Bayesian Rare Variant Analysis Identifies Novel Schizophrenia Putative Risk Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. SCHEMA Sample
2.2. Applying MIRAGE on SCHEMA Sample
3. Results
3.1. MIRAGE Identifies Novel Putative Risk Genes for Schizophrenia
3.2. MIRAGE Risk Genes Largely Overlapping with Risk Genes in SCHEMA Results
3.3. Gene Set Enrichment of MIRAGE FDR Genes
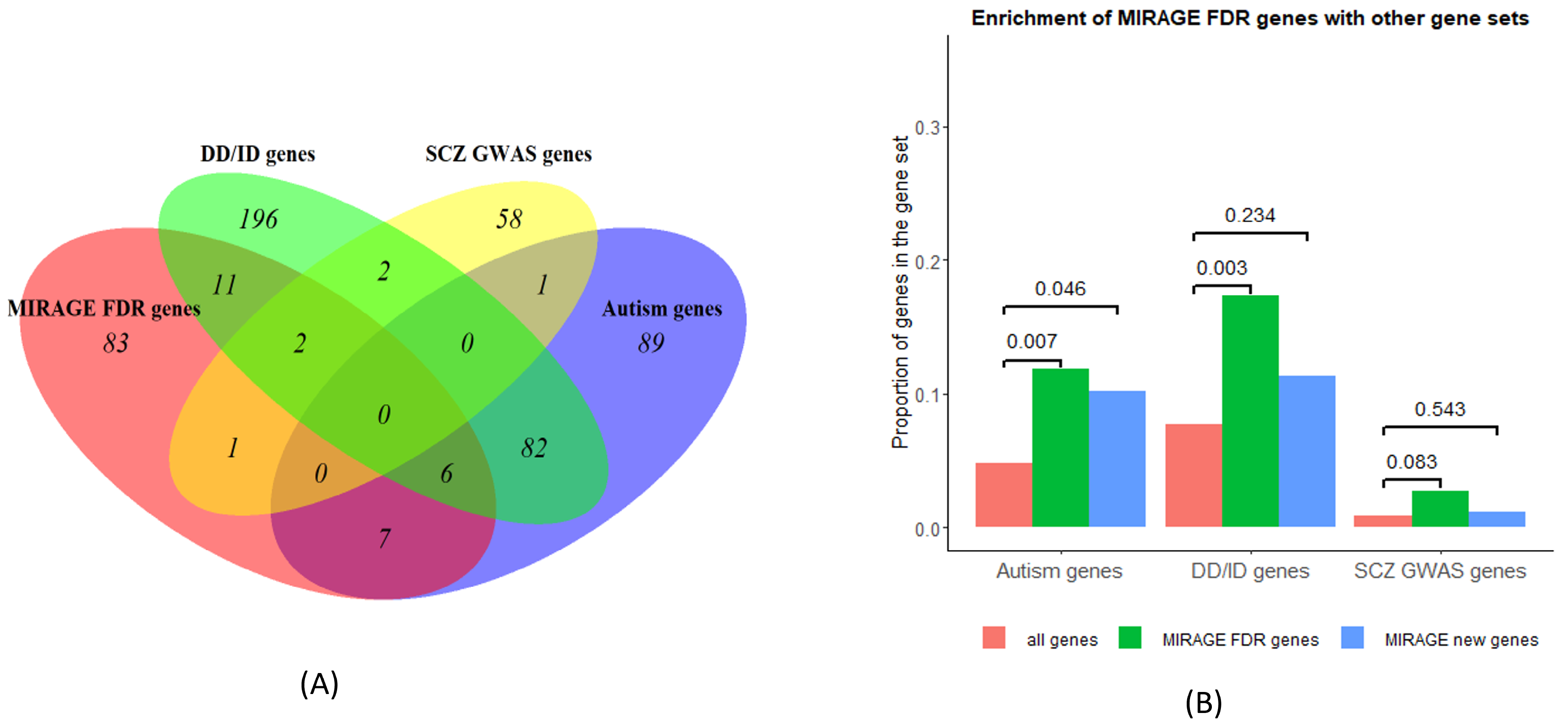
3.3.1. MIRAGE FDR Genes Significantly Enriched with Autism and Other Disorder Genes
3.3.2. GO Enrichment Analysis
4. Discussion and Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCHEMA | schizophrenia exome meta-analysis |
| SCZ | schizophrenia |
| GWAS | genome-wide association study |
| SNV | single nucleotide variants |
| CNV | copy number variation |
| FDR | false discovery rate |
| URV | ultra-rare variants |
| MIRAGE | mixture model based rare variant analysis on genes |
| MAC | minor allele counts |
| GO | gene ontology |
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Institute of Health Metrics and Evaluation (IHME). Global Health Data Exchange (GHDx). Available online: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/27a7644e8ad28e739382d31e77589dd7 (accessed on 25 September 2021).
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Genovese, G.; Fromer, M.; Stahl, E.A.; Ruderfer, D.M.; Chambert, K.; Landén, M.; Moran, J.L.; Purcell, S.M.; Sklar, P.; Sullivan, P.F.; et al. Increased burden of ultra-rare protein-altering variants among 4877 individuals with schizophrenia. Nat. Neurosci. 2016, 19, 1433–1441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marshall, C.R.; Howrigan, D.P.; Merico, D.; Thiruvahindrapuram, B.; Wu, W.; Greer, D.S.; Antaki, D.; Shetty, A.; Holmans, P.A.; Pinto, D.; et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 2017, 49, 27–35, Erratum in Nat. Genet. 2017, 49, 651; Erratum in Nat. Genet. 2017, 49, 1558. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Walters, J.T.R.; Johnstone, M.; Curtis, D.; Suvisaari, J.; Torniainen, M.; Rees, E.; Iyegbe, C.; Blackwood, D.; McIntosh, A.M.; et al. The contribution of rare variants to risk of schizophrenia in individuals with and without intellectual disability. Nat. Genet. 2017, 49, 1167–1173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rees, E.; Han, J.; Morgan, J.; Carrera, N.; Escott-Price, V.; Pocklington, A.J.; Duffield, M.; Hall, L.S.; Legge, S.E.; Pardiñas, A.F.; et al. De novo mutations identified by exome sequencing implicate rare missense variants in SLC6A1 in schizophrenia. Nat. Neurosci. 2020, 23, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Poterba, T.; Curtis, D.; Akil, H.; Al Eissa, M.; Barchas, J.D.; Bass, N.; Bigdeli, T.B.; Breen, G.; Bromet, E.J.; et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 2022, 604, 509–516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, J.M.; Satterstrom, F.K.; Peng, M.; Brand, H.; Collins, R.L.; Dong, S.; Wamsley, B.; Klei, L.; Wang, L.; Hao, S.P.; et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat. Genet. 2022, 54, 1320–1331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trost, B.; Thiruvahindrapuram, B.; Chan, A.J.S.; Engchuan, W.; Higginbotham, E.J.; Howe, J.L.; Loureiro, L.O.; Reuter, M.S.; Roshandel, D.; Whitney, J.; et al. Genomic architecture of autism from comprehensive whole-genome sequence annotation. Cell 2022, 185, 4409–4427.e18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.; Knoblauch, N.; Wang, G.; Zhao, S.; Liu, Y.; Xