Abstract
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder potentially linked to gut dysbiosis. This comparative cross-sectional study profiled the gut microbiota in 24 treatment-naïve Thai children diagnosed with ADHD and 24 healthy ones matched by age and gender (median age: 7 years). Fecal microbial compositions were genetically analyzed using 16s rRNA gene amplicon sequencing. The study findings indicated no statistically significant differences in microbial diversity between groups, although Firmicutes and Actinobacteria appeared dominant in both groups. Moreover, ADHD patients exhibited enrichment in Alloprevotella, CAG-352, Succinivibrio, and Acidaminococcus genera, while healthy controls had higher levels of Megamonas, Enterobacter, Eubacterium hallii, and Negativibacillus genera. Spearman correlation analysis demonstrated a significant positive association between CAG-352 and inattention and hyperactivity/impulsivity scores, whereas the Eubacterium hallii group and Megamonas exhibited negative correlations with these symptomatology domains. Beta-carotene intake was associated with the Eubacterium hallii group and Succinivibrio: likewise, vitamin B2 intake was associated with Alloprevotella. Additional research should aim to elucidate the underlying mechanisms influencing clinical biomarkers that signify alterations in specific gut microbiome profiles linked to ADHD.
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is currently the most common childhood-onset neurodevelopmental disorder [1]. ADHD is characterized by 3 clusters of developmentally inappropriate and impairing clinical manifestations: inattention, hyperactivity, and impulsivity. Based on the types of symptoms, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) mainly categorizes ADHD into three presentations: predominantly attentive presentation, predominantly hyperactive/impulsive presentation and combined presentation [1,2,3]. A recent meta-analysis study revealed the estimated data of worldwide ADHD prevalence among school-aged children, with a rate of approximately 7.6% [2]. In Thailand, a national survey of ADHD estimated its prevalence among Thai primary school children at 8.1% in 2012 [4]. The global prevalence of ADHD is increasing in society and involves a very high cost to the individual and society. ADHD might be a life-long issue for many patients treated inadequately. At least half of children with ADHD have symptoms persisting into adolescence and adulthood. These symptoms will manifest conduct disorders, failed relationships, workplace underachievement, substance abuse, and low self-esteem. Moreover, there are many problematic impacts on families and patients, including reduced social, academic, and occupational functioning, and overall quality of life [1,5].
Similar to all complex disorders, a single risk factor is insufficient to explain the etiology of ADHD. It has been shown that ADHD results from multifactorial factors such as genetic, environmental, and neurobiological interactions [6]. Multiple causative factors including gestational age, delivery mode, type of feeding, diet, antibiotic usage, epigenetics, infection as well as neurotransmission system dysfunctions may influence the risk of ADHD development and the variation of ADHD manifestations [7,8]. Nonetheless, although the precise mechanisms underpinning ADHD pathogenesis remain elusive, emerging evidence points towards a potential association between ADHD symptoms and alterations in gut microbiota [9]. Numerous investigations underscore the pivotal role of gut microbiota in the bidirectional gut-brain axis communication, influencing metabolic processes, inflammatory responses, the hypothalamic-pituitary-adrenal axis, and neurotransmitter systems [9,10,11]. The gut microbiota exerts neurobiological influence by modulating neurotransmitter levels essential for cognitive and emotional regulation, including dopamine and serotonin [12]. Despite serotonin’s primarily cerebral synthesis, a considerable portion originates in the gut [13]. Established associations between the gut microbiome, neurotransmitters, and neuropsychiatric disorders have been demonstrated by animal studies showing microbiome alterations related to anxiety and social behavior, with concomitant neurotransmission changes in relevant brain regions [14,15,16,17,18,19,20,21,22]. In humans, links between microbiome composition and emotion regulation have been discerned in relation to its capacity to release dopamine and serotonin [23,24]. While the relationship between the gut microbiome and executive function (EF) remains less elucidated in humans, evidence from rodent studies supports a dopamine-mediated influence on EF, further linking the gut microbiome to EF-related behaviors [25,26]. Additionally, the association between the gut microbiome and neuropsychiatric disorders like Autism Spectrum Disorder (ASD), characterized by impaired EF, accentuates its potential impact on EF and emotion regulation [27,28,29,30,31,32,33,34]. Due to the shared symptoms between ADHD and other neuropsychiatric conditions, notably ASD, investigating the involvement of the gut microbiome in ADHD could shed light on its underlying mechanisms and potential treatments [35,36].
Numerous studies have employed 16S ribosomal RNA (rRNA) sequencing to investigate the relationship between bacterial composition and the pathological mechanisms of ADHD [37,38,39,40,41]. Aarts et al. identified elevated levels of Actinobacteria and Bifidobacterium in individuals with ADHD [37], whereas Jiang et al. reported decreased levels of Faecalibacterium, Dialister, and Sutterella, without significant differences in overall microbial diversity [38]. Prehn-Kristensen et al. found significant differences in both alpha and beta diversity, noting increased levels of Bacteroidaceae and Neisseriaceae in ADHD patients [40]. In addition, gene-set enrichment analysis linked ADHD to Desulfovibrio and Clostridiales and revealed variations in Bacteroides and Sutterella between ADHD patients and healthy controls [41]. Bifidobacterium, involved in the dopamine reward system, along with genera such as Faecalibacterium, Anaerotaenia, and Gracilibacter, has been found to be associated with attention deficits, suggesting that gut microbiota dysbiosis may contribute to ADHD pathophysiology [39].
However, findings on alpha diversity remain inconclusive due to study heterogeneity. These inconsistencies, influenced by demographic factors, medication use, and nutritional variations, underscore the need for further research to elucidate the underlying mechanisms. Therefore, this study aimed to determine gut microbiome profiles in treatment-naïve Thai ADHD children. We proposed that treatment-naïve Thai children with ADHD may exhibit distinct gut microbial compositions based on their ADHD symptomatology compared to healthy counterparts. In addition, this study was conducted to delineate differential patterns of gut microbiome and dietary intakes between ADHD children and the controls.
2. Subjects and Methods
2.1. Study Design and Participants
This study was carried out as a comparative cross-sectional study approved by the Institutional Review Board (IRB) of the Faculty of Medicine Ramathibodi Hospital, Mahidol University, and the IRB of Buddhasothorn Hospital in Thailand. The procedures were conducted according to the 1996 Declaration of Helsinki. All participants who met the criteria for recruitment and their parents were fully informed of the purpose, procedures, and hazards involved in the study. Written informed consent was obtained from the participants and their parents before enrollment. Participants in the ADHD group were recruited from eligible patients aged between 6 and 12 years who were treated in the outpatient department of the Division of Child and Adolescent Psychiatry at Buddhasothorn Hospital in Thailand. The inclusion criteria for ADHD patients were as follows: (1) a clinical diagnosis of ADHD according to the DSM-5 classification system assessed by a certified child and adolescent psychiatrist; (2) having scored above the standard clinical cutoffs for ADHD symptoms on the Thai version of Swanson, Nolan, and Pelham Rating Scale-IV (SNAP-IV) [42]; and (3) having not received any medication yet. We excluded patients who had: (1) been diagnosed with primary psychiatric disorders or significant physical illness such as intellectual disability, genetic disorders, cerebral palsy, autism, neuromuscular diseases, epilepsy, brain trauma, encephalitis, or chronic atopic diseases; (2) taken any medications or probiotics within the two months before the fecal collection; and (3) obesity. The control group comprised healthy children aged between 6 and 12 years studying in regular classes at primary schools. They did not have childhood developmental disorders as confirmed by the Thai version of SNAP-IV criteria. In addition, children in the control group were not children diagnosed with other comorbid conditions. They had no known behavioral, academic, or social difficulties. As with the ADHD group, those currently taking medications or probiotic supplements were excluded.
Demographic data, including age, gender, home type, early feeding practices, mode of delivery, gestational age (and premature birth status if applicable), birth weight, and health behaviors such as bowel habits, were reported by the children’s parents or guardians using study-specific questionnaires. Bowel habits were assessed using the Bristol Stool Chart, which was employed to identify stool shapes and types and functioned as a diagnostic tool for constipation [43]. For dietary information, a nutritionist interviewed the parents or guardians to record dietary consumption during the month before the visit, utilizing semi-quantitative food frequency questionnaires (FFQs). Additionally, three non-consecutive 24-h dietary recalls (two weekdays and one weekend day) documented the menu, ingredients, and quantities of each meal, including breakfast, mid-morning snack, lunch, mid-afternoon snack, dinner, and after-dinner snack, before fecal sample collection. For participants under ten years of age, dietary intake information was provided by their parents. Energy, macronutrient, and micronutrient intakes were calculated using the INMUCAL-Nutrients software V.4.0 from the Institute of Nutrition, Mahidol University [44]. Due to the absence of prebiotic and probiotic information in the INMUCAL-Nutrients software, the intakes of these nutrients were estimated using nutrition labels on food products.
2.2. Sample Collection and 16S rRNA Gene Amplicon Sequencing
The stool samples were collected from the children assisted by their parents, kept in a sterile plastic tube with a tightly closing lid, stored in an insulated bag, and then immediately frozen at home. The samples were held in an icebox, transported to the hospital laboratory unit, and stored at −80 °C for further analysis. According to the manufacturer’s instructions, total fecal bacterial DNA was extracted from 200 mg of feces using the QIAamp®Fast DNA stool mini kit (Qiagen, Hilden, Germany). DNA concentration was measured using the Qubit®2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) and stored at −20 °C before analysis.
The bacterial 16S rRNA gene V3-V4 region was amplified with a barcoded primer set using 338FACTCCTACGGGAGGCAGCAG and 806R GGACTACHVGGGTWTCTAAT. Approximately 4 μL of 5 × FastPfu Buffer, 0.8 μL of 2.5 mM deoxyribonucleotide triphosphates (dNTPs), 0.8 μL of 5 μM reverse primer, 0.4 μL of FastPfu Polymerase, and a 10-ng template were employed in the polymerase chain reaction (PCR). The PCR cycling conditions comprised an initial denaturation step of 3 min at 95 °C, followed by 27 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 seconds, extension at 72 °C for 45 s, and a final extension step of 10 min at 72 °C. Subsequently, after the initial PCR clean-up procedure, the samples underwent amplification with dual indices employing an Illumina sequencing adapter integrated with the Nextera XT Index Kit (Illumina Inc., San Diego, CA, USA). The resulting Amplicon Library was pooled, normalized, supplemented with PhiX Control, and subjected to sequencing. Sequencing of the 16S rRNA was executed utilizing the Illumina MiSeq platform, employing the TruSeqTM DNA Sample Prep Kit (Illumina Inc., San Diego, CA, USA) [38].
2.3. Bioinformatics and Statistical Analysis
The sequence data analysis commenced with the sequence quality control (QC) of raw paired-end (PE) sequences using FASTP [45]. Cleaned PE sequences were merged into single-end reads utilizing FLASH [46]. The ASV table was then constructed via DADA2 [47] and implemented in QIIME2 version 2023.2 [48]. The alpha diversity index and the rarefaction curve of the saturated microbiome were calculated based on the subsampling method of 43,798 reads per sample. Bacterial profiles were classified using consensus blast against the Silva database, version 132 (latest release on 2 November 2020), applying a percent identity cutoff of 80%. The gut microbiome was investigated either within-sample diversity (alpha diversity) or between-sample diversity (beta diversity). To determine alpha diversity, three metrics, including richness, Chao1 index, and Shannon index, were computed and compared using the Kruskal-Wallis test. Beta diversity was assessed using Principal Coordinates Analysis (PCoA) plots based on Weighted UniFrac, Unweighted UniFrac, and Bray-Curtis distances. Group differences were evaluated for significance with pairwise permutational multivariate analysis of variance (PERMANOVA) using 999 random permutations. Additionally, microbiome community comparisons and cladograms were conducted using linear discriminant analysis effect size (LEfSe). Functional abundances and KEGG pathway predictions were performed using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) pipeline in the QIIME2 plugin. Taxonomic and functional profiles generated by PICRUSt2 were analyzed and visualized using Statistical Analysis of Taxonomic and Functional Profiles (STAMP). Basic statistical analyses were conducted using SPSS version 19.0 software (SPSS Inc., Armonk, NY, USA). Depending on the data type, categorical and continuous variables between groups were compared using chi-squared, nonparametric, and Student’s t-tests. Correlations between bacterial DNA concentration and the average scores on the inattention and hyperactivity/impulsivity subsets, as well as nutrient consumption, were analyzed using Spearman’s rank test.
3. Results
The study was performed between March 2019 and March 2022 in Chachoengsao, Thailand. Among 48 participants enrolled in the study, 24 participants were treatment-naïve patients with ADHD referred to the outpatient unit at the Division of Child and Adolescent, Department of Psychiatry, Buddhasothorn Hospital, Chachoengsao, and 24 were age- and sex-matched healthy children recruited from children with normal development, studying in regular classes at primary schools.
The median age of the participants was 7 years old. Most participants were male. There were no significant differences in gender, age, anthropometric data, type of delivery, gestational age, birth weight, birth length, head circumference, continued breastfeeding time, and constipation. Accordingly, there was a significantly higher severity score on the SNAP-IV scale rated by parents and teachers in the ADHD group than in the healthy group. In the ADHD group, 75% of participants had a predominantly combined type of ADHD, 20.8% predominantly inattentive ADHD, and 4.2% had predominantly hyperactive-impulsive ADHD. The general characteristics of the study participants are presented in Table 1.

Table 1.
Demographic data for 48 participants in this study.
