Detection and Characterization of Extracellular Vesicles in Sputum Samples of COPD Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of Sputum Extracellular Vesicles
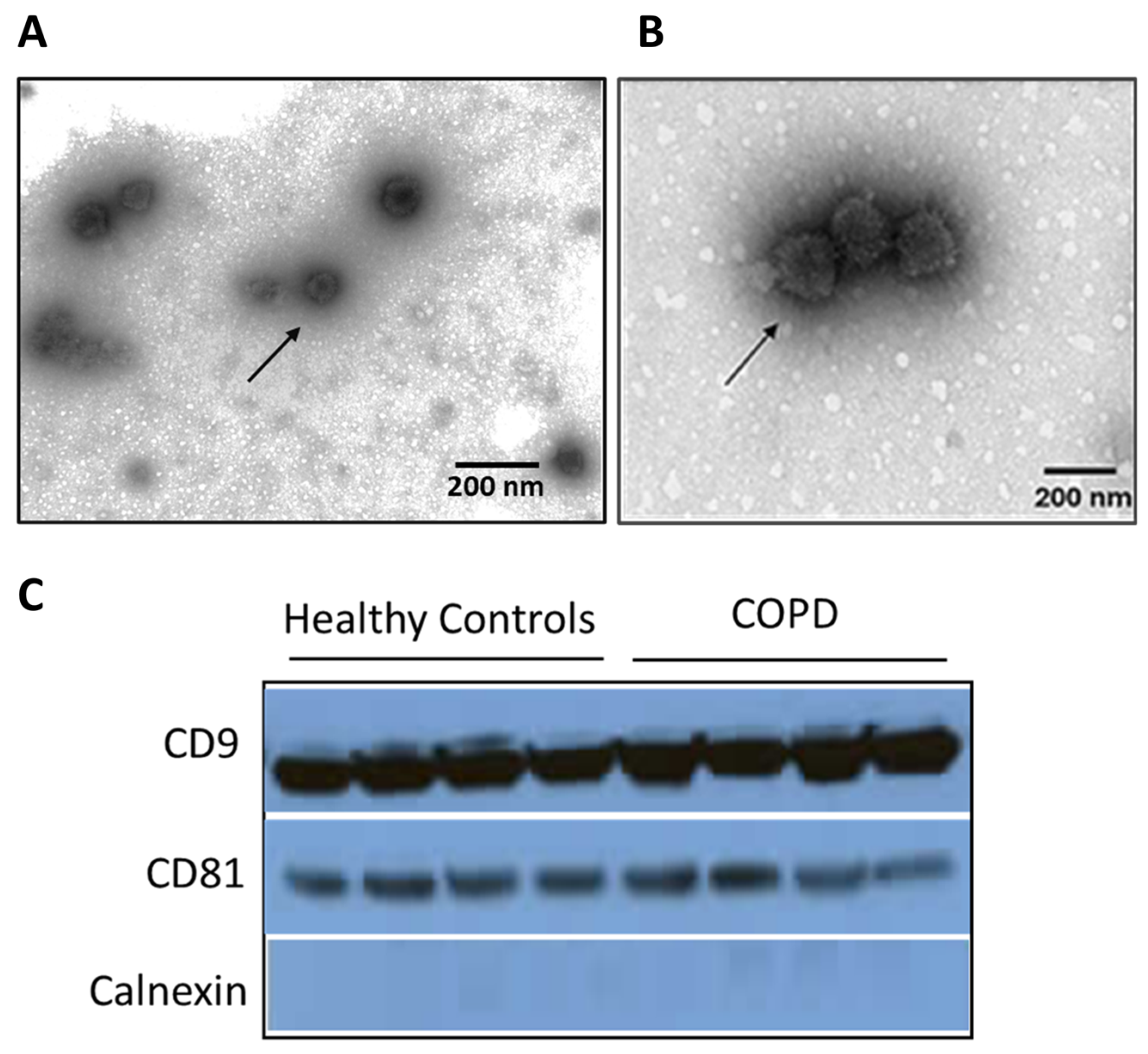
2.3. Transmission Electron Microscopy
2.4. Protein Quantification and Western Blot Analysis
2.5. Statistical Analysis
3. Results
3.1. Characterization of Sputum-Derived EVs from COPD Patients
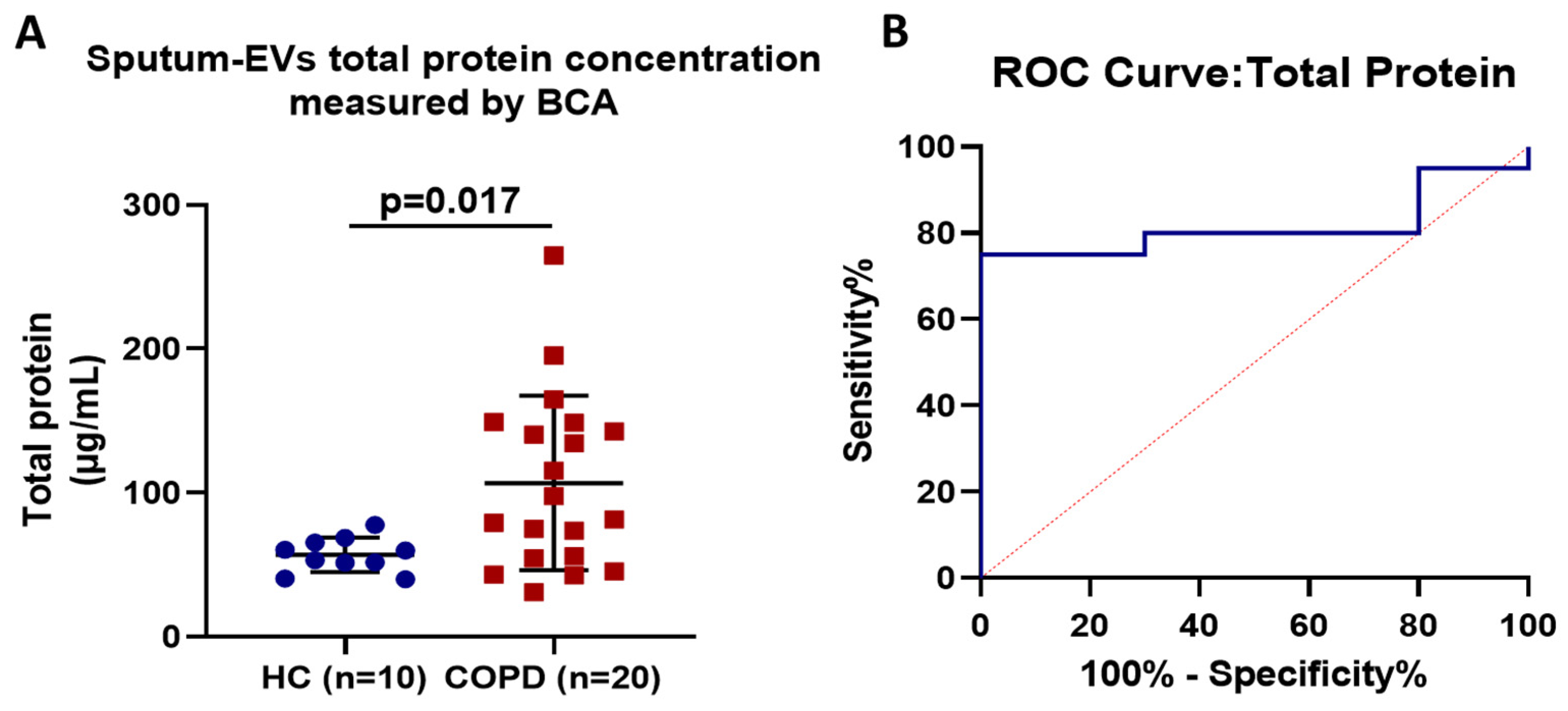
3.2. Assessment and Diagnostic Accuracy of Sputum EV-Associated Total Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GINA Guidelines 2024. Available online: https://ginasthma.org/reports/ (accessed on 19 July 2024).
