Cerebral Oxygen Saturation Associates with Changes in Oxygen Transport Parameters during Cardiopulmonary Bypass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anesthesia
2.3. Extracorporeal Circulation
2.4. Transesophageal Echocardiography (TEE)
2.5. Near-Infrared Spectroscopy
2.6. Measurements
2.7. Statistical Analysis
3. Results
3.1. Perioperative Hemodynamics
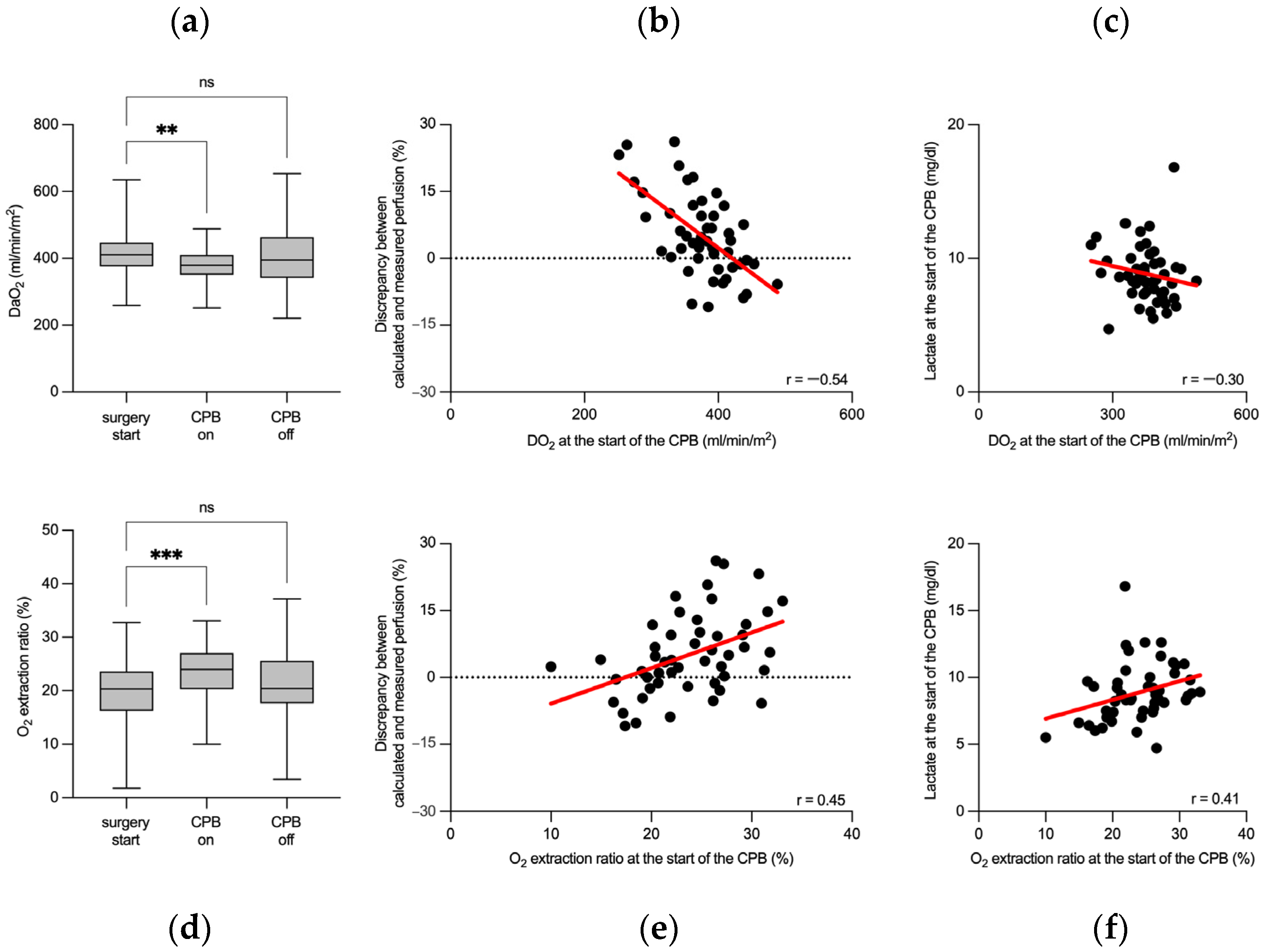
3.2. Flow Rate-Sensitive Discrepancy between Calculated and Measured Perfusion Flow Rates
3.3. Cerebral Oxygen Saturation Associates with Perioperative Changes in Oxygen Transport Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, J.C.; Talebi, F.M.; Jang, D.H.; Spelde, A.E.; Gordon, E.K.; Horak, J.; Acker, M.A.; Kilbaugh, T.J.; Shofer, F.S.; Augoustides, J.G.T.; et al. Anaerobic Lactate Production Is Associated with Decreased Microcirculatory Blood Flow and Decreased Mitochondrial Respiration Following Cardiovascular Surgery with Cardiopulmonary Bypass. Crit. Care Med. 2024; ahead of print. [Google Scholar] [CrossRef]
- Demirjian, S.; Bakaeen, F.; Tang, W.H.W.; Donaldson, C.; Taliercio, J.; Huml, A.; Gadegbeku, C.A.; Gillinov, A.M.; Insler, S. Hemodynamic Determinants of Cardiac Surgery-Associated Acute Kidney Injury. Crit. Care Explor. 2024, 6, e1063. [Google Scholar] [CrossRef] [PubMed]
- Noe, K.M.; Don, A.; Cochrane, A.D.; Zhu, M.Z.L.; Ngo, J.P.; Smith, J.A.; Thrift, A.G.; Vogiatjis, J.; Martin, A.; Bellomo, R.; et al. Intraoperative Hemodynamics and Risk of Cardiac Surgery-associated Acute Kidney Injury: An Observation Study and a Feasibility Clinical Trial. Clin. Exp. Pharmacol. Physiol. 2023, 50, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Searles, B.; Darling, E.M.; Riley, J.B.; Wiles, J.R. Survey of the Routine Practice Limits for Physiologic and Technical Parameters Managed by Clinical Perfusionists during Adult Cardiopulmonary Bypass. J. Extra-Corpor. Technol. 2020, 52, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.E.; Chaney, M.A.; London, M.J. Anesthetic Management During Cardiopulmonary Bypass. Anesth. Analg. 2015, 120, 749–769. [Google Scholar] [CrossRef] [PubMed]
- Kurusz, M. Standards Update on Perfusion Equipment and Practice. Perfusion 2005, 20, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Magruder, J.T.; Weiss, S.J.; DeAngelis, K.G.; Haddle, J.; Desai, N.D.; Szeto, W.Y.; Acker, M.A.; Group, P.P.T.W.; Magruder, J.T.; Acker, A.; et al. Correlating Oxygen Delivery on Cardiopulmonary Bypass with Society of Thoracic Surgeons Outcomes Following Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2022, 164, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Condello, I.; Santarpino, G.; Nasso, G.; Moscarelli, M.; Speziale, G.; Lorusso, R. Goal-Directed