The cGAS/STING Pathway—A New Potential Biotherapeutic Target for Gastric Cancer?
Abstract
:1. Backgrounds
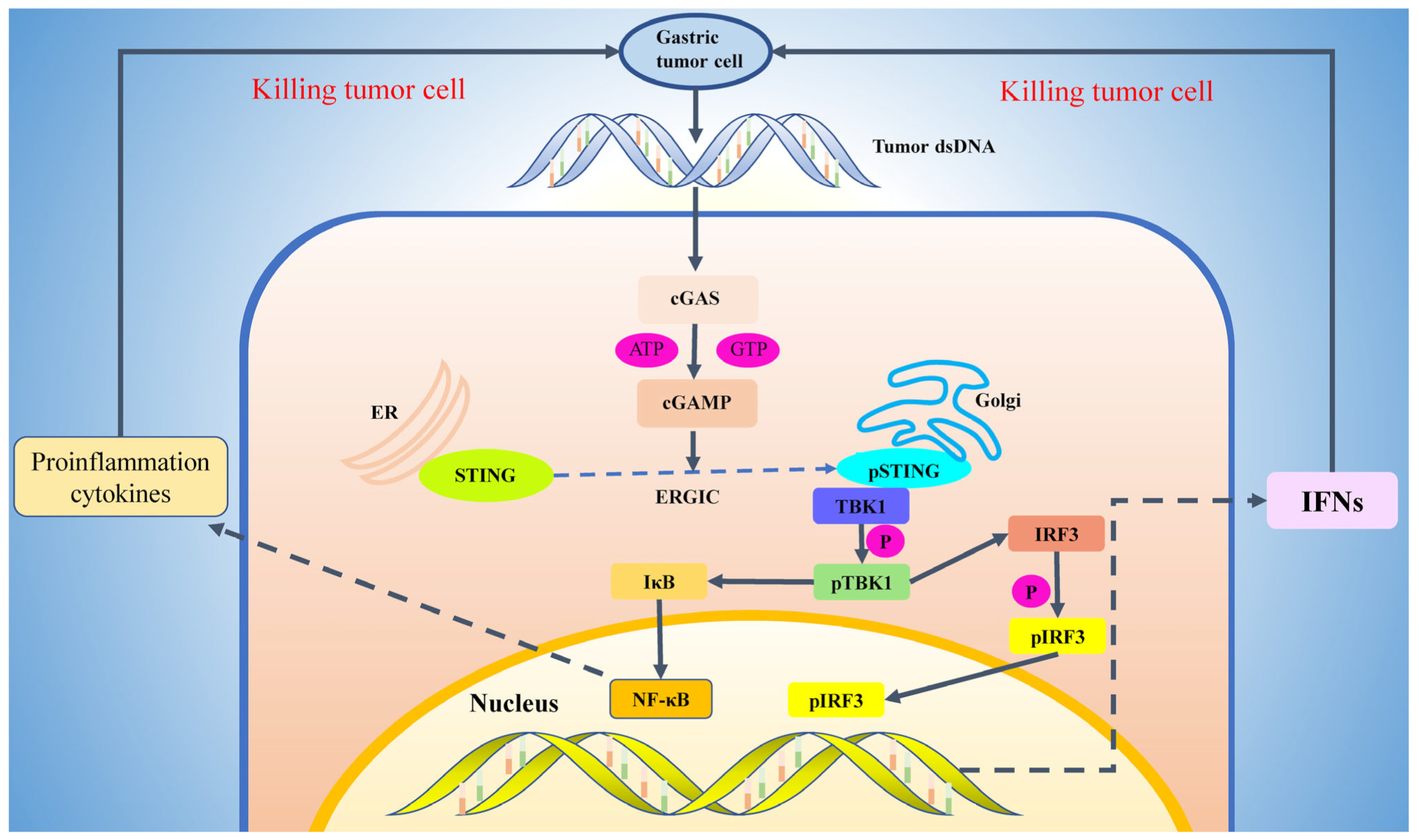
2. Activation Mechanism of cGAS-STING Pathway
3. The Role of cGAS-STING Signaling Pathway in Tumor Biotherapy
4. The cGAS-STING Pathway in Gastric Cancer
5. Types of STING Agonists and Their Related Clinical Trials
6. Application of Nano Drug Delivery Technology
7. Conclusions and Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TME | Tumor microenvironment |
| cGAS | cyclic GMP–AMP synthase |
| STING | stimulator of interferon genes |
| DAMP | damage-associated molecular pattern |
| dsDNA | double-stranded DNA |
| cGAMP | cyclic GMP-AMP |
| ATP | Adenosine Triphosphate |
| CTD | C-terminal nucleotide transferase domain |
| ER | Endoplasmic reticulum |
| CTT | C-terminal tail |
| ERGIC | endoplasmic reticulum Golgi intermediate chamber |
| TBK1 | tank-binding kinase 1 |
| IKK | inhibitor of kappa B kinas |
| IRF3 | interferon regulatory factor 3 |
| IFN | interferon |
| NF-kB | nuclear factor kappa B |
| TAK1 | TGF-beta-activated kinase 1 |
| IKK | I kappa B kinase |
| NK | cells natural killer cells |
| CRC | colorectal cancer |
| CDN | cyclic dinucleotides |
| NCDN | non- cyclic dinucleotides |
| OS | overall survival |
| HER2 | human epidermalal growth factor receptor receptor 2 |
| PD-1 | programmed death-1 |
| PD-L1 | Programmed cell death ligand 1 |
| MFC | mouse forestomach carcinoma |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W.; Hong, S.J.; Kim, S.H. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology 2024, 166, 313–322.e3. [Google Scholar] [CrossRef]
- He, S.; Xia, C.; Li, H.; Cao, M.; Yang, F.; Yan, X.; Zhang, S.; Teng, Y.; Li, Q.; Chen, W. Cancer profiles in China and comparisons with the USA: A comprehensive analysis in the incidence, mortality, survival, staging, and attribution to risk factors. Sci. China Life Sci. 2024, 67, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Zhang, X.T.; Li, Y.F.; Tang, L.; Qu, X.J.; Ying, J.E.; Zhang, J.; Sun, L.Y.; Lin, R.B.; Qiu, H.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021, 41, 747–795. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.E.; Strong, V.E. Gastric Cancer Etiology and Management in Asia and the West. Annu. Rev. Med. 2019, 70, 353–367. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wu, X.B. Pembrolizumab plus chemotherapy for advanced gastric cancer. Lancet Oncol. 2024, 25, e50. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef]
- Samson, N.; Ablasser, A. The cGAS-STING pathway and cancer. Nat. Cancer 2022, 3, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, X.C.; Chen, Z.J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Ergun, S.L.; Li, L. Structural Insights into STING Signaling. Trends Cell Biol. 2020, 30, 399–407. [Google Scholar] [CrossRef]
- Vashi, N.; Bakhoum, S.F. The Evolution of STING Signaling and Its Involvement in Cancer. Trends Biochem. Sci. 2021, 46, 446–460. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.T.; Grishin, N.V.; et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015, 347, aaa2630. [Google Scholar] [CrossRef]
- Tanaka, Y.; Chen, Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012, 5, ra20. [Google Scholar] [CrossRef]
- Bowie, A. The STING in the tail for cytosolic DNA-dependent activation of IRF3. Sci. Signal. 2012, 5, pe9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.C.; Nandakumar, R.; Reinert, L.S.; Huang, J.; Laustsen, A.; Gao, Z.L.; Sun, C.L.; Jensen, S.B.; Troldborg, A.; Assil, S.; et al. STEEP mediates STING ER exit and activation of signaling. Nat. Immunol. 2020, 21, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Dang, Y.; Chang, B.; Wang, F.; Xu, J.; Chen, L.; Su, H.; Li, J.; Ge, B.; Chen, C.; et al. TAK1 is an essential kinase for STING trafficking. Mol. Cell 2023, 83, 3885–3903.e3885. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, G.; Gui, X.; Zhang, X.; Bai, X.C.; Chen, Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature 2019, 567, 394–398. [Google Scholar] [CrossRef]
- King, K.R.; Aguirre, A.D.; Ye, Y.X.; Sun, Y.; Roh, J.D.; Ng, R.P., Jr.; Kohler, R.H.; Arlauckas, S.P.; Iwamoto, Y.; Savol, A.; et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 2017, 23, 1481–1487. [Google Scholar] [CrossRef]
- Balka, K.R.; Louis, C.; Saunders, T.L.; Smith, A.M.; Calleja, D.J.; D’Silva, D.B.; Moghaddas, F.; Tailler, M.; Lawlor, K.E.; Zhan, Y.; et al. TBK1 and IKKε Act Redundantly to Mediate STING-Induced NF-κB Responses in Myeloid Cells. Cell Rep. 2020, 31, 107492. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Hou, Y.; Cui, K.; Yang, X.; Li, X.; Chen, L.; Liu, S.; Zhang, Z.; Jia, Y.; et al. The mechanism of STING autoinhibition and activation. Mol. Cell 2023, 83, 1502–1518.e10. [Google Scholar] [CrossRef] [PubMed]
- Bulgakova, O.; Kausbekova, A.; Kussainova, A.; Kalibekov, N.; Serikbaiuly, D.; Bersimbaev, R. Involvement of Circulating Cell-Free Mitochondrial DNA and Proinflammatory Cytokines in Pathogenesis of Chronic Obstructive Pulmonary Disease and Lung Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Oduro, P.K.; Zheng, X.; Wei, J.; Yang, Y.; Wang, Y.; Zhang, H.; Liu, E.; Gao, X.; Du, M.; Wang, Q. The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy. Acta Pharm. Sin. B 2022, 12, 50–75. [Google Scholar] [CrossRef] [PubMed]
- Gonugunta, V.K.; Sakai, T.; Pokatayev, V.; Yang, K.; Wu, J.; Dobbs, N.; Yan, N. Trafficking-Mediated STING Degradation Requires Sorting to Acidified Endolysosomes and Can Be Targeted to Enhance Anti-tumor Response. Cell Rep. 2017, 21, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 2020, 78, 1019–1033. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Lorenzi, S.; Mattei, F.; Sistigu, A.; Bracci, L.; Spadaro, F.; Sanchez, M.; Spada, M.; Belardelli, F.; Gabriele, L.; Schiavoni, G. Type I IFNs control antigen retention and survival of CD8α(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J. Immunol. 2011, 186, 5142–5150. [Google Scholar] [CrossRef]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef]
- Simpson, J.A.; Al-Attar, A.; Watson, N.F.; Scholefield, J.H.; Ilyas, M.; Durrant, L.G. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut 2010, 59, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Laengle, J.; Stift, J.; Bilecz, A.; Wolf, B.; Beer, A.; Hegedus, B.; Stremitzer, S.; Starlinger, P.; Tamandl, D.; Pils, D.; et al. DNA damage predicts prognosis and treatment response in colorectal liver metastases superior to immunogenic cell death and T cells. Theranostics 2018, 8, 3198–3213. [Google Scholar] [CrossRef]
- Gordon-Alonso, M.; Hirsch, T.; Wildmann, C.; van der Bruggen, P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017, 8, 793. [Google Scholar] [CrossRef]
- Clancy-Thompson, E.; Perekslis, T.J.; Croteau, W.; Alexander, M.P.; Chabanet, T.B.; Turk, M.J.; Huang, Y.H.; Mullins, D.W. Melanoma Induces, and Adenosine Suppresses, CXCR3-Cognate Chemokine Production and T-cell Infiltration of Lungs Bearing Metastatic-like Disease. Cancer Immunol. Res. 2015, 3, 956–967. [Google Scholar] [CrossRef]
- Vornholz, L.; Isay, S.E.; Kurgyis, Z.; Strobl, D.C.; Loll, P.; Mosa, M.H.; Luecken, M.D.; Sterr, M.; Lickert, H.; Winter, C.; et al. Synthetic enforcement of STING signaling in cancer cells appropriates the immune microenvironment for checkpoint inhibitor therapy. Sci. Adv. 2023, 9, eadd8564. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Xu, W.; Atkinson, V.G.; Menzies, A.M. Intratumoural immunotherapies in oncology. Eur. J. Cancer 2020, 127, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, F.; Zhang, L.; Wang, M.; Yin, L.; Dong, X.; Xiao, H.; Xing, N. Targeting DNA Damage and Repair Machinery via Delivering WEE1 Inhibitor and Platinum (IV) Prodrugs to Stimulate STING Pathway for Maximizing Chemo-Immunotherapy in Bladder Cancer. Adv. Mater. 2024, 36, e2308762. [Google Scholar] [CrossRef]
- Dooyema, S.D.R.; Noto, J.M.; Wroblewski, L.E.; Piazuelo, M.B.; Krishna, U.; Suarez, G.; Romero-Gallo, J.; Delgado, A.G.; Peek, R.M. Helicobacter pylori actively suppresses innate immune nucleic acid receptors. Gut Microbes 2022, 14, 2105102. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, P.; Tang, Z.; Zhao, J.; Wu, W.; Li, H.; Shao, M.; Li, L.; Yang, C.; Duan, F.; et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci. Rep. 2017, 7, 39858. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, Y.; Chen, X.; Li, L.; Wu, H.; Liu, Y.; Xu, H.; Zhou, G.; Fan, X.; Xia, H. Prognostic Perspectives of STING and PD-L1 Expression and Correlation with the Prognosis of Epstein-Barr Virus-Associated Gastric Cancers. Gut Liver 2022, 16, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.S.; Xu, C.Q.; Lv, J. Identification and validation of the prognostic value of cyclic GMP-AMP synthase-stimulator of interferon (cGAS-STING) related genes in gastric cancer. Bioengineered 2021, 12, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, Z.; Zhang, H.; Huang, S.; Xu, M.; Pan, H. Transcriptomic characterization and construction of M2 macrophage-related prognostic and immunotherapeutic signature in ovarian metastasis of gastric cancer. Cancer Immunol. Immunother. CII 2023, 72, 1121–1138. [Google Scholar] [CrossRef]