- Kumar, M.A.; Baba, S.K. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [PubMed]
- Rafieezadeh, D.; Rafieezadeh, A. Extracellular vesicles and their therapeutic applications: A review article (part1). Int. J. Physiol. Pathophysiol. Pharmacol. 2024, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Panagiotidou, S.; Theoharides, T.C. Exosomes in neurologic and psychiatric disorders. Clin. Ther. 2014, 36, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Natelson, B.; Theoharides, T.C. Exosome-associated mitochondrial DNA from patients with mylagic encephalomyelitis/chronic fatigue syndrome stimulates human microglia to release IL-1beta. Eur. J. Neurosci. 2022, 56, 5784–5794. [Google Scholar] [CrossRef]
- Rajabi, H.; Konyalilar, N. Emerging role of exosomes in the pathology of chronic obstructive pulmonary diseases; destructive and therapeutic properties. Stem Cell Res. Ther. 2022, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Paul, S. Beyond Macromolecules: Extracellular Vesicles as Regulators of Inflammatory Diseases. Cells 2023, 12, 1963. [Google Scholar] [CrossRef]
- Lucchetti, D.; Santini, G. Detection and characterisation of extracellular vesicles in exhaled breath condensate and sputum of COPD and severe asthma patients. Eur. Respir. J. 2021, 58, 2003024. [Google Scholar] [CrossRef]
- Enderle, D.; Spiel, A. Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS ONE 2015, 10, e0136133. [Google Scholar] [CrossRef]
- Xiao, C.T.; Lai, W. MicroRNA derived from circulating exosomes as noninvasive biomarkers for diagnosing renal cell carcinoma. Oncotargets Ther. 2020, 13, 10765–10774. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J. Circulating exosomal microRNA profiles associated with acute soft tissue injury. Cell J. 2021, 23, 474–484. [Google Scholar]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Zuba-Surma, E.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.R.; Araldi, R.P.; Vigerelli, H.; D’Ámelio, F.; Mendes, T.B.; Gonzaga, V.; Policíquio, B.; Colozza-Gama, G.A.; Valverde, C.W.; Kerkis, I. Exosomes in the Tumor Microenvironment: From Biology to Clinical Applications. Cells 2021, 10, 2617. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; James, V.; Onion, D.; Fairclough, L.C. Extracellular vesicles and chronic obstructive pulmonary disease (COPD): A systematic review. Respir. Res. 2022, 23, 82. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A. Exosomes: Mediators of communication in eukaryotes. Biol. Res. 2013, 46, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- van den Boorn, J.G.; Daßler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Gordon, C.; Gudi, K.; Krause, A.; Sackrowitz, R.; Harvey, B.G.; Strulovici-Barel, Y.; Mezey, J.G.; Crystal, R.G. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am. J. Respir. Crit. Care Med. 2011, 184, 224–232. [Google Scholar] [CrossRef]
- Takahashi, T.; Kobayashi, S.; Fujino, N.; Suzuki, T.; Ota, C.; He, M.; Yamada, M.; Suzuki, S.; Yanai, M.; Kurosawa, S.; et al. Increased circulating endothelial microparticles in COPD patients: A potential biomarker for COPD exacerbation susceptibility. Thorax 2012, 67, 106. [Google Scholar] [CrossRef]
- Strulovici-Barel, Y.; Staudt, M.R.; Krause, A.; Gordon, C.; Tilley, A.E.; Harvey, B.G.; Kaner, R.J.; Hollmann, C.; Mezey, J.G.; Bitter, H.; et al. Persistence of circulating endothelial microparticles in COPD despite smoking cessation. Thorax 2016, 71, 1137. [Google Scholar] [CrossRef]