Extracorporeal Circulation: Transferring the Knowledge and Experience from Daily Cardiac Surgery to Extracorporeal Membrane Oxygenation. Perfusion 2023, 38, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Pratomo, B.Y.; Sudadi, S.; Setianto, B.Y.; Novenanto, T.T.; Raksawardana, Y.K.; Rayhan, A.; Kurniawaty, J. Intraoperative Goal-Directed Perfusion in Cardiac Surgery with Cardiopulmonary Bypass: The Roles of Delivery Oxygen Index and Cardiac Index. Ann. Thorac. Cardiovasc. Surg. 2024, 30, ra.23-00188. [Google Scholar] [CrossRef] [PubMed]
- Dijoy, L.; Dean, J.S.; Bistrick, C.; Sistino, J.J. The History of Goal-Directed Therapy and Relevance to Cardiopulmonary Bypass. J. Extra-Corpor. Technol. 2014, 47, 90–94. [Google Scholar] [CrossRef]
- Mukaida, H.; Matsushita, S.; Yamamoto, T.; Minami, Y.; Sato, G.; Asai, T.; Amano, A. Oxygen Delivery-Guided Perfusion for the Prevention of Acute Kidney Injury: A Randomized Controlled Trial. J. Thorac. Cardiovasc. Surg. 2023, 165, 750–760.e5. [Google Scholar] [CrossRef]
- Alston, R.P.; Anderson, A.; Sanger, K. Is Body Surface Area Still the Best Way to Determine Pump Flow Rate during Cardiopulmonary Bypass? Perfusion 2006, 21, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, S.; Larsson, A.; Andreasen, J.; Bundgaard, W.; Boegsted, M.; Rasmussen, B. Should Blood Flow during Cardiopulmonary Bypass Be Individualized More than to Body Surface Area? Perfusion 2011, 26, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Hessel, E.A.; Groom, R.C. Optimal Perfusion During Cardiopulmonary Bypass: An Evidence-Based Approach. Anesth. Analg. 2009, 108, 1394–1417. [Google Scholar] [CrossRef] [PubMed]
- Jarry, S.; Couture, E.J.; Falih, J.; Lebon, J.-S.; Ayoub, C.; Rochon, A.; Rousseau-Saine, N.; Denault, A. New Development in Brain Monitoring for Cardiac Surgery. Can. J. Cardiol. 2023, 39, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Murkin, J.M.; Arango, M. Near-Infrared Spectroscopy as an Index of Brain and Tissue Oxygenation. Br. J. Anaesth. 2009, 103, i3–i13. [Google Scholar] [CrossRef] [PubMed]
- Lanning, K.M.; Ylikauma, L.A.; Erkinaro, T.M.; Ohtonen, P.P.; Vakkala, M.A.; Kaakinen, T.I. Changes in Transcranial Near-infrared Spectroscopy Values Reflect Changes in Cardiac Index during Cardiac Surgery. Acta Anaesthesiol. Scand. 2023, 67, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Aron, J.H.; Fink, G.W.; Swartz, M.F.; Ford, B.; Hauser, M.C.; O’Leary, C.E.; Puskas, F. Cerebral Oxygen Desaturation After Cardiopulmonary Bypass in a Patient with Raynaud’s Phenomenon Detected by Near-Infrared Cerebral Oximetry. Anesth. Analg. 2007, 104, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-W.; Chang, H.-H.; Chen, Y.-J.; Chang, W.-K.; Chan, K.-H.; Chen, P.-T. Clinical Value of Application of Cerebral Oximetry in Total Replacement of the Aortic Arch and Concomitant Vessels. Acta Anaesthesiol. Taiwanica 2008, 46, 178–183. [Google Scholar] [CrossRef]