- Fukai, S.; Nakajima, S.; Saito, M.; Saito, K.; Kase, K.; Nakano, H.; Sato, T.; Sakuma, M.; Kaneta, A.; Okayama, H.; et al. Down-regulation of stimulator of interferon genes (STING) expression and CD8(+) T-cell infiltration depending on HER2 heterogeneity in HER2-positive gastric cancer. Gastric Cancer 2023, 26, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Guo, X.L.; Chen, J.H.; He, Y.; Liu, Z.Q.; Zhang, H.P.; Ren, J.; Xu, Q. Anlotinib suppresses proliferation, migration, and immune escape of gastric cancer cells by activating the cGAS-STING/IFN-β pathway. Neoplasma 2022, 69, 807–819. [Google Scholar] [CrossRef]
- Hong, S.; Bi, M.; Yu, H.; Yan, Z.; Wang, H. Radiation therapy enhanced therapeutic efficacy of anti-PD1 against gastric cancer. J. Radiat. Res. 2020, 61, 851–859. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, L.; Li, C.; Wang, T.; Lv, J.; Liu, W.; Lin, Y.; Yin, Y.; Tao, K. Metformin promotes cGAS/STING signaling pathway activation by blocking AKT phosphorylation in gastric cancer. Heliyon 2023, 9, e18954. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Imani, M.; Pourfarzi, F.; Jafari, N.; AbedianKenari, S.; Safarzadeh, E. Combination of IFN-gamma with STING agonist and PD-1 immune checkpoint blockade: A potential immunotherapy for gastric cancer. Med. Oncol. 2024, 41, 110. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, P.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; He, Y.; et al. cGAS-STING, an important pathway in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zhao, L.; Hu, H.G.; Li, W.H.; Li, Y.M. Agonists and inhibitors of the STING pathway: Potential agents for immunotherapy. Med. Res. Rev. 2020, 40, 1117–1141. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Z.; Ma, J.; Wang, Y.; Cheng, N. A next-generation STING agonist MSA-2: From mechanism to application. J. Control. Release 2024, 371, 273–287. [Google Scholar] [CrossRef]
- Zaver, S.A.; Woodward, J.J. Cyclic dinucleotides at the forefront of innate immunity. Curr. Opin. Cell Biol. 2020, 63, 49–56. [Google Scholar] [CrossRef]
- Xin, G.F.; Chen, N.N.; Li, L.L.; Liu, X.C.; Che, C.C.; Wu, B.D.; You, Q.D.; Xu, X.L. An updated patent review of stimulator of interferon genes agonists (2021-present). Expert Opin. Ther. Pat. 2024, 34, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Zhang, J.; Quan, H.; Yang, L.; Gao, Y.Q. CDNs-STING Interaction Mechanism Investigations and Instructions on Design of CDN-Derivatives. J. Phys. Chem. B 2018, 122, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Sweis, R.F.; Hodi, F.S.; Messersmith, W.A.; Andtbacka, R.H.I.; Ingham, M.; Lewis, N.; Chen, X.; Pelletier, M.; Chen, X.; et al. Phase I Dose-Escalation Trial of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients with Advanced/Metastatic Solid Tumors or Lymphomas. Clin. Cancer Res. 2022, 28, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yang, H.; Kim, W.R.; Lee, Y.S.; Lee, W.S.; Kong, S.J.; Lee, H.J.; Kim, J.H.; Cheon, J.; Kang, B.; et al. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J. Immunother. Cancer 2021, 9, e002195. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Sweis, R.F.; Kasper, S.; Hamid, O.; Bhatia, S.; Dummer, R.; Stradella, A.; Long, G.V.; Spreafico, A.; Shimizu, T.; et al. Combination of the STING Agonist MIW815 (ADU-S100) and PD-1 Inhibitor Spartalizumab in Advanced/Metastatic Solid Tumors or Lymphomas: An Open-Label, Multicenter, Phase Ib Study. Clin. Cancer Res. 2023, 29, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Altman, M.D.; Lesburg, C.A.; Perera, S.A.; Piesvaux, J.A.; Schroeder, G.K.; Wyss, D.F.; Cemerski, S.; Chen, Y.; DiNunzio, E.; et al. Discovery of MK-1454: A Potent Cyclic Dinucleotide Stimulator of Interferon Genes Agonist for the Treatment of Cancer. J. Med. Chem. 2022, 65, 5675–5689. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chelvanambi, M.; Filderman, J.N.; Storkus, W.J.; Luke, J.J. STING Agonists as Cancer Therapeutics. Cancers 2021, 13, 2695. [Google Scholar] [CrossRef]
- Challa, S.V.; Zhou, S.; Sheri, A.; Padmanabhan, S.; Meher, G.; Gimi, R.; Schmidt, D.; Cleary, D.; Afdhal, N.; Iyer, R. Preclinical studies of SB 11285, a novel STING agonist for immuno-oncology. J. Clin. Oncol. 2017, 35, e14616. [Google Scholar] [CrossRef]
- Cherney, E.C.; Zhang, L.; Lo, J.; Huynh, T.; Wei, D.; Ahuja, V.; Quesnelle, C.; Schieven, G.L.; Futran, A.; Locke, G.A.; et al. Discovery of Non-Nucleotide Small-Molecule STING Agonists via Chemotype Hybridization. J. Med. Chem. 2022, 65, 3518–3538. [Google Scholar] [CrossRef]
- Shih, A.Y.; Damm-Ganamet, K.L.; Mirzadegan, T. Dynamic Structural Differences between Human and Mouse STING Lead to Differing Sensitivity to DMXAA. Biophys. J. 2018, 114, 32–39. [Google Scholar] [CrossRef]
- Tian, X.; Ai, J.; Tian, X.; Wei, X. cGAS-STING pathway agonists are promising vaccine adjuvants. Med. Res. Rev. 2024, 44, 1768–1799. [Google Scholar] [CrossRef] [PubMed]
- Garland, K.M.; Sheehy, T.L.; Wilson, J.T. Chemical and Biomolecular Strategies for STING Pathway Activation in Cancer Immunotherapy. Chem. Rev. 2022, 122, 5977–6039. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, L. Nanodelivery of cGAS-STING activators for tumor immunotherapy. Trends Pharmacol. Sci. 2022, 43, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Yi, J.; Cao, Y.; Qin, Z.; Zhong, Z.; Yang, W. Nanoparticle-Mediated STING Activation for Cancer Immunotherapy. Adv. Healthc. Mater. 2023, 12, e2300260. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shao, N.; Huang, Y.; Chen, J.; Wang, D.; Hu, G.; Zhang, H.; Luo, L.; Xiao, Z. Overcoming challenges in the delivery of STING agonists for cancer immunotherapy: A comprehensive review of strategies and future perspectives. Mater. Today. Bio 2023, 23, 100839. [Google Scholar] [CrossRef] [PubMed]
