Biobanks as an Indispensable Tool in the “Era” of Precision Medicine: Key Role in the Management of Complex Diseases, Such as Melanoma
Abstract
1. Introduction
2. Biobanks: General Aspects
2.1. Ethical and Legal Regulation
- ✧
- Description of the materials to be biobanked;
- ✧
- Principles of data sharing with other institutions;
- ✧
- Principles for ‘researchers’ access to data;
- ✧
- Privacy regulations;
- ✧
- Possibility of withdrawal from the study;
- ✧
- Dispositions in case of death;
- ✧
- Principles of non-profit.
2.2. Privacy Policy
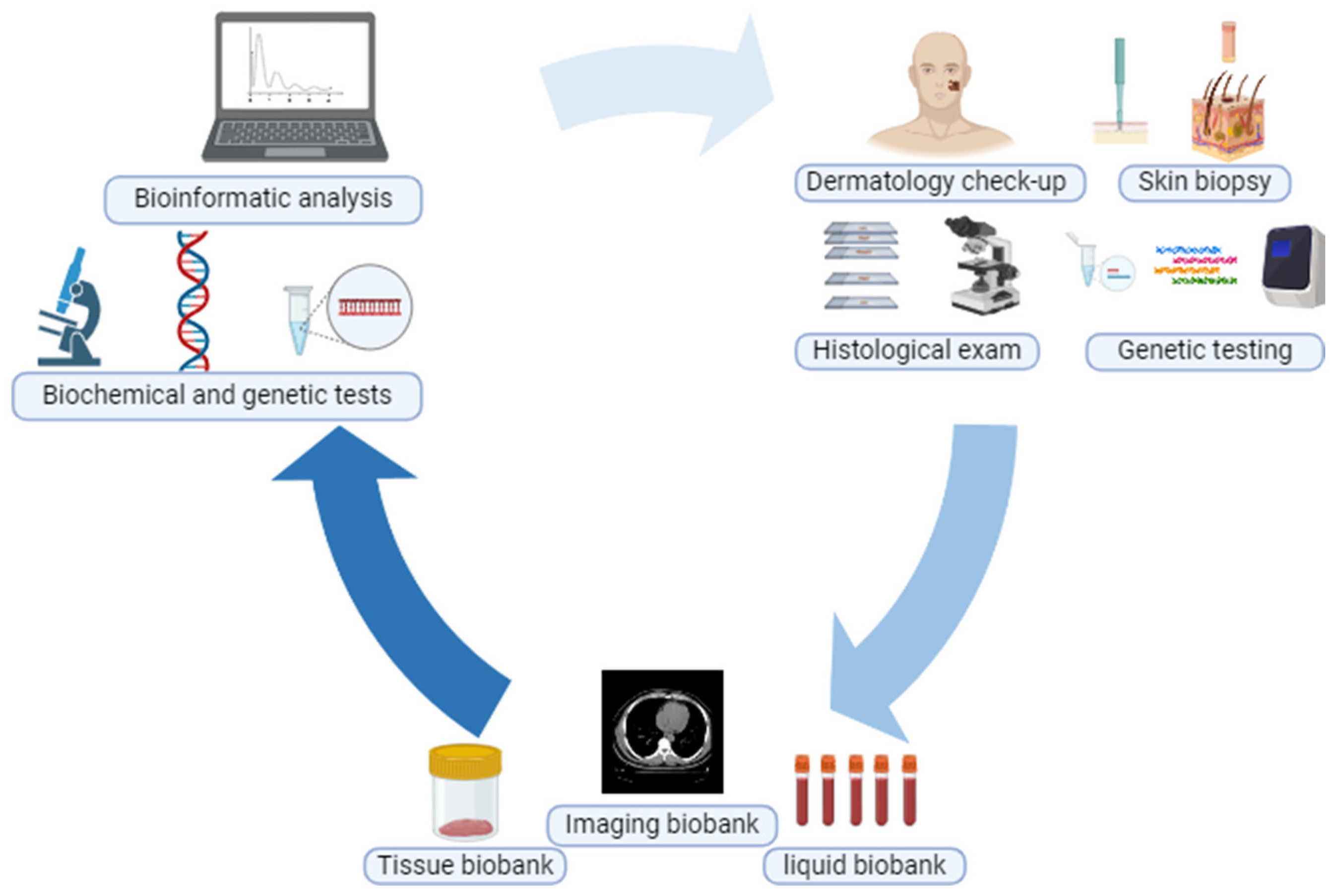
3. Biobank Types
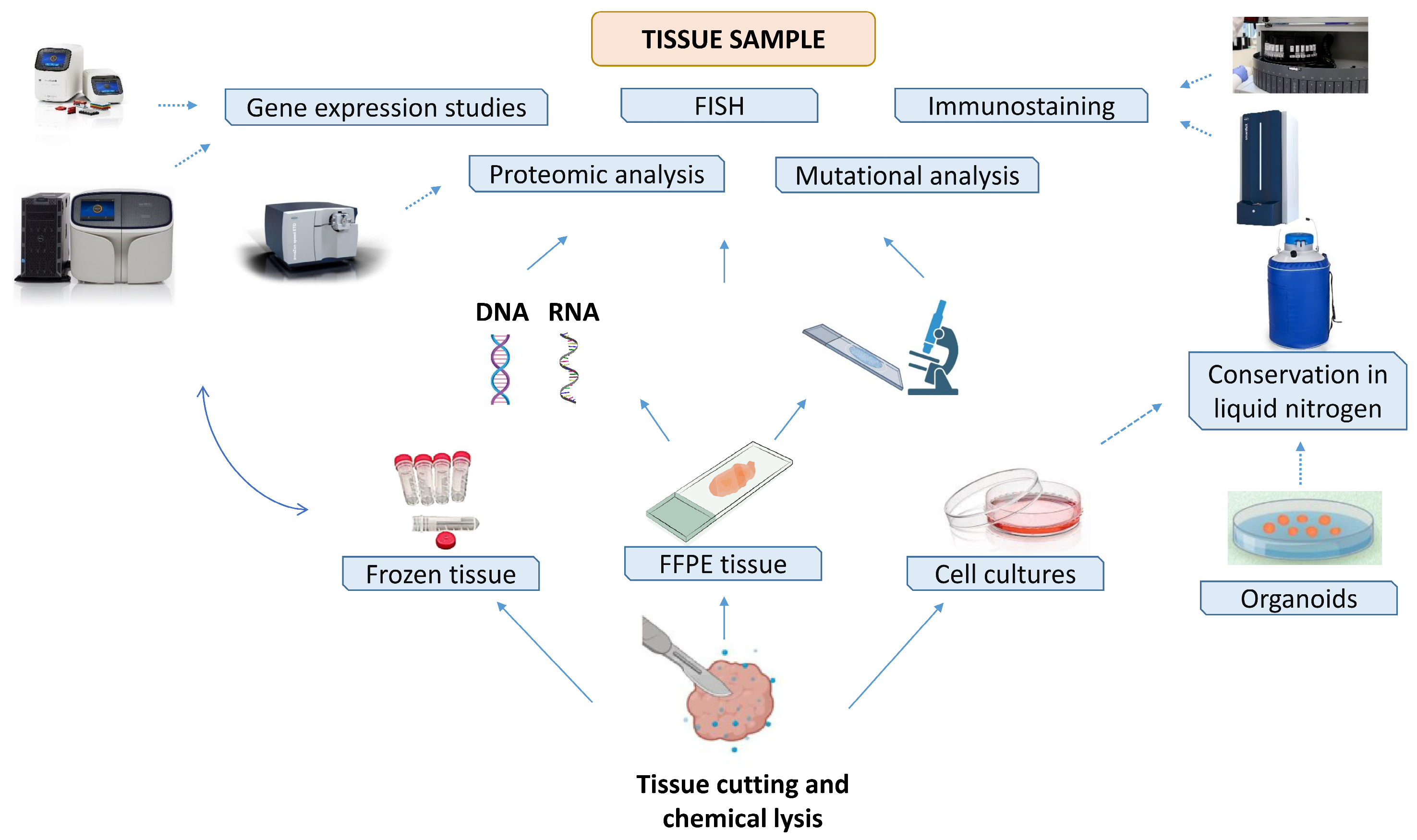
3.1. Tissue Biobank
3.2. Cell and Organoid Biobank
3.3. Liquid Biobank
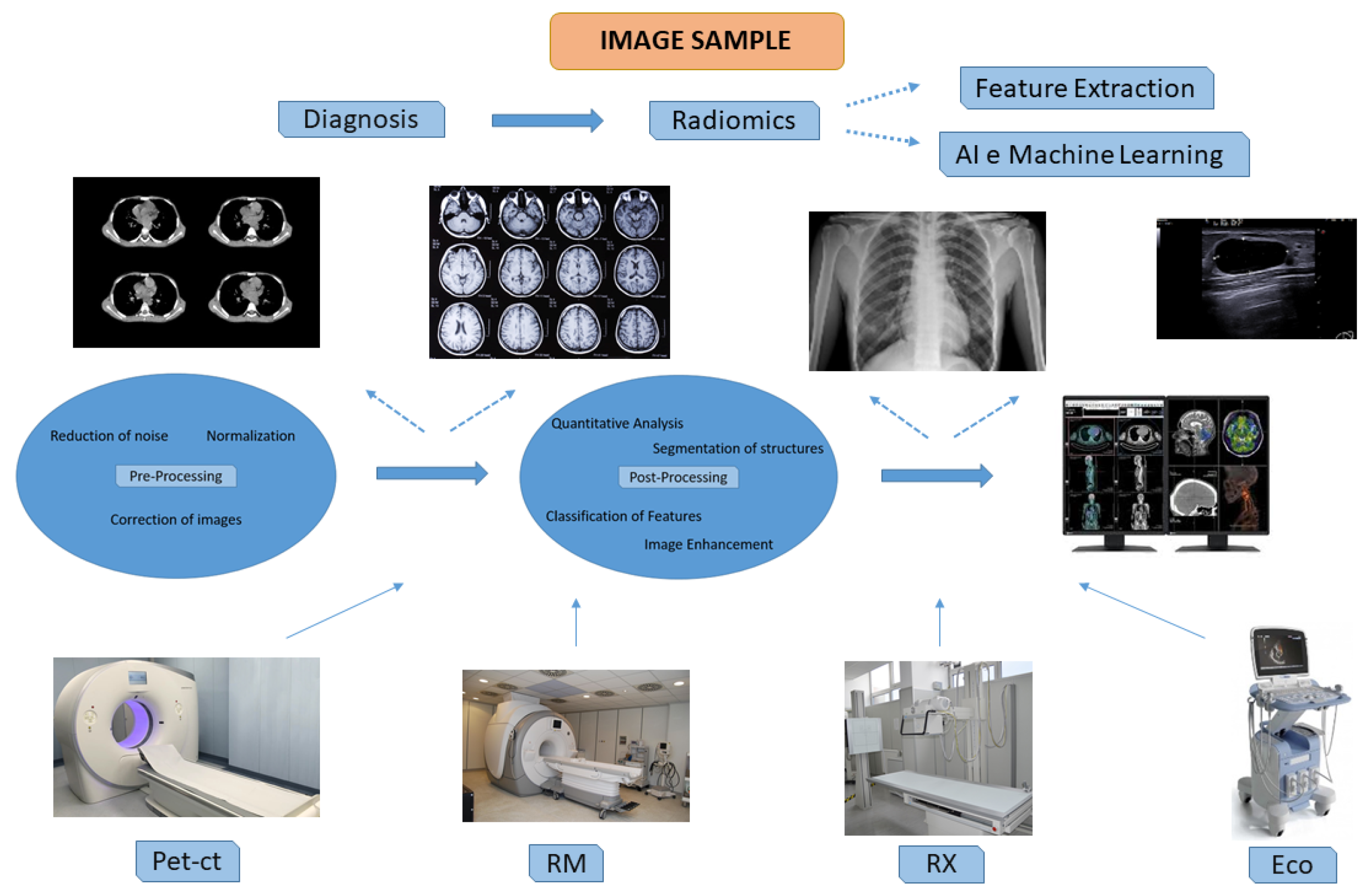
3.4. Imaging Biobank
3.5. Digital Biobank
- Cancer Genome Atlas (TCGA) is a digital biobank focused on oncological research that provides public access to an extensive catalog of genomic and epigenomic data (https://portal.gdc.cancer.gov, accessed on 1 July 2024) [45];
- The European Genome and Phenome Archive (EGA) is a digital biobank focused on storing genetic, phenotypic and clinical data from different research projects while maintaining control over access to data at the sender level (https://ega-archive.org, accessed on 1 July 2024) [46];
- The Global Alliance for Genomics and Health (GA4GH) is an advanced digital biobank that addresses the challenges of the growing production of sequencing data from diverse study populations (https://www.ga4gh.org, accessed on 1 July 2024) [47];
- U.K. Biobank aims to collect, store and manage information and samples from 5,000,000 participants to enable genetic and nongenetic investigations of diseases of aging. It is among the most efficient digital entities and enables sharing through online platforms and systems (https://www.ukbiobank.ac.uk, accessed on 1 July 2024) [48].
4. Biobank Sustainability
5. Melanoma Precision Medicine: Biobanking Role
6. Future Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asslaber, M.; Zatloukal, K. Biobanks: Transnational, European and global networks. Brief. Funct. Genom. Proteomic 2007, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cambon-Thomsen, A. The social and ethical issues of post-genomic human biobanks. Nat. Rev. Genet. 2004, 5, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Annaratone, L.; De Palma, G.; Bonizzi, G.; Sapino, A.; Botti, G.; Berrino, E.; Mannelli, C.; Arcella, P.; Di Martino, S.; Steffan, A.; et al. Basic principles of biobanking: From biological samples to precision medicine for patients. Virchows Arch. 2021, 479, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Shabihkhani, M.; Lucey, G.M.; Wei, B.; Mareninov, S.; Lou, J.J.; Vinters, H.V.; Singer, E.J.; Cloughesy, T.F.; Yong, W.H. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin. Biochem. 2014, 47, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S. Biospecimen repositories and cytopathology. Cancer Cytopathol. 2015, 123, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Vaught, J.; Lockhart, N.C. The evolution of biobanking best practices. Clin. Chim. Acta 2012, 413, 1569–1575. [Google Scholar] [CrossRef]
- Carey, D.J.; Fetterolf, S.N.; Davis, F.D.; Faucett, W.A.; Kirchner, H.L.; Mirshahi, U.; Murray, M.F.; Smelser, D.T.; Gerhard, G.S.; Ledbetter, D.H. The Geisinger MyCode community health initiative: An electronic health record-linked biobank for precision medicine research. Genet. Med. 2016, 18, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Cianflone, A.; Grimaldi, A.M.; Incoronato, M.; Bevilacqua, P.; Messina, F.; Baselice, S.; Soricelli, A.; Mirabelli, P.; Salvatore, M. Biobanking in health care: Evolution and future directions. J. Transl. Med. 2019, 17, 172. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, Z.; Chen, Y.; Jiang, E.; Chen, T.; Wang, C. Positive association between research competitiveness of Chinese academic hospitals and the scale of their biobanks: A national survey. Clin. Transl. Sci. 2022, 15, 2909–2917. [Google Scholar] [CrossRef]
- Valenti, F.; Falcone, I.; Ungania, S.; Desiderio, F.; Giacomini, P.; Bazzichetto, C.; Conciatori, F.; Gallo, E.; Cognetti, F.; Ciliberto, G.; et al. Precision Medicine and Melanoma: Multi-Omics Approaches to Monitoring the Immunotherapy Response. Int. J. Mol. Sci. 2021, 22, 3837. [Google Scholar] [CrossRef]
- Falcone, I.; Conciatori, F.; Bazzichetto, C.; Ferretti, G.; Cognetti, F.; Ciuffreda, L.; Milella, M. Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy. Cancers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Harati, M.D.; Williams, R.R.; Movassaghi, M.; Hojat, A.; Lucey, G.M.; Yong, W.H. An Introduction to Starting a Biobank. Methods Mol. Biol. 2019, 1897, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.H.; Barnes, R.O. A proposed schema for classifying human research biobanks. Biopreserv. Biobank. 2011, 9, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Mendy, M.; Caboux, E.; Lawlor, R.T.; Wright, J.; Wild, C.P. Common Minimum Technical Standards and Protocols for Biobanks Dedicated to Cancer Research; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- De Souza, Y.G.; Greenspan, J.S. Biobanking past, present and future: Responsibilities and benefits. AIDS 2013, 27, 303–312. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Hansson, M.G. Ethics and biobanks. Br. J. Cancer 2009, 100, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Cai, W. Privacy Protection and Secondary Use of Health Data: Strategies and Methods. Biomed. Res. Int. 2021, 2021, 6967166. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Hu, Z.; Muallem, H.; Gulley, M.L. Quality assurance of RNA expression profiling in clinical laboratories. J. Mol. Diagn. 2012, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Naber, S.P. Continuing role of a frozen-tissue bank in molecular pathology. Diagn. Mol. Pathol. 1996, 5, 253–259. [Google Scholar] [CrossRef]
- Shi, S.R.; Liu, C.; Pootrakul, L.; Tang, L.; Young, A.; Chen, R.; Cote, R.J.; Taylor, C.R. Evaluation of the value of frozen tissue section used as “gold standard” for immunohistochemistry. Am. J. Clin. Pathol. 2008, 129, 358–366. [Google Scholar] [CrossRef]
- Bruschini, S.; di Martino, S.; Pisanu, M.E.; Fattore, L.; De Vitis, C.; Laquintana, V.; Buglioni, S.; Tabbi, E.; Cerri, A.; Visca, P.; et al. CytoMatrix for a reliable and simple characterization of lung cancer stem cells from malignant pleural effusions. J. Cell Physiol. 2020, 235, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The boom in mini stomachs, brains, breasts, kidneys and more. Nature 2015, 523, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; van der Ent, C.K.; Beekman, J.M. Novel opportunities for CFTR-targeting drug development using organoids. Rare Dis. 2013, 1, e27112. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Zhu, Y.J.; Xiao, Z.Z.; Chen, Y.D.; Chang, X.S.; Liu, Y.H.; Tang, Q.; Zhang, H.B. The pivotal application of patient-derived organoid biobanks for personalized treatment of gastrointestinal cancers. Biomark. Res. 2022, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; Piccotti, F.; Allevi, R.; Truffi, M.; Sorrentino, L.; Russo, L.; Agozzino, M.; Signati, L.; Bonizzi, A.; Villani, L.; et al. Establishment and Morphological Characterization of Patient-Derived Organoids from Breast Cancer. Biol. Proced. Online 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Beshiri, M.L.; Tice, C.M.; Tran, C.; Nguyen, H.M.; Sowalsky, A.G.; Agarwal, S.; Jansson, K.H.; Yang, Q.; McGowen, K.M.; Yin, J.; et al. A PDX/Organoid Biobank of Advanced Prostate Cancers Captures Genomic and Phenotypic Heterogeneity for Disease Modeling and Therapeutic Screening. Clin. Cancer Res. 2018, 24, 4332–4345. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204.e122. [Google Scholar] [CrossRef]
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers 2020, 12, 2727. [Google Scholar] [CrossRef]
- Ricciardi, E.; Giordani, E.; Ziccheddu, G.; Falcone, I.; Giacomini, P.; Fanciulli, M.; Russillo, M.; Cerro, M.; Ciliberto, G.; Morrone, A.; et al. Metastatic Melanoma: Liquid Biopsy as a New Precision Medicine Approach. Int. J. Mol. Sci. 2023, 24, 4014. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.T.; Smith, M.T.; Eskenazi, B.; Bastaki, M. Biological sample collection and processing for molecular epidemiological studies. Mutat. Res. 2003, 543, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Gillio-Meina, C.; Cepinskas, G.; Cecchini, E.L.; Fraser, D.D. Translational research in pediatrics II: Blood collection, processing, shipping, and storage. Pediatrics 2013, 131, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Zattoni, L.; Capra, M.; Cassi, C.; Taliento, G.; Ivanova, M.; Guerini-Rocco, E.; Fumagalli, M.; Monturano, M.; Albini, A.; et al. Standard operating procedures for biobank in oncology. Front. Mol. Biosci. 2022, 9, 967310. [Google Scholar] [CrossRef] [PubMed]
- Halsall, A.; Ravetto, P.; Reyes, Y.; Thelwell, N.; Davidson, A.; Gaut, R.; Little, S. The quality of DNA extracted from liquid or dried blood is not adversely affected by storage at 4 degrees C for up to 24 h. Int. J. Epidemiol. 2008, 37 (Suppl. 1), i7–i10. [Google Scholar] [CrossRef] [PubMed]
- Guerrisi, A.; Russillo, M.; Loi, E.; Ganeshan, B.; Ungania, S.; Desiderio, F.; Bruzzaniti, V.; Falcone, I.; Renna, D.; Ferraresi, V.; et al. Exploring CT Texture Parameters as Predictive and Response Imaging Biomarkers of Survival in Patients With Metastatic Melanoma Treated With PD-1 Inhibitor Nivolumab: A Pilot Study Using a Delta-Radiomics Approach. Front. Oncol. 2021, 11, 704607. [Google Scholar] [CrossRef] [PubMed]
- European Society of Radiology (ESR). ESR Position Paper on Imaging Biobanks. Insights Imaging 2015, 6, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Longabaugh, W.J.R.; Pot, D.; Clunie, D.A.; Pieper, S.D.; Gibbs, D.L.; Bridge, C.; Herrmann, M.D.; Homeyer, A.; Lewis, R.; et al. National Cancer Institute Imaging Data Commons: Toward Transparency, Reproducibility, and Scalability in Imaging Artificial Intelligence. Radiographics 2023, 43, e230180. [Google Scholar] [CrossRef]
- Gabelloni, M.; Faggioni, L.; Borgheresi, R.; Restante, G.; Shortrede, J.; Tumminello, L.; Scapicchio, C.; Coppola, F.; Cioni, D.; Gomez-Rico, I.; et al. Bridging gaps between images and data: A systematic update on imaging biobanks. Eur. Radiol. 2022, 32, 3173–3186. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Holliday, J.; Gibson, L.M.; Garratt, S.; Oesingmann, N.; Alfaro-Almagro, F.; Bell, J.D.; Boultwood, C.; Collins, R.; Conroy, M.C.; et al. The UK Biobank imaging enhancement of 100,000 participants: Rationale, data collection, management and future directions. Nat. Commun. 2020, 11, 2624. [Google Scholar] [CrossRef]
- Borgheresi, R.; Barucci, A.; Colantonio, S.; Aghakhanyan, G.; Assante, M.; Bertelli, E.; Carlini, E.; Carpi, R.; Caudai, C.; Cavallero, D.; et al. NAVIGATOR: An Italian regional imaging biobank to promote precision medicine for oncologic patients. Eur. Radiol. Exp. 2022, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Marti-Bonmati, L.; Alberich-Bayarri, A.; Ladenstein, R.; Blanquer, I.; Segrelles, J.D.; Cerda-Alberich, L.; Gkontra, P.; Hero, B.; Garcia-Aznar, J.M.; Keim, D.; et al. PRIMAGE project: Predictive in silico multiscale analytics to support childhood cancer personalised evaluation empowered by imaging biomarkers. Eur. Radiol. Exp. 2020, 4, 22. [Google Scholar] [CrossRef]
- Kondylakis, H.; Kalokyri, V.; Sfakianakis, S.; Marias, K.; Tsiknakis, M.; Jimenez-Pastor, A.; Camacho-Ramos, E.; Blanquer, I.; Segrelles, J.D.; Lopez-Huguet, S.; et al. Data infrastructures for AI in medical imaging: A report on the experiences of five EU projects. Eur. Radiol. Exp. 2023, 7, 20. [Google Scholar] [CrossRef]