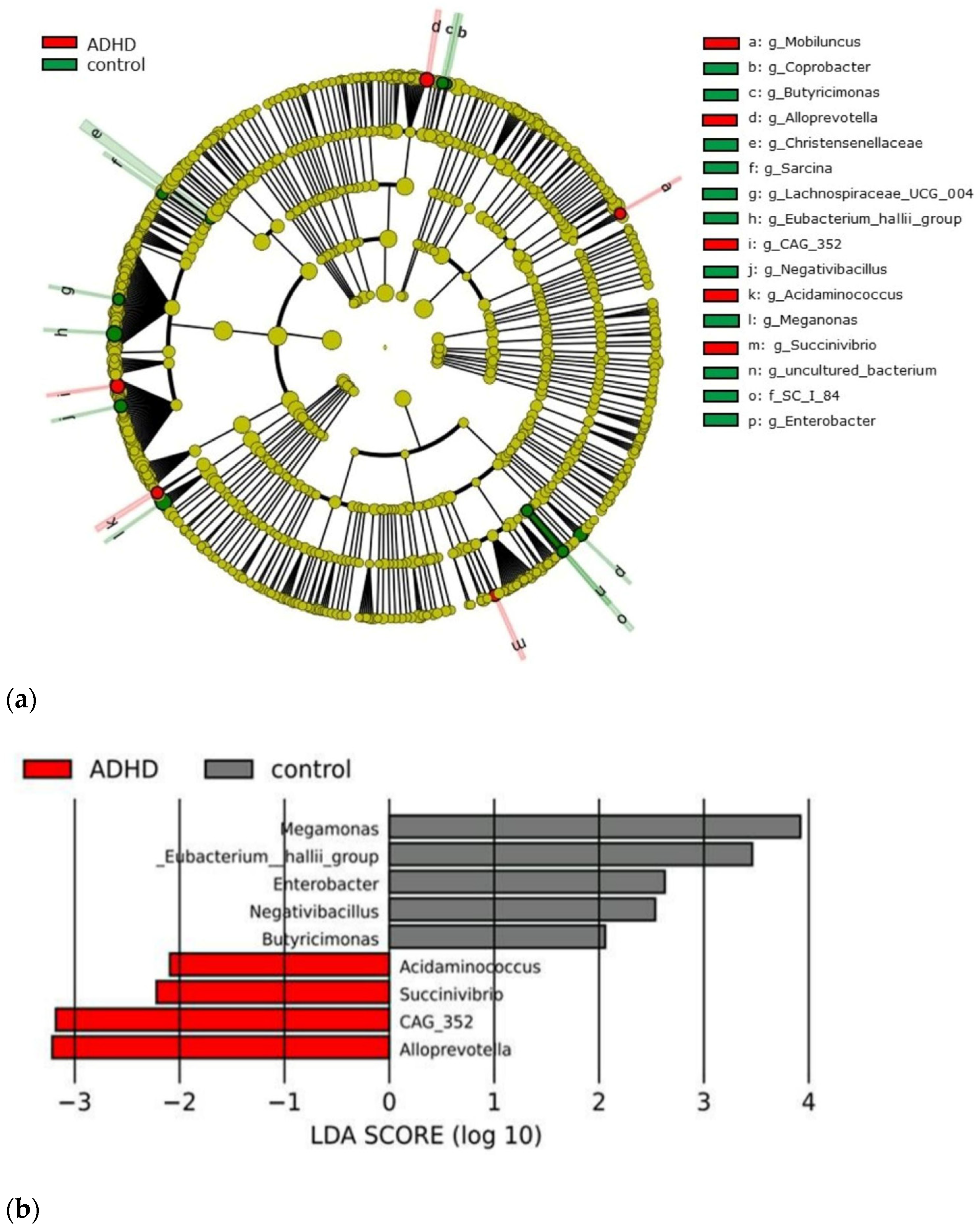
Based on the sequencing data, there were 5,284,081 sequencing reads clustered into 2,078 operational taxonomic units (OTUs), 28 phyla, 53 classes, 134 orders, 231 families, 499 genera, and 620 species. The relative abundance at the phylum level in ADHD patients exhibited a dominance of Firmicutes (61.96%), Actinobacteria (25.71%), Bacteroidetes (9.75%), and Proteobacteria (1.62%). The relative abundances of these major phyla in the control group were largely similar to the ADHD group. Therefore, the gut microbiome profiles of the ADHD patient group and the control group were similar. A LEfSe plot was developed and presented for relative abundance comparison to distinguish the genus variation between groups. The relative abundance of Alloprevotella, CAG-352, Succinivibrio, and Acidaminococcus was predominant in the ADHD group. On the other hand, the relative abundance of Megamonas, Eubacterium hallii group, Enterobacter, Negativibacillus, and Butyricimonas become the notable genus in the healthy control group. The LEfSe plot is shown in Figure 1.
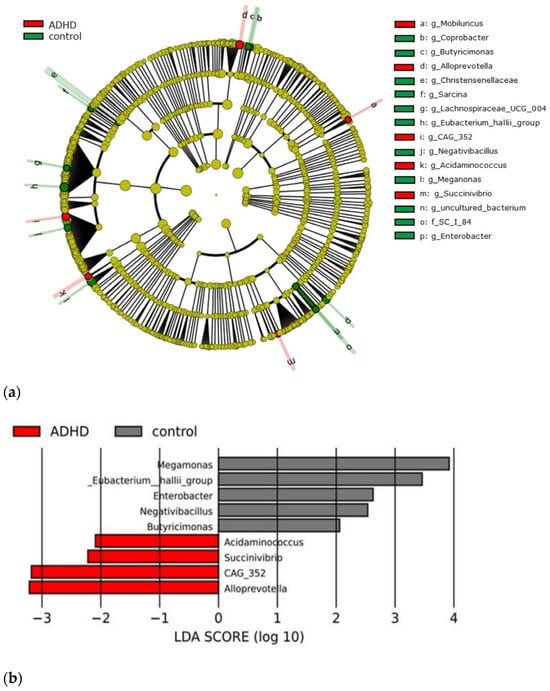
Figure 1.
A cladogram illustrating differentially abundant taxa in the gut microbiota is presented. (a) Genus-level taxon features were identified by LEfSe with an LDA score greater than 2, comparing treatment-naïve Thai children with attention-deficit hyperactivity disorder (ADHD) to age- and sex-matched healthy controls. (b) Red bars indicate taxa with significantly higher expression in children with ADHD, while grey bars represent those in healthy controls. Group differences were assessed using the Kruskal-Wallis test, with a significance threshold of p < 0.05. LDA denotes linear discriminant analysis, and LEfSe represents linear discriminant analysis effect size.