- Hassan, M.A.A.; Rozario, C.; Elsayed, H.; Morcos, K.; Millner, R. A Novel Application of Cerebral Oximetry in Cardiac Surgery. Ann. Thorac. Surg. 2010, 90, 1700–1701. [Google Scholar] [CrossRef] [PubMed]
- Prabhune, A.; Sehic, A.; Spence, P.A.; Church, T.; Edmonds, H.L. Cerebral Oximetry Provides Early Warning of Oxygen Delivery Failure during Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2002, 16, 204–206. [Google Scholar] [CrossRef]
- Bennett, S.R.; Smith, N.; Bennett, M.R. Cerebral Oximetry in Adult Cardiac Surgery to Reduce the Incidence of Neurological Impairment and Hospital Length-of-Stay: A Prospective, Randomized, Controlled Trial. J. Intensiv. Care Soc. 2022, 23, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Donnelly, J.; Brady, K.M.; Akiyoshi, K.; Bush, B.; Koehler, R.C.; Lee, J.K.; Hogue, C.W.; Czosnyka, M.; Smielewski, P.; et al. Comparison of Different Metrics of Cerebral Autoregulation in Association with Major Morbidity and Mortality after Cardiac Surgery. Br. J. Anaesth. 2022, 129, 22–32. [Google Scholar] [CrossRef]
- Arthursson, H.; Kjellberg, G.; Tovedal, T.; Lennmyr, F. Cerebral Oxygenation and Autoregulation during Rewarming on Cardiopulmonary Bypass. Perfusion 2023, 38, 523–529. [Google Scholar] [CrossRef]
- Brady, K.; Joshi, B.; Zweifel, C.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Hogue, C.W. Real-Time Continuous Monitoring of Cerebral Blood Flow Autoregulation Using Near-Infrared Spectroscopy in Patients Undergoing Cardiopulmonary Bypass. Stroke 2010, 41, 1951–1956. [Google Scholar] [CrossRef]
- Steiner, L.A.; Pfister, D.; Strebel, S.P.; Radolovich, D.; Smielewski, P.; Czosnyka, M. Near-Infrared Spectroscopy Can Monitor Dynamic Cerebral Autoregulation in Adults. Neurocritical Care 2008, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Putnam, B.; Bricker, S.; Fedorka, P.; Zelada, J.; Shebrain, S.; Omari, B.; Bongard, F. The Correlation of Near-Infrared Spectroscopy with Changes in Oxygen Delivery in a Controlled Model of Altered Perfusion. Am. Surg. 2007, 73, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Parulkar, S.D.; Bebawy, J.; Sherwani, S.; Hogue, C.W. Cerebral Neuromonitoring During Cardiac Surgery: A Critical Appraisal With an Emphasis on Near-Infrared Spectroscopy. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2313–2322. [Google Scholar] [CrossRef]
