%); T2BA was significantly higher among patients with embolic stroke than those without a history of stroke (10.5% vs. 5.0%). |
| Gold M et al., 2018 [33] | SUA | CT/MRI | 119 group BAA+: 22 patients with BAA; group BAA−: 135 patients without BAA; | General prevalence of BAA: 33%; The most common etiologies of cardioembolic stroke were atrial fibrillation—67%, congestive heart failure with ejection fraction <30–15%; BAA patients had a 50% chance of having a left or right hemisphere stroke; No statistically significant difference in cardio-emboli stroke laterality in BAA patients was demonstrated; In patients with standard AA, there was a trend toward right hemisphere lesions, but this was not statistically significant. |
| Matakas JD et al., 2020 [34] | SUA | CT/MRI |
615 group of patients with BAA: 191; group of patients with normal AA: 424; | Among patients with normal AA, the distribution of stroke was left in 43.6%, right in 45.1%, and bilateral in 11.3% of cases; In the group of patients with BAA, the stroke distribution was left in 51.3%, right in 35.6%, and bilateral in 13.1% of cases; 41% of BAA patients were black, and there was a statistically significant association of black race with BAA. |
| Samadhiya S et al., 2022 [35] | India | CT/MRI | 200 | Standard AA—type I (with the 3 variants, types 1, 2, 3) was the most frequent, registering a prevalence of 85.5% in the studied population; BAA (with the 3 variants, A, B, C) was the most frequent branching variation of AA: 13.5%; The age at presentation of stroke in type 1 (distance is less than 1 diameter) was 61.83 years; The age at presentation of stroke in type 2 (distance is between 1 and 2 LCCA diameters) was 59.8 years; The age at presentation of stroke in type 3 (distance is greater than 2 LCCA diameters) was 60.96 years. The age at presentation of stroke in type A (LCCA originating from BT) was 53.33 years; The age at presentation of stroke in type B (common origin of BT and LCCA) was 53.36 years; The age at presentation of stroke in type C (true BAA) was 63.25 years. |
| Zhu J et al., 2022 [36] | China | CT/surgical records | 896 | 9% of patients presented abnormalities of AA, of whom 3.9% BAA; Among all patients with AA abnormalities, those with BAA had the highest perioperative mortality (14%) and the highest incidence of neurological complications (16%). |
| Montorsi P et al., 2014 [37] | Italy | CTA | 505 | 11.9% of the 505 patients with LICA and BAA stenosis were treated by CAS through the right radial approach (6.4%) or right brachial approach (5.5%); CAS under cerebral protection (a distal filter or proximal MO.MA system) performed via a radial or brachial approach had a 98.3% success rate; The MO.MA system proved too short in a tall patient with a radial approach, and a filter was used; Clinical success without adverse events was 96.7% due to one retinal embolism and one minor stroke; Vascular complications occurred in 3.3% of patients in the brachial approach group; Over a mean follow-up period of 18.7, the median event-free survival rate was 93%. |
| Burzotta F et al., 2015 [38] | Italy | Angiography | 282 | Of 282 CAS, 54% were under proximal balloon occlusion and 42.2% under distal filter neuroprotection; General prevalence of BAA: 20.5%; CMT was significantly influenced by LICA in patients with BAA (49.2 min in patients with BAA vs. 37.7 min in patients with normal AA anatomy); CMT was the only independent predictor of adverse outcomes at 30 days. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotundu, A.; Nedelcu, A.H.; Tepordei, R.T.; Moraru, M.C.; Chiran, D.A.; Oancea, A.; Maștaleru, A.; Costache, A.-D.; Chirica, C.; Grosu, C.; et al. Medical–Surgical Implications of Branching Variation of Human Aortic Arch Known as Bovine Aortic Arch (BAA). J. Pers. Med. 2024, 14, 678. https://doi.org/10.3390/jpm14070678
Rotundu A, Nedelcu AH, Tepordei RT, Moraru MC, Chiran DA, Oancea A, Maștaleru A, Costache A-D, Chirica C, Grosu C, et al. Medical–Surgical Implications of Branching Variation of Human Aortic Arch Known as Bovine Aortic Arch (BAA). Journal of Personalized Medicine. 2024; 14(7):678. https://doi.org/10.3390/jpm14070678
Chicago/Turabian StyleRotundu, Andreea, Alin Horatiu Nedelcu, Razvan Tudor Tepordei, Marius Constantin Moraru, Dragos Andrei Chiran, Andra Oancea, Alexandra Maștaleru, Alexandru-Dan Costache, Costin Chirica, Cristina Grosu, and et al. 2024. "Medical–Surgical Implications of Branching Variation of Human Aortic Arch Known as Bovine Aortic Arch (BAA)" Journal of Personalized Medicine 14, no. 7: 678. https://doi.org/10.3390/jpm14070678
APA StyleRotundu, A., Nedelcu, A. H., Tepordei, R. T., Moraru, M. C., Chiran, D. A., Oancea, A., Maștaleru, A., Costache, A.-D., Chirica, C., Grosu, C., Mitu, F., & Leon, M. M. (2024). Medical–Surgical Implications of Branching Variation of Human Aortic Arch Known as Bovine Aortic Arch (BAA). Journal of Personalized Medicine, 14(7), 678. https://doi.org/10.3390/jpm14070678