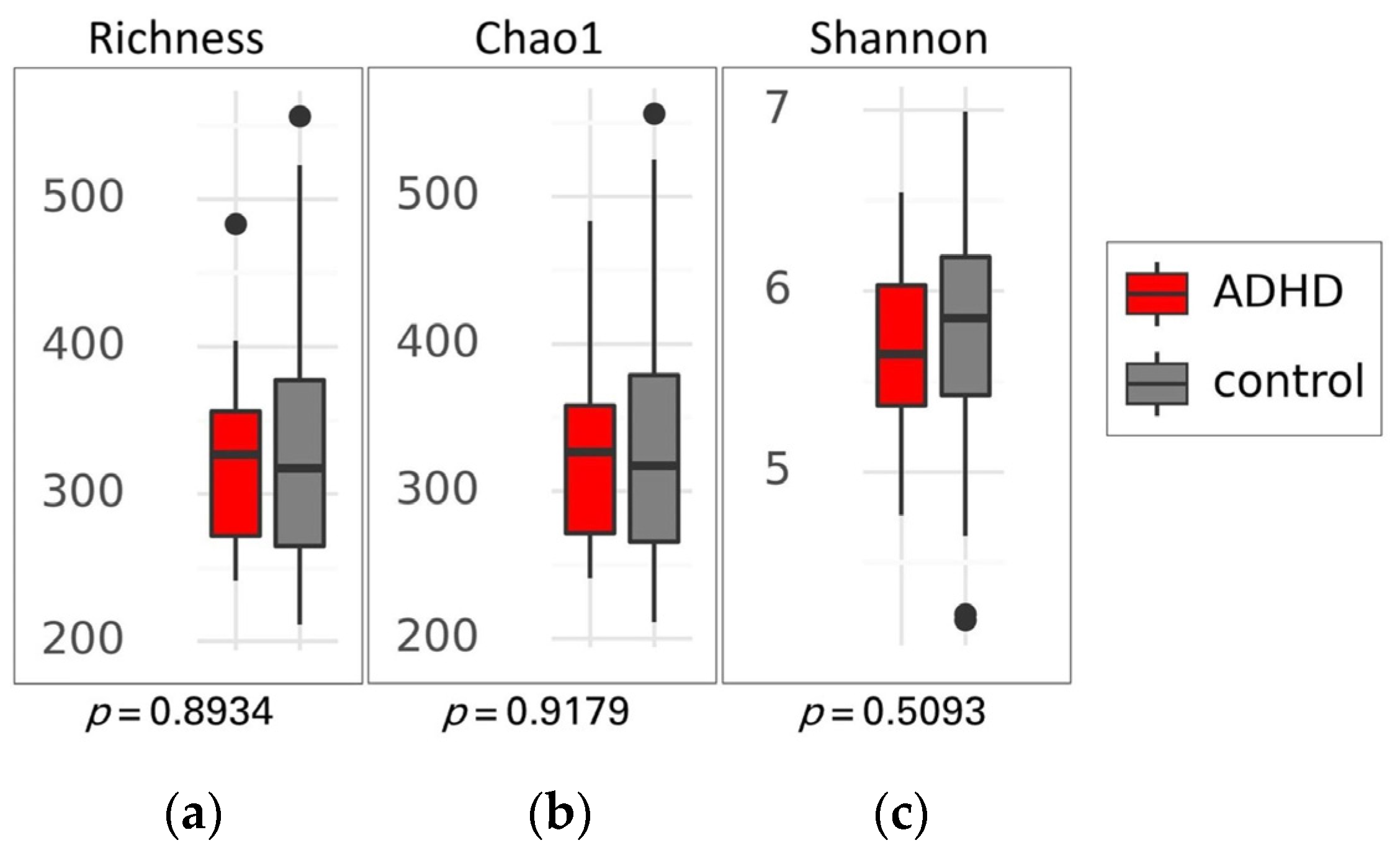
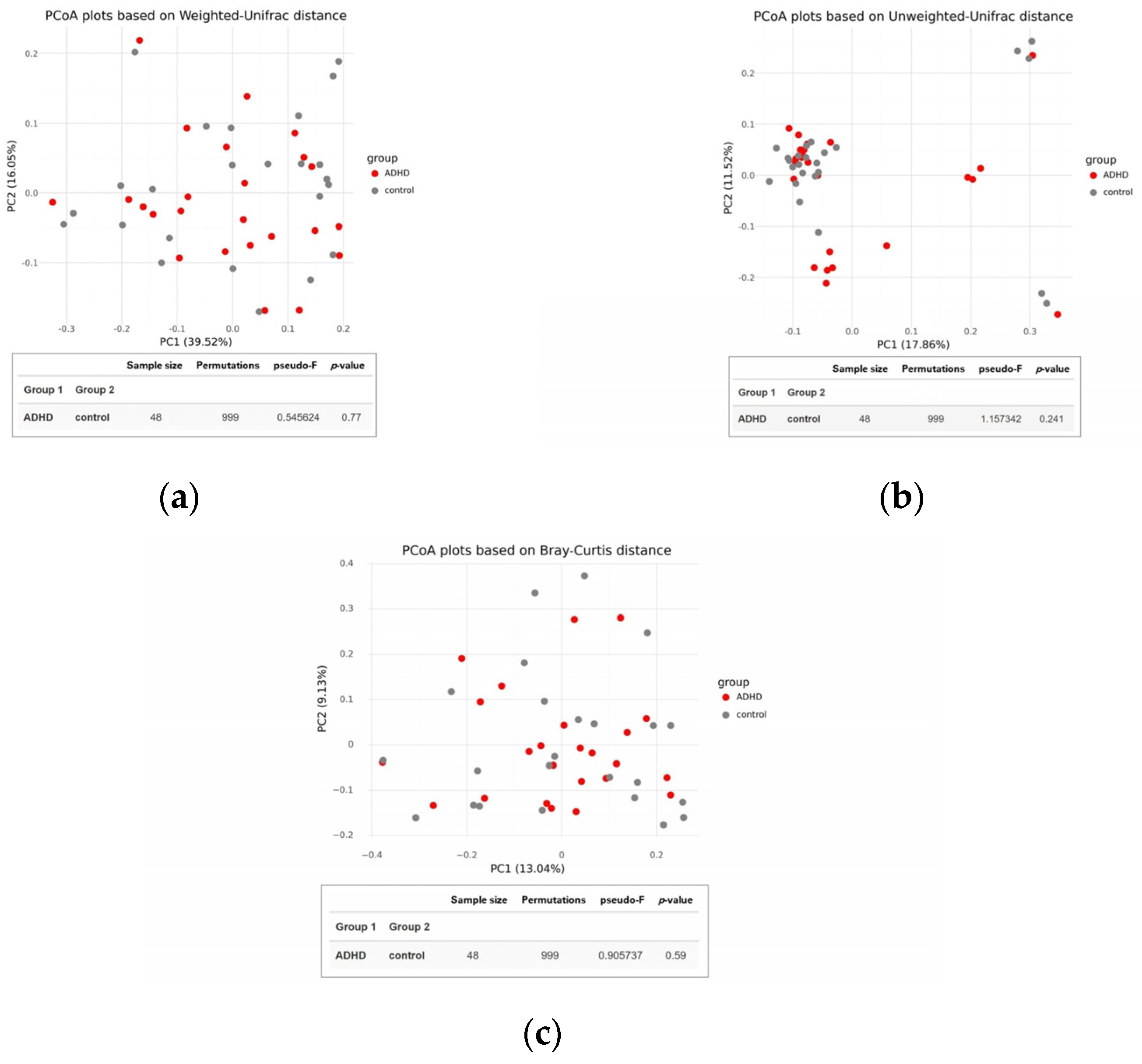
There was no significant difference in alpha diversity between the two groups (Figure 2), suggesting that the composition of gut microbiota was similar in both ADHD and healthy children. This finding was supported by the PCoA plot and statistical analysis using permutational multivariate analysis of variance (PERMANOVA) based on Weighted UniFrac, Unweighted UniFrac, and Bray-Curtis distances, which showed no distinct clustering with p-values of 0.770, 0.241, and 0.590, respectively (Figure 3).
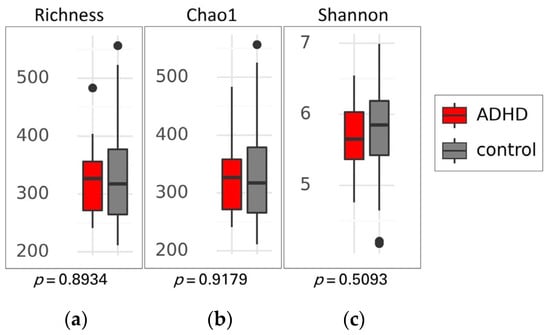
Figure 2.
Alpha diversity of gut microbiota between patients with attention-deficit hyperactivity disorder (ADHD) and healthy children (control). Box plots show the richness (a), Chao1 index (b), and Shannon index (c), along with their respective p-values. Differences between groups were analyzed using the Kruskal-Wallis test. The boxes display the interquartile range (IQR) from the 25th to the 75th percentiles, with a line representing the median. Notches indicate the 95% confidence interval for the median. Whiskers extend to 1.5 times the IQR, and any potential outliers are depicted as dots.
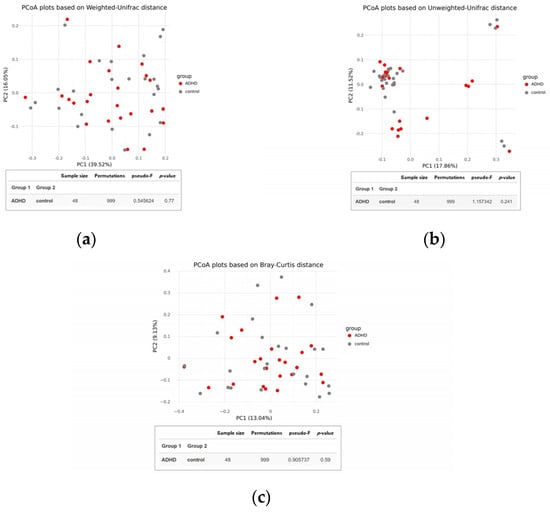
Figure 3.
Beta diversity of the gut microbiota between children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. PCoA plots were constructed using (a) Weighted UniFrac, (b) Unweighted UniFrac, and (c) Bray-Curtis distances. Group differences were evaluated through permutational multivariate analysis of variance (PERMANOVA).