- Metkus, T.S.; Thibault, D.; Grant, M.C.; Badhwar, V.; Jacobs, J.P.; Lawton, J.; O’Brien, S.M.; Thourani, V.; Wegermann, Z.K.; Zwischenberger, B.; et al. Transesophageal Echocardiography in Patients Undergoing Coronary Artery Bypass Graft Surgery. J. Am. Coll. Cardiol. 2021, 78, 112–122. [Google Scholar] [CrossRef]
- Coskun, C.; Borulu, F.; Emir, I.; Hanedan, M.; Mataraci, I. Investigation of the Relationship between Cerebral Near-Infrared Spectroscopy Measurements and Cerebrovascular Event in Coronary Artery Bypass Grafting Operation in Patients without Carotid Stenosis and Patients with Carotid Stenosis below Surgical Margins. Braz. J. Cardiovasc. Surg. 2020, 35, 465–470. [Google Scholar] [CrossRef]
- Nicoara, A.; Skubas, N.; Ad, N.; Finley, A.; Hahn, R.T.; Mahmood, F.; Mankad, S.; Nyman, C.B.; Pagani, F.; Porter, T.R.; et al. Guidelines for the Use of Transesophageal Echocardiography to Assist with Surgical Decision-Making in the Operating Room: A Surgery-Based Approach from the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Anesthesiologists and the Society of Thoracic Surgeons. J. Am. Soc. Echocardiogr. 2020, 33, 692–734. [Google Scholar] [CrossRef]
- Somer, F.D. What Is Optimal Flow and How to Validate This. J. Extra-Corpor. Technol. 2007, 39, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Primeau, C.; Hesse, U.; Hougen, H.P.; Lynnerup, N.; Hesse, B. Body Surface Area Determined by Whole-body CT Scanning: Need for New Formulae? Clin. Physiol. Funct. Imaging 2017, 37, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Mertes, P.-M.; Kindo, M.; Amour, J.; Baufreton, C.; Camilleri, L.; Caus, T.; Chatel, D.; Cholley, B.; Curtil, A.; Grimaud, J.-P.; et al. Guidelines on Enhanced Recovery after Cardiac Surgery under Cardiopulmonary Bypass or Off-Pump. Anaesth. Crit. Care Pain Med. 2022, 41, 101059. [Google Scholar] [CrossRef] [PubMed]
- Elsebaie, A.; Shakeel, A.; Zhang, S.; Alarie, M.; Tahan, M.E.; El-Diasty, M. Effect of Oxygen Delivery during Cardiopulmonary Bypass on Postoperative Neurological Outcomes in Patients Undergoing Cardiac Surgery: A Scoping Review of the Literature. Perfusion 2024, 2676591241239279. [Google Scholar] [CrossRef]
- Livingston, E.H.; Lee, S. Body Surface Area Prediction in Normal-Weight and Obese Patients. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E586–E591. [Google Scholar] [CrossRef]
- Aubourg, C.; Collard, A.; Léger, M.; Gros, A.; Fouquet, O.; Sargentini, C.; Rineau, E. Risk Factors and Consequences of Late-Onset Hyperlactatemia After Cardiac Surgery With Cardiopulmonary Bypass: A Single-Center Retrospective Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4077–4084. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yu, G.-L.; Zhou, Y.-P.; Zhang, Q.-M.; Wang, C.-G.; Zhang, S. Association between Lactic Acidosis and Multiple Organ Dysfunction Syndrome after Cardiopulmonary Bypass. PeerJ 2024, 12, e16769. [Google Scholar] [CrossRef] [PubMed]