- Dane, E.L.; Belessiotis-Richards, A.; Backlund, C.; Wang, J.; Hidaka, K.; Milling, L.E.; Bhagchandani, S.; Melo, M.B.; Wu, S.; Li, N.; et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat. Mater. 2022, 21, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sato, T.; Endo, R.; Sasaki, S.; Takahashi, N.; Sato, Y.; Hyodo, M.; Hayakawa, Y.; Harashima, H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 2021, 9, e002852. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, P.; Liu, Y.; Liu, Z.; Tang, J.; Xu, L.; Liu, J. Multifunctional hybrid exosomes enhanced cancer chemo-immunotherapy by activating the STING pathway. Biomaterials 2023, 301, 122259. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, K.M.; Che, S.P.Y.; LeBleu, V.S.; Kalluri, R. Effective delivery of STING agonist using exosomes suppresses tumor growth and enhances antitumor immunity. J. Biol. Chem. 2021, 296, 100523. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Hu, M.; Yang, Y.; McCabe, E.; Zhang, L.; Withrow, A.M.; Ting, J.P.; Liu, R. Universal STING mimic boosts antitumour immunity via preferential activation of tumour control signalling pathways. Nat. Nanotechnol. 2024, 19, 856–866. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, Q.; Li, Y.; Lu, J.; Xiong, S.; Yue, Y. Exosome co-delivery of a STING agonist augments immunogenicity elicited by CVB3 VP1 vaccine via promoting antigen cross-presentation of CD8 (+) DCs. Int. J. Biol. Macromol. 2024, 261 Pt 1, 129518. [Google Scholar] [CrossRef] [PubMed]

| Sample Source (Cells, Animal Model or Clinical Patients) | Specific Information | Expression Level of cGAS-STING | Function of cGAS-STING | Reference |
|---|---|---|---|---|
| mice | Helicobacter pylori infection | The expression of STING protein increased | Suppression of infection | [48] |
| Xenograft tumor | STING expression was low | Anlotinib activates STING and improves the efficacy of anti-PD-L1 | [53] | |
| MFC | STING expression was low | Radiation therapy activates STING, thereby promoting the expression of PD-1/PD-L1 | [54] | |
| cells | BGC823, AGS, and SGC7901 | STING expression was low | Metformin activates STING to inhibit cell proliferation and invasion | [55] |
| AGS and HS746T | STING expression was low | Anlotinib activates STING and inhibits tumor cell proliferation and invasion | [53] | |
| clinical patients | no | STING expression was increased in tumor tissue of patients | STING-positive patients had better TNM stage and OS | [48] |
| EBV infection | STING expression was increased in tumor tissue of patients with EBV positive gastric cancer | STING positive expression showed better OS rate regardless of EBV infection | [49] | |
| Metastatic ovarian carcinoma | The expression of TBK1 and IRF3 was low | Patients with lower M2GO, which includes TBK1 and IRF3, had worse outcomes | [51] | |