The scores in the two domains of ADHD symptoms, inattention and hyperactivity/impulsivity, and the relative abundance of 9 bacterial taxons identified as showing a significant difference by the LEfSe plot were used for investigating the association between gut microbiota and clinical manifestation of ADHD. The analyses revealed that the relative abundance of Eubacterium hallii group, Megamonas, and Negativibacillus was negatively correlated with inattention scores reported by parents and teachers, whereas CAG-352 and Alloprevotella levels were positively correlated with these scores. Similarly, in the domain of hyperactivity/impulsivity symptoms, the result showed that the relative abundance of the Eubacterium hallii group, Megamonas, and Butyricimonas, was negatively correlated with hyperactivity/impulsivity scores reported by parents and teachers. On the other hand, the CAG-352 level was the only genera that positively correlated with these modules (Table 2).

Table 2.
The association between the average scores on the inattention and hyperactivity/impulsivity subsets and the relative abundance of each genus in participants.
Based on semi-FFQ data designed to assess habitual diet based on particular food items, there was no significant difference in types and portions of dietary intake between ADHD patients and healthy children. The 3-day food record data to determine nutrient intake details showed no significant differences in the daily energy and macronutrient intake. The percentage of the energy distribution of macronutrients in both groups was 49:19:32 for carbohydrate, protein, and fat, respectively, which was within the recommendations for Thai school-age children from Thai Dietary Reference Intakes (DRIs) [49,50]. However, there were significant differences in micronutrient intakes. The median intake of copper, vitamin B2, and vitamin B3 was significantly lower in the ADHD group compared to the control group.
Finding this significance led to the analysis of daily micronutrient intake categorized by adequate consumption among participants. According to the recommended energy and nutrient intake values in Southeast Asian countries with the Estimated Average Requirement (EAR) or the Average Intake (AI) for children aged 6–12 years, ADHD patients and healthy children were reclassified into adequacy or inadequacy groups [51]. There was no statistically significant difference in the proportion of ADHD patients and healthy children between the adequacy and inadequacy groups for each micronutrient (Table 3). Despite no statistical significance of DRI compliance analysis, there was a difference in participant proportions by the adequacy of vitamin B3 consumption between ADHD patients and healthy counterparts. Vitamin B3 intake analysis shows that 95.8% of healthy children and 79.2% of ADHD patients had adequate vitamin B3 consumption (p = 0.081).

Table 3.
Daily dietary intake analysis between ADHD patients and healthy children in this study.
Furthermore, a statistically significant correlation between nutrient consumption and gut microbiome profiles was detected, as shown in Table 4. Specifically, there was a positive correlation noted between beta-carotene intake and the relative abundance of the Eubacterium hallii group, alongside a negative correlation with the relative abundance of Succinivibrio. Conversely, a negative correlation was identified between vitamin B2 intake and the relative abundance of the Alloprevotella genera.

Table 4.
The association between the dietary intake and the relative abundance of each genera.
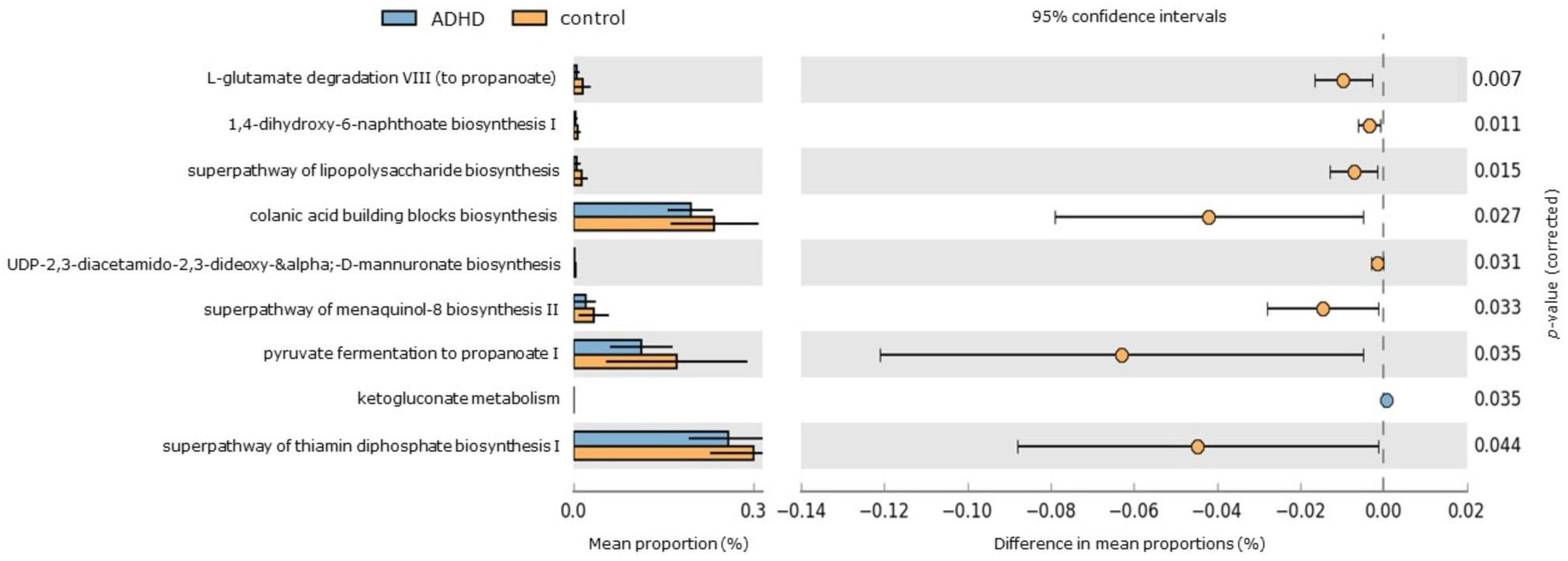
PICRUSt2 analysis was utilized to predict functional KEGG pathways of gut microbiota [52], aiming to evaluate whether taxonomic variations correlate with potential functional changes (Figure 4). Among the healthy control group, significant enrichment was observed in 8 pathways. These pathways include functions related to short-chain fatty acid “propionate” production (such as pyruvate fermentation to propanoate I and L-glutamate degradation VIII to propanoate), thiamin synthesis in bacteria (superpathway of thiamin diphosphate biosynthesis), biosynthesis of polyanionic heteropolysaccharides containing repeat units with D-glucose, L-fucose, D-galactose, and D-glucuronate sugars (colanic acid building blocks biosynthesis), lipopolysaccharide biosynthesis (superpathway of lipopolysaccharide biosynthesis and UDP-2,3-diacetamido-2,3-dideoxy-α-D-mannuronate biosynthesis), and menaquinone biosynthesis (including 1,4-dihydroxy-6-naphthoate biosynthesis I and superpathway of menaquinol-8 biosynthesis II). In contrast, the pentose phosphate pathway (associated with ketogluconate metabolism) was significantly elevated in the ADHD group.
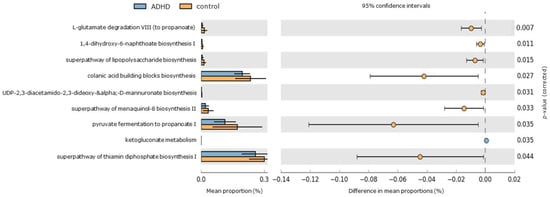
Figure 4.
Differentially abundant KEGG pathways in ADHD and healthy control groups. An extended error bar plot was generated to visualize significantly different KEGG pathways identified through PICRUSt2 analysis. The left sidebar plots illustrate the mean proportions of each KEGG pathway. On the right, dot plots depict the differences in mean proportions between the specified groups, with only those showing p-values < 0.05 (Welch’s test) displayed.