- Fuernau, G. Lactate and Other Biomarkers as Treatment Target in Cardiogenic Shock. Curr. Opin. Crit. Care 2019, 25, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.J.; Lam, S.W.; Bauer, S.R.; Guzman, J.A. Lactic Acidosis: Clinical Implications and Management Strategies. Clev Clin. J. Med. 2015, 82, 615–624. [Google Scholar] [CrossRef]
- Engoren, M.C.; Habib, R.H.; Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Durham, S.J. Effect of Blood Transfusion on Long-Term Survival after Cardiac Operation. Ann. Thorac. Surg. 2002, 74, 1180–1186. [Google Scholar] [CrossRef]
- Seghrouchni, A.; Atmani, N.; Moutakiallah, Y.; Belmekki, A.; Bekkali, Y.E.; Houssa, M.A. Does Severe Hyperlactatemia during Cardiopulmonary Bypass Predict a Worse Outcome? Ann. Med. Surg. 2022, 73, 103198. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Wu, D.J.; Chen, X.; Xu, M.; Sun, L.; Guo, Z.; He, B.; Wu, J. Target Blood Pressure Management during Cardiopulmonary Bypass Improves Lactate Levels after Cardiac Surgery: A Randomized Controlled Trial. BMC Anesthesiol. 2021, 21, 309. [Google Scholar] [CrossRef] [PubMed]



| Number of Patients | 50 |
| Female/male (%) | 7 (14%)/43 (86%) |
| Age (years) | 66 (61−75) |
| BSA (m2) | 2.1 (1.9−2.2) |
| NYHA I (%) | 7 (14%) |
| NYHA II (%) | 20 (40%) |
| NYHA III (%) | 19 (38%) |
| NYHA not classified (%) | 4 (8%) |
| History of single-vessel CAD | 1 (2%) |
| History of double-vessel CAD | 6 (12%) |
| History of triple-vessel CAD | 43 (86%) |
| History of hypertension | 47 (94%) |
| History of diabetes mellitus | 13 (26%) |
| History of carotid artery stenosis | 4 (8%) |
| ACC duration (minutes) | 57 (47−77) |
| CPB duration (minutes) | 102 (83−121) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisner, C.; Adam, H.; Weigand, M.A.; Zivkovic, A.R. Cerebral Oxygen Saturation Associates with Changes in Oxygen Transport Parameters during Cardiopulmonary Bypass. J. Pers. Med. 2024, 14, 691. https://doi.org/10.3390/jpm14070691
Eisner C, Adam H, Weigand MA, Zivkovic AR. Cerebral Oxygen Saturation Associates with Changes in Oxygen Transport Parameters during Cardiopulmonary Bypass. Journal of Personalized Medicine. 2024; 14(7):691. https://doi.org/10.3390/jpm14070691
Chicago/Turabian StyleEisner, Christoph, Heimo Adam, Markus A. Weigand, and Aleksandar R. Zivkovic. 2024. "Cerebral Oxygen Saturation Associates with Changes in Oxygen Transport Parameters during Cardiopulmonary Bypass" Journal of Personalized Medicine 14, no. 7: 691. https://doi.org/10.3390/jpm14070691