- Gao, G.F.; Parker, J.S.; Reynolds, S.M.; Silva, T.C.; Wang, L.B.; Zhou, W.; Akbani, R.; Bailey, M.; Balu, S.; Berman, B.P.; et al. Before and After: Comparison of Legacy and Harmonized TCGA Genomic Data Commons’ Data. Cell Syst. 2019, 9, 24–34.e10. [Google Scholar] [CrossRef] [PubMed]
- Freeberg, M.A.; Fromont, L.A.; D‘Altri, T.; Romero, A.F.; Ciges, J.I.; Jene, A.; Kerry, G.; Moldes, M.; Ariosa, R.; Bahena, S.; et al. The European Genome-phenome Archive in 2021. Nucleic Acids Res. 2022, 50, D980–D987. [Google Scholar] [CrossRef] [PubMed]
- Rehm, H.L.; Page, A.J.H.; Smith, L.; Adams, J.B.; Alterovitz, G.; Babb, L.J.; Barkley, M.P.; Baudis, M.; Beauvais, M.J.S.; Beck, T.; et al. GA4GH: International policies and standards for data sharing across genomic research and healthcare. Cell Genom. 2021, 1, 100029. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Abdaljaleel, M.; Singer, E.J.; Yong, W.H. Sustainability in Biobanking. Methods Mol. Biol. 2019, 1897, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Vaught, J.; Tulskie, B.; Olson, D.; Odeh, H.; McLean, J.; Moore, H.M. Critical Financial Challenges for Biobanking: Report of a National Cancer Institute Study. Biopreserv. Biobank. 2019, 17, 129–138. [Google Scholar] [CrossRef]
- Rush, A.; Catchpoole, D.R.; Ling, R.; Searles, A.; Watson, P.H.; Byrne, J.A. Improving Academic Biobank Value and Sustainability Through an Outputs Focus. Value Health 2020, 23, 1072–1078. [Google Scholar] [CrossRef]
- Ostrowski, S.M.; Fisher, D.E. Biology of Melanoma. Hematol. Oncol. Clin. North Am. 2021, 35, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shklovskaya, E.; Lee, J.H.; Pedersen, B.; Stewart, A.; Ming, Z.; Irvine, M.; Shivalingam, B.; Saw, R.P.M.; Menzies, A.M.; et al. The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma. Nat. Commun. 2023, 14, 1516. [Google Scholar] [CrossRef] [PubMed]
- Tangella, L.P.; Clark, M.E.; Gray, E.S. Resistance mechanisms to targeted therapy in BRAF-mutant melanoma—A mini review. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129736. [Google Scholar] [CrossRef] [PubMed]
- Shannan, B.; Perego, M.; Somasundaram, R.; Herlyn, M. Heterogeneity in Melanoma. Cancer Treat. Res. 2016, 167, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Helkkula, T.; Christensen, G.; Ingvar, C.; Isaksson, K.; Harbst, K.; Persson, B.; Ingvar, A.; Hafstrom, A.; Carneiro, A.; Gaspar, V.; et al. BioMEL: A translational research biobank of melanocytic lesions and melanoma. BMJ Open 2024, 14, e069694. [Google Scholar] [CrossRef]
- Seviiri, M.; Scolyer, R.A.; Bishop, D.T.; Newton-Bishop, J.A.; Iles, M.M.; Lo, S.N.; Stretch, J.R.; Saw, R.P.M.; Nieweg, O.E.; Shannon, K.F.; et al. Higher polygenic risk for melanoma is associated with improved survival in a high ultraviolet radiation setting. J. Transl. Med. 2022, 20, 403. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.S.; Klein, K.; Weide, B.; Haydu, L.E.; Pflugfelder, A.; Tang, Y.H.; Palmer, J.M.; Whiteman, D.C.; Scolyer, R.A.; Mann, G.J.; et al. The Prognostic and Predictive Value of Melanoma-related MicroRNAs Using Tissue and Serum: A MicroRNA Expression Analysis. EBioMedicine 2015, 2, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Versluis, J.M.; Blankenstein, S.A.; Dimitriadis, P.; Wilmott, J.S.; Elens, R.; Blokx, W.A.M.; van Houdt, W.; Menzies, A.M.; Schrage, Y.M.; Wouters, M.; et al. Interferon-gamma signature as prognostic and predictive marker in macroscopic stage III melanoma. J. Immunother. Cancer 2024, 12, 8125. [Google Scholar] [CrossRef] [PubMed]