4. Discussion
This comparative cross-sectional study represents the inaugural situational analysis of the gut microbiome, employing a 16S rRNA sequencing platform to delineate fecal microbiota composition in treatment-naïve Thai children with ADHD and their healthy counterparts. Our investigation revealed no significant disparity in gut microbial diversity, encompassing both alpha and beta diversity, between the two groups. Notably, the composition of the gut microbiota exhibited similarity across both groups, with Firmicutes emerging as the predominant phylum, followed sequentially by Actinobacteria, Bacteroidetes, and Proteobacteria. This finding aligns with prior research on longitudinal gut microbiota dynamics during childhood conducted in the Netherlands. Houtman et al. [53] explored gut microbial diversity in 193 healthy mother-infant pairs over the initial 12 years of life. Their study highlighted Firmicutes as the most prevalent phylum among participants aged 6 to 10 years, which corresponds well with the life stage of our participants.
Moreover, our investigation identified a heightened relative abundance of the genera Alloprevotella, CAG-352, Succinivibrio, and Acidaminococcus in the ADHD group compared to healthy children. Conversely, the relative abundance of the genera Megamonas, Eubacterium hallii group, Enterobacter, and Negativibacillus was notably higher in healthy children. This analysis offers valuable insights into the potential correlation between gut microbiota composition and ADHD symptomatology, particularly concerning inattention and hyperactivity/impulsivity. Correlation analysis indicated a significant positive relationship with the CAG-352 genera. In contrast, the Eubacterium hallii group and the Megamonas genera exhibited negative associations with inattention and hyperactivity/impulsivity scores reported by parents and teachers. Additionally, the level of the Eubacterium hallii group in children with ADHD was significantly lower than that in healthy children.
According to the results of this study, it is possible that the CAG-352 genus could be a significant contributing factor associated with the symptoms of ADHD. Although no previous studies have shown a direct link between the CAG-352 genus and the pathophysiology of ADHD, there are studies showing that the Ruminococcaceae family, which includes the CAG-352 genus, was correlated with symptoms of inattention in ADHD and various other psychiatric disorders such as autism, bipolar disorder, anxiety, depression, and schizophrenia [54,55,56]. Szophinska-Tokov J, et al. reported results suggesting that the Ruminococcaceae family was related to inattention symptoms and might have a potential role in ADHD conditions [57]. Additionally, a study conducted by Tengeler AC, et al. demonstrated that introducing the gut microbiota of ADHD-afflicted individuals into germ-free mice significantly heightened anxious behavior [58]. Notably, the relative abundance of the Ruminococcaceae family was linked to abnormal emotional and behavioral symptoms associated with mental health conditions, including ADHD. Further investigation is required to explore the correlation between the abundance of the CAG-352 genus and ADHD.
The influence of dietary intake on both the composition and functionality of the gut microbiota warrants consideration. Our investigation showed no discrepancy in dietary intake between individuals diagnosed with ADHD and their healthy counterparts. However, individuals with ADHD exhibited markedly lower median intake levels of copper, beta-carotene, vitamin B2, and vitamin B3 compared to the healthy group. In particular, there was a positive association between beta-carotene intake and the increased prevalence of the Eubacterium hallii group, predominantly found in healthy children, while simultaneously exhibiting a negative correlation with Succinivibrio, which displayed higher abundance in individuals with ADHD. Moreover, there was a negative correlation of vitamin B12 intake with Alloprevotella, a genus more abundantly present in individuals diagnosed with ADHD.
Beta-carotene, a fat-soluble provitamin A carotenoid, is renowned for its antioxidant and anti-inflammatory properties [59]. The evidence suggests that cognitive deficits and disruptions in sensory processing, progressive maturation, and social behavior may be linked to low vitamin A levels in children with ADHD [60]. Additionally, studies on gut microbiota have underscored the role of beta-carotene in promoting gut health through immunoglobulin A production, contributing to gut immune system maturation, and mitigating gut dysbiosis in inflammatory conditions [59,61,62,63,64]. Likewise, riboflavin or vitamin B2 plays a pivotal role in antioxidant effects as a precursor of flavin nucleotide and flavin adenine dinucleotide, coenzymes of glutathione reductase implicated in scavenging reactive oxygen species for cellular homeostasis [65,66]. Furthermore, the involvement of vitamin B2 in the kynurenine pathway, in intrinsic neuroprotection against glutamate excitotoxicity [67], and its impact on tryptophan catabolism underscores its relevance to ADHD development [68].
Previous research has indicated that short-chain fatty acids (SCFAs), such as butyrate, propionate, and acetate, produced through anaerobic bacterial fermentation of dietary fiber in the intestine, are critical microbial metabolites linking gut microbial composition alterations to brain dysfunction [69,70,71,72,73]. These SCFAs can cross the blood-brain barrier, serve as primary energy sources for glial cells and neurons, and play a vital role in brain development, particularly in early life [74]. The PICRUSt2 analysis in the present study showed the significant upregulation of pathways involved in the biosynthesis of the SCFA propionate, specifically pyruvate fermentation to propanoate I and L-glutamate degradation VIII to propanoate, in the healthy control group. Propionate, a three-carbon short-chain fatty acid (SCFA), is generated during microbial fermentation of dietary fibers by gut microbiota such as Eubacterium hallii [75,76], recently reclassified as Anaerobutyricum hallii, an anaerobic, Gram-positive bacterium within the Lachnospiraceae family [77]. Eubacterium hallii exhibits versatility in fermenting various substrates, producing key metabolites like butyrate and propionate, which are crucial for gut health, metabolic functions, and immune modulation [78,79]. Utilizing the acrylate and succinate-propionate pathways, this bacterium converts pyruvate and other substrates into propionate, essential for energy metabolism, glucose, and lipid regulation, and maintaining gut microbial balance [80]. The ability of propionate to cross the blood-brain barrier highlights its role in gut-brain axis communication and neurological function [69,81].
Reduced plasma SCFA levels, including propionate, in individuals with ADHD underscore the importance of Eubacterium hallii in SCFA production and interactions within the gut-brain axis [82,83]. In addition, the increased abundance of the Eubacterium hallii group in healthy controls may be related to gut-brain axis interactions via butyrate production. Butyrate, recognized for its anti-inflammatory and barrier-enhancing properties, functions as a histone deacetylase inhibitor [84]. In peripheral blood mononuclear cell (PBMC) models, butyrate effectively reduces pro-inflammatory cytokine production while promoting the release of anti-inflammatory cytokines [85,86]. Butyrate also influences T-cell differentiation through GPR109A signaling, fostering the development of regulatory T-cells and Interleukin-10 (IL-10)-producing T-cells [87]. Moreover, butyrate can enhance brain-derived neurotrophic factor (BDNF) production, encouraging neural synapse formation and differentiation, potentially improving brain health, and mitigating neurodegenerative conditions associated with ADHD [69,88]. Future research should explore the link between ADHD and these SCFAs, particularly propionate and butyrate, to assess their potential in modulating ADHD and preventing neurodevelopmental diseases.
To our knowledge, this is the first study on gut microbiome profiles in Thai ADHD children compared with healthy counterparts. The present study has several strengths. Firstly, we designed the investigation with an age- and sex-matched control condition to ameliorate age and gender heterogeneities. Secondly, we excluded factors interfering with gut microbiome profiles, such as obesity, allergic diseases, and atypical mental disorders. In addition, our participants had not used any medications or food supplements that might affect microbial diversity for at least two months before fecal collection. We actively gave consideration to limiting the magnitude of differences between groups from the onset of our analysis. However, we recognize that this study has certain limitations. It is a cross-sectional study conducted at a single time point in Thai children, which limits the generalizability of the findings to other ethnic populations and does not capture changes in the gut microbiome profile over time. Due to the global COVID-19 pandemic during sample collection, the small sample size is another limitation. Another one is that the widespread availability of the QIAamp® DNA Stool Mini Kit in Thailand and the need for standardized procedures necessitated its use despite its known limitations. Notably, bead-beating intensity significantly influences the observed microbial community structure, giving results that correlate strongly with bacterial cell wall strength and improving DNA detection from Gram-positive bacteria. It is important to note that some gastrointestinal bacteria, particularly those in the Firmicutes phylum, have robust peptidoglycan layers, making them difficult to lyse without mechanical disruption [89]. Thus, the QIAamp® Fast DNA Stool Mini Kit’s reliance on enzymatic digestion may lead to an underestimation of these bacteria. Future investigations will use a kit incorporating mechanical lysis, such as the QIAamp® PowerFecal Pro DNA Kit from QIAGEN.