- Lacedonia, D.; Carpagnano, G.E.; Trotta, T.; Palladino, G.P.; Panaro, M.A.; Zoppo, L.D.; Foschino Barbaro, M.P.; Porro, C. Microparticles in sputum of COPD patients: A potential biomarker of the disease? Int. J. Chron. Obstr. Pulm. Dis. 2016, 11, 527–533. [Google Scholar]
- Finicelli, M.; Digilio, F.A.; Galderisi, U.; Peluso, G. The Emerging Role of Macrophages in Chronic Obstructive Pulmonary Disease: The Potential Impact of Oxidative Stress and Extracellular Vesicle on Macrophage Polarization and Function. Antioxidants 2022, 11, 464. [Google Scholar] [CrossRef]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Roda, M.A.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef]
- Sundar, I.K.; Li, D.; Rahman, I. Small RNA-sequence analysis of plasma-derived extracellular vesicle miRNAs in smokers and patients with chronic obstructive pulmonary disease as circulating biomarkers. J. Extracell. Vesicles 2019, 8, 1684816. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Fujita, Y.; Kadota, T.; Araya, J.; Kuwano, K. Intercellular Communication by Vascular Endothelial Cell-Derived Extracellular Vesicles and Their MicroRNAs in Respiratory Diseases. Front. Mol. Biosci. 2021, 7, 619697. [Google Scholar] [CrossRef]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef]
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large extracellular vesicles: Size matters in tumor progression. Cytokine Growth Factor. Rev. 2020, 51, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Grunewald, J.; Thyberg, J.; Gripenbäck, S.; Tornling, G.; Eklund, A.; Scheynius, A.; Gabrielsson, S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur. Respir. J. 2003, 22, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
| Parameters | COPD Patients (n = 20) | Controls (n = 10) | p-Value |
|---|---|---|---|
| Gender | |||
| Males, n (%) | 19 (95) | 9 (90) | 0.569 |
| Females, n (%) | 1 (5) | 1 (10) | |
| Age (years) | 70 ± 8 | 65 ± 5 | 0.342 |
| BMI (Kg/m2) | 31 ± 6 | 28 ± 7 | 0.423 |
| Commorbidities, yes, n (%) | |||
| Atrial Hypertention | 18 (90) | 5 (50) | 0.140 |
| Hyperlipidemia | 15 (70) | 5 (50) | 0.240 |
| Obstructive sleep apnea | 10 (50) | 3 (30) | 0.321 |
| Depression | 9 (45) | 2 (20) | 0.150 |
| Pys | 77 ± 42 | 8 ± 2 | 0.001 |
| FEV1/FVC | 66 ± 5 | 76 ± 2 | 0.001 |
| FEV1 (%) | 72 ± 8 | 110 ± 2 | 0.001 |
| GOLD stage | - | - | |
| Stage I, n (%) | 10 (50) | ||
| Stage II, n (%) | 9 (45) | ||
| Stage III, n (%) | 1(5) | ||
| Stage IV, n (%) | 0 (0) | ||
| CAT score | 16 ± 9 | - | - |
| Εxacerbation the last year, yes, n (%) | 6 (30) | - | - |
| Hospitalization the last year, yes, n (%) | 6 (30) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsiou, O.S.; Katsanaki, K.; Tsiggene, A.; Papathanasiou, S.; Rouka, E.; Antonopoulos, D.; Gerogianni, I.; Balatsos, N.A.A.; Gourgoulianis, K.I.; Tsilioni, I. Detection and Characterization of Extracellular Vesicles in Sputum Samples of COPD Patients. J. Pers. Med. 2024, 14, 820. https://doi.org/10.3390/jpm14080820
Kotsiou OS, Katsanaki K, Tsiggene A, Papathanasiou S, Rouka E, Antonopoulos D, Gerogianni I, Balatsos NAA, Gourgoulianis KI, Tsilioni I. Detection and Characterization of Extracellular Vesicles in Sputum Samples of COPD Patients. Journal of Personalized Medicine. 2024; 14(8):820. https://doi.org/10.3390/jpm14080820
Chicago/Turabian StyleKotsiou, Ourania S., Katerina Katsanaki, Aikaterini Tsiggene, Sophia Papathanasiou, Erasmia Rouka, Dionysios Antonopoulos, Irene Gerogianni, Nikolaos A. A. Balatsos, Konstantinos I. Gourgoulianis, and Irene Tsilioni. 2024. "Detection and Characterization of Extracellular Vesicles in Sputum Samples of COPD Patients" Journal of Personalized Medicine 14, no. 8: 820. https://doi.org/10.3390/jpm14080820