- Marcell Szasz, A.; Malm, J.; Rezeli, M.; Sugihara, Y.; Betancourt, L.H.; Rivas, D.; Gyorffy, B.; Marko-Varga, G. Challenging the heterogeneity of disease presentation in malignant melanoma-impact on patient treatment. Cell Biol. Toxicol. 2019, 35, 1–14. [Google Scholar] [CrossRef]
- Prendergast, C.M.; Capaccione, K.M.; Lopci, E.; Das, J.P.; Shoushtari, A.N.; Yeh, R.; Amin, D.; Dercle, L.; De Jong, D. More than Just Skin-Deep: A Review of Imaging’s Role in Guiding CAR T-Cell Therapy for Advanced Melanoma. Diagnostics 2023, 13, 992. [Google Scholar] [CrossRef]
- Durot, C.; Mule, S.; Soyer, P.; Marchal, A.; Grange, F.; Hoeffel, C. Metastatic melanoma: Pretreatment contrast-enhanced CT texture parameters as predictive biomarkers of survival in patients treated with pembrolizumab. Eur. Radiol. 2019, 29, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Peisen, F.; Gerken, A.; Dahm, I.; Nikolaou, K.; Eigentler, T.; Amaral, T.; Moltz, J.H.; Othman, A.E.; Gatidis, S. Pre-treatment 18F-FDG-PET/CT parameters as biomarkers for progression free survival, best overall response and overall survival in metastatic melanoma patients undergoing first-line immunotherapy. PLoS ONE 2024, 19, e0296253. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.K.; Goldring, K.; Simeon-Dubach, D. Advancing Professionalization of Biobank Business Operations: A Worldwide Survey. Biopreserv. Biobank. 2019, 17, 71–75. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenti, A.; Falcone, I.; Valenti, F.; Ricciardi, E.; Di Martino, S.; Maccallini, M.T.; Cerro, M.; Desiderio, F.; Miseo, L.; Russillo, M.; et al. Biobanks as an Indispensable Tool in the “Era” of Precision Medicine: Key Role in the Management of Complex Diseases, Such as Melanoma. J. Pers. Med. 2024, 14, 731. https://doi.org/10.3390/jpm14070731
Valenti A, Falcone I, Valenti F, Ricciardi E, Di Martino S, Maccallini MT, Cerro M, Desiderio F, Miseo L, Russillo M, et al. Biobanks as an Indispensable Tool in the “Era” of Precision Medicine: Key Role in the Management of Complex Diseases, Such as Melanoma. Journal of Personalized Medicine. 2024; 14(7):731. https://doi.org/10.3390/jpm14070731
Chicago/Turabian StyleValenti, Alessandro, Italia Falcone, Fabio Valenti, Elena Ricciardi, Simona Di Martino, Maria Teresa Maccallini, Marianna Cerro, Flora Desiderio, Ludovica Miseo, Michelangelo Russillo, and et al. 2024. "Biobanks as an Indispensable Tool in the “Era” of Precision Medicine: Key Role in the Management of Complex Diseases, Such as Melanoma" Journal of Personalized Medicine 14, no. 7: 731. https://doi.org/10.3390/jpm14070731
APA StyleValenti, A., Falcone, I., Valenti, F., Ricciardi, E., Di Martino, S., Maccallini, M. T., Cerro, M., Desiderio, F., Miseo, L., Russillo, M., & Guerrisi, A. (2024). Biobanks as an Indispensable Tool in the “Era” of Precision Medicine: Key Role in the Management of Complex Diseases, Such as Melanoma. Journal of Personalized Medicine, 14(7), 731. https://doi.org/10.3390/jpm14070731