Furthermore, the 16S metagenomic profiling method itself has limitations as it does not adequately reflect the function or activity of the microbiota. To elucidate the functional roles of the intestinal microbiota and establish causal relationships between ADHD and the gut microbiome, metagenomic analysis combined with metabolomic profiling of individual samples is required. Additionally, the LEfSe data analysis method can be influenced by null inflation, where many zero values in relative abundance data may impact the outcomes. Alternative approaches, such as ANCOM-BC derived from ANCOM (Analysis of Composition of Microbiomes), have addressed this issue by integrating bias correction techniques, thereby reducing potential biases like false positives and null inflation.
Regarding dietary analysis limitations, we employed semi-quantitative food frequency questionnaires (semi-FFQs) and 3-day food records without measuring plasma micronutrient levels. Additionally, there is a potential for underestimating dietary intake among study participants compared to reference values due to limitations in the dietary database. This method may not accurately assess the impact of nutritional metabolites on the gut microbiome, as FFQs might be less effective than plasma micronutrient measurements for evaluating dietary influences. We recommend that future research explore the association between plasma nutrients and the gut microbiome, highlighting the need for rigorously designed intervention trials to establish causality. Further studies should consider larger sample sizes, genetic variations, and biochemical pathways to support the current findings and elucidate the role of gut microbiota in ADHD manifestation and treatment.
5. Conclusions
The results of the present study suggest a potential link between the gut microbiome profiles and the clinical symptoms observed in treatment-naïve Thai children diagnosed with ADHD. There was a positive correlation between CAG-352 and ADHD as well as a negative correlation between the Eubacterium hallii group and ADHD. Moreover, the intake of beta-carotene and vitamin B2 was associated with the abundance of particular gut microbiota in ADHD children. Subsequent investigations are warranted to validate these findings and to explore the influence of nutrients, therefore providing further insights into the mechanistic role of gut microbiota in ADHD.
Author Contributions
Conceptualization, N.C., J.P. and K.K.; methodology, J.P., S.T. (Siriporn Tuntipopipat) and N.C.; validation, J.P., S.T. (Sithichoke Tangphatsornruang) and W.M.; formal analysis, J.P. and W.M.; investigation, J.P.; data curation, J.P. and N.C.; writing—original draft preparation, J.P.; writing—review and editing, N.C. and K.K.; visualization, W.M.; supervision, N.C., K.K. and S.T. (Sithichoke Tangphatsornruang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Medicine Ramathibodi Hospital, Mahidol University (grant number: RF_63076), and the Nutrition Association of Thailand under the patronage of Her Royal Highness Princess Maha Chakri Sirindhorn (grant number: 3/2563).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Faculty of Medicine Ramathibodi Hospital, Mahidol University (MURA2019/512).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this study were uploaded to the Sequence Read Archive of the National Center for Biotechnology Information (NCBI) with the Bioproject accession number PRJNA988940 and are available on request from the corresponding author.
Acknowledgments
The author would like to thank the participants and laboratory technicians at Buddhasothorn Hospital, Chachoengsao, Thailand, for their valuable contributions to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Antolini, G.; Colizzi, M. Where Do Neurodevelopmental Disorders Go? Casting the Eye Away from Childhood towards Adulthood. Healthcare 2023, 11, 1015. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Abdoli, N.; Rahmani, A.; Shiri, M.H.; Hashemian, A.H.; Akbari, H.; Mohammadi, M. The global prevalence of ADHD in children and adolescents: A systematic review and meta-analysis. Ital. J. Pediatr. 2023, 49, 48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Visanuyothin, T.; Wachiradilok, P.; Pavasuthiapaisit, C. 597–Prevalence of adhd and odd: A national survey in thailand 2012. Eur. Psychiatry 2013, 28, 1. [Google Scholar] [CrossRef]
- Singh, A.; Yeh, C.J.; Verma, N.; Das, A.K. Overview of Attention Deficit Hyperactivity Disorder in Young Children. Health Psychol. Res. 2015, 3, 2115. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.S.; Grevet, E.H.; Silva, L.C.F.; Ramos, J.K.N.; Rovaris, D.L.; Bau, C.H.D. An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder. Discov. Ment. Health 2023, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Cecil, C.A.M.; Nigg, J.T. Epigenetics and ADHD: Reflections on Current Knowledge, Research Priorities and Translational Potential. Mol. Diagn. Ther. 2022, 26, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Ikeda, K.; Miyakawa, T. Behavioral phenotype, intestinal microbiome, and brain neuronal activity of male serotonin transporter knockout mice. Mol. Brain 2023, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Peay, D.N.; Acuna, A.; Reynolds, C.M.; Willis, C.; Takalkar, R.; Bryce Ortiz, J.; Conrad, C.D. Chronic stress leads to persistent and contrasting stellate neuron dendritic hypertrophy in the amygdala of male and female rats, an effect not found in the hippocampus. Neurosci. Lett. 2023, 812, 137403. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Ryan, F.J.; Hoban, A.E.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Microbes & neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav. Immun. 2015, 50, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Philip, V.; Newton, D.F.; Oh, H.; Collins, S.M.; Bercik, P.; Sibille, E. Transcriptional markers of excitation-inhibition balance in germ-free mice show region-specific dysregulation and rescue after bacterial colonization. J. Psychiatr. Res. 2021, 135, 248–255. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Tang, W.; Wu, W.L. Searching for the gut microbial contributing factors to social behavior in rodent models of autism spectrum disorder. Dev. Neurobiol. 2018, 78, 474–499. [Google Scholar] [CrossRef] [PubMed]
- Roman, P.; Rueda-Ruzafa, L.; Cardona, D.; Cortes-Rodríguez, A. Gut-brain axis in the executive function of austism spectrum disorder. Behav. Pharmacol. 2018, 29, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, M.U.; Hosie, S.; Shindler, A.E.; Wood, J.L.; Franks, A.E.; Hill-Yardin, E.L. Comparing the Gut Microbiome in Autism and Preclinical Models: A Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 905841. [Google Scholar] [CrossRef]
- Chernikova, M.A.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-Zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef]
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; De Giacomo, A.; Laguardia, M.; Schettini, F.; Francavilla, R.; Cristofori, F. Intestinal Barrier Dysfunction and Microbiota-Gut-Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder. Nutrients 2023, 15, 1620. [Google Scholar] [CrossRef] [PubMed]
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav. Immun. 2018, 73, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Pazienza, V.; Calamandrei, G.; Ricceri, L. A snapshot of gut microbiota data from murine models of Autism Spectrum Disorder: Still a blurred picture. Neurosci. Biobehav. Rev. 2023, 147, 105105. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.L.; Owen, M.J. Genomic insights into the overlap between psychiatric disorders: Implications for research and clinical practice. Genome Med. 2014, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Reiersen, A.M.; Todd, R.D. Co-occurrence of ADHD and autism spectrum disorders: Phenomenology and treatment. Expert Rev. Neurother. 2008, 8, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Zhou, Y.Y.; Zhou, G.L.; Li, Y.C.; Yuan, J.; Li, X.H.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Richarte, V.; Sánchez-Mora, C.; Corrales, M.; Fadeuilhe, C.; Vilar-Ribó, L.; Arribas, L.; Garcia, E.; Rosales-Ortiz, S.K.; Arias-Vasquez, A.; Soler-Artigas, M.; et al. Gut microbiota signature in treatment-naïve attention-deficit/hyperactivity disorder. Transl. Psychiatry 2021, 11, 382. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Zimmermann, A.; Tittmann, L.; Lieb, W.; Schreiber, S.; Baving, L.; Fischer, A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS ONE 2018, 13, e0200728. [Google Scholar] [CrossRef]
- Cheng, S.; Han, B.; Ding, M.; Wen, Y.; Ma, M.; Zhang, L.; Qi, X.; Cheng, B.; Li, P.; Kafle, O.P.; et al. Identifying psychiatric disorder-associated gut microbiota using microbiota-related gene set enrichment analysis. Brief. Bioinform. 2020, 21, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Pityaratstian, N.; Booranasuksakul, T.; Juengsiragulwit, D.; Benyakorn, S. ADHD screening properties of the Thai version of Swanson, Nolan, and Pelham IV scale (SNAP-IV) and Strengths and Difficulties Questionnaire (SDQ). J. Psychiatr. Assoc. Thail. 2014, 2, 97–110. [Google Scholar]
- Shokouhi, N.; Mohammadi, S.; Ghanbari, Z.; Montazeri, A. Development of a new version of the Bristol Stool Form Scale: Translation, content validity, face validity, and reliability of the Persian version. BMJ Open Gastroenterol. 2022, 9, e001017. [Google Scholar] [CrossRef] [PubMed]
- Nutrient Calculation Computer Software INMUCAL-Nutrients V 4.0 Database NB1; Institute of Nutrition, Mahidol University: Nakhon Pathom, Thailand, 2018.