| HER-2 | The expression of STING was lower in HER2-positive patients | Patients with lower STING expression had worse prognosis | [52] |
| Type | STING Agonist | Advantage | Insufficiency | Reference |
|---|---|---|---|---|
| CDN | ADU-S100/MIW815, MK-1454, SB11285, BMS-986301, BI-1387446, IMSA-101, JNJ-67544412, et al. | It belongs to natural ligand and has obvious effect; it is suitable for intratumoral drug delivery therapy | It has poor stability, poor cell targeting, and low cell uptake efficiency | [59,60] |
| NCDN | DMXAA, MK-2118, GSK-3745417, SNX281, TAK-676, E7766, SR-717, RVU3128603, et al. | It has better oral absorption prospects and lower production costs | The clinical trial of DMXAA failed, and the clinical trial results of other drugs were not clear | [61] |
| Type of Agonists | Name | Tumor | Combination Therapy | Phase | NCT Code |
|---|---|---|---|---|---|
| CDN | MIW815 | Advanced/Metastatic solid tumors or lymphomas | Spartalizumab(PD-1 Inhibitor) | I | NCT03172936 |
| Head and neck squamous cell carcinoma | Pembrolizumab(PD-1 Inhibitor) | II | NCT03937141 | ||
| MK-1454 | Advanced/Metastatic solid tumors or lymphomas | Pembrolizumab | I | NCT03010176 | |
| Head and neck squamous cell carcinoma | Pembrolizumab | II | NCT04220866 | ||
| BMS-986301 | Advanced solid tumors | Nivolumab | I | NCT03956680 | |
| SB-11285 | Advanced solid tumors | Atezolizumab (PD-L1 Inhibitor) | I | NCT04096638 | |
| NCDN | DMXAA | Non-small cell lung cancer | Docetaxel or PC | III | NCT00738387, NCT00662597, NCT00832494 |
| Advanced solid tumors | Docetaxel or PC | I | NCT01290380, NCT01278849, NCT01240642, NCT01031212 | ||
| MK-2118 | Advanced/Metastatic solid tumors or lymphomas | Pembrolizumab | I | NCT03249792 | |
| GSK-3745417 | Advanced solid tumors | Dostarlimab | II | NCT03843359 | |
| SNX281 | Advanced/Metastatic solid tumors or lymphomas | Pembrolizumab | I | NCT04609579 | |
| TAK-676 | Advanced solid tumors | Pembrolizumab | I | NCT04420884 | |
| Non-small cell lung cancer | Pembrolizumab | I | NCT04879849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Zhang, S.; Tan, F. The cGAS/STING Pathway—A New Potential Biotherapeutic Target for Gastric Cancer? J. Pers. Med. 2024, 14, 736. https://doi.org/10.3390/jpm14070736
Tian M, Zhang S, Tan F. The cGAS/STING Pathway—A New Potential Biotherapeutic Target for Gastric Cancer? Journal of Personalized Medicine. 2024; 14(7):736. https://doi.org/10.3390/jpm14070736
Chicago/Turabian StyleTian, Mengxiang, Shuai Zhang, and Fengbo Tan. 2024. "The cGAS/STING Pathway—A New Potential Biotherapeutic Target for Gastric Cancer?" Journal of Personalized Medicine 14, no. 7: 736. https://doi.org/10.3390/jpm14070736
APA StyleTian, M., Zhang, S., & Tan, F. (2024). The cGAS/STING Pathway—A New Potential Biotherapeutic Target for Gastric Cancer? Journal of Personalized Medicine, 14(7), 736. https://doi.org/10.3390/jpm14070736