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2. In Microbiome Analysis: Methods and Protocols; Beiko, R.G., Hsiao, W., Parkinson, J., Eds.; Springer: New York, NY, USA, 2018; pp. 113–129. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Nutrition, Department of Health, Ministry of Public Health. Dietary Reference Intake Tables for Thais 2020; A.V. Progressive Ltd.: Bangkok, Thailand, 2020. [Google Scholar]
- Tee, E.S.; Florentino, R.F.; Chongviriyaphan, N.; Ridwan, H.; Appukutty, M.; Mai, T.T. Review of recommended energy and nutrient intake values in Southeast Asian countries. Malays. J. Nutr. 2023, 29, 163–241. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut microbiota and BMI throughout childhood: The role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kosciolek, T.; Maldonado, Y.; Daly, R.E.; Martin, A.S.; McDonald, D.; Knight, R.; Jeste, D.V. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr. Res. 2019, 204, 23–29. [Google Scholar] [CrossRef]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.; Li, G.; Li, G.; Liang, J. Two-sample Mendelian randomization analysis investigates causal associations between gut microbiota and attention deficit hyperactivity disorder. Front. Microbiol. 2023, 14, 1144851. [Google Scholar] [CrossRef] [PubMed]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Bloemendaal, M.; Aarts, E.; et al. Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms. Microorganisms 2020, 8, 406, Erratum in Microorganisms 2021, 9, 1358. [Google Scholar] [CrossRef] [PubMed]
- Tengeler, A.C.; Dam, S.A.; Wiesmann, M.; Naaijen, J.; van Bodegom, M.; Belzer, C.; Dederen, P.J.; Verweij, V.; Franke, B.; Kozicz, T.; et al. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 2020, 8, 44. [Google Scholar] [CrossRef]
- Eroglu, A.; Al’Abri, I.S.; Kopec, R.E.; Crook, N.; Bohn, T. Carotenoids and Their Health Benefits as Derived via Their Interactions with Gut Microbiota. Adv. Nutr. 2023, 14, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Mawson, A.R. Toward a theory of childhood learning disorders, hyperactivity, and aggression. ISRN Psychiatry 2012, 2012, 589792. [Google Scholar] [CrossRef]
- Hegde, P.S.; Agni, M.B.; Rai, P.; Kumar B, M.; KM, D.G. Impact of carotenoids on gut microbiome: Implications in human health and disease. J. Appl. Nat. Sci. 2022, 14, 1085–1099. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases. Nutrients 2023, 15, 2265. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, L.; Wang, F.; Shen, X.; Lin, D. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp. Biol. Med. 2018, 243, 613–620. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef]
- Marashly, E.T.; Bohlega, S.A. Riboflavin Has Neuroprotective Potential: Focus on Parkinson’s Disease and Migraine. Front. Neurol. 2017, 8, 333. [Google Scholar] [CrossRef]
- Cavaleri, D.; Crocamo, C.; Morello, P.; Bartoli, F.; Carrà, G. The Kynurenine Pathway in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Blood Concentrations of Tryptophan and Its Catabolites. J. Clin. Med. 2024, 13, 583. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Wortelboer, K.; Koopen, A.M.; Herrema, H.; de Vos, W.M.; Nieuwdorp, M.; Kemper, E.M. From fecal microbiota transplantation toward next-generation beneficial microbes: The case of Anaerobutyricum soehngenii. Front. Med. 2022, 9, 1077275. [Google Scholar] [CrossRef]
- Engels, C.; Ruscheweyh, H.J.; Beerenwinkel, N.; Lacroix, C.; Schwab, C. The Common Gut Microbe Eubacterium hallii also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016, 7, 713. [Google Scholar] [CrossRef]
- Seegers, J.; Bui, N.; De Vos, W. Remarkable Metabolic Versatility of the Commensal Bacteria Eubacterium hallii and Intestinimonas butyriciproducens: Potential Next-Generation Therapeutic Microbes. In Probiotic Bacteria and Postbiotic Metabolites: Role in Animal and Human Health. Microorganisms for Sustainability; Springer: Singapore, 2021; pp. 139–151. [Google Scholar]
- Wei, Y.H.; Ma, X.; Zhao, J.C.; Wang, X.Q.; Gao, C.Q. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes 2023, 15, 2190300. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Yang, L.L.; Stiernborg, M.; Skott, E.; Gillberg, T.; Landberg, R.; Giacobini, M.; Lavebratt, C. Lower plasma concentrations of short-chain fatty acids (SCFAs) in patients with ADHD. J. Psychiatr. Res. 2022, 156, 36–43. [Google Scholar] [CrossRef]
- Yang, L.L.; Stiernborg, M.; Skott, E.; Xu, J.; Wu, Y.; Landberg, R.; Arefin, S.; Kublickiene, K.; Millischer, V.; Nilsson, I.A.K.; et al. Effects of a Synbiotic on Plasma Immune Activity Markers and Short-Chain Fatty Acids in Children and Adults with ADHD—A Randomized Controlled Trial. Nutrients 2023, 15, 1293. [Google Scholar] [CrossRef]
- Korsten, S.; Vromans, H.; Garssen, J.; Willemsen, L.E.M. Butyrate Protects Barrier Integrity and Suppresses Immune Activation in a Caco-2/PBMC Co-Culture Model While HDAC Inhibition Mimics Butyrate in Restoring Cytokine-Induced Barrier Disruption. Nutrients 2023, 15, 2760. [Google Scholar] [CrossRef]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol. Investig. 2016, 45, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Matsuda, K. How Microbes Affect Depression: Underlying Mechanisms via the Gut-Brain Axis and the Modulating Role of Probiotics. Int. J. Mol. Sci. 2022, 23, 1172. [Google Scholar] [CrossRef]
- Albertsen, M.; Karst, S.M.; Ziegler, A.S.; Kirkegaard, R.H.; Nielsen, P.H. Back to Basics--The Influence of DNA Extraction and Primer Choice on Phylogenetic Analysis of Activated Sludge Communities. PLoS ONE 2015, 10, e0132783. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).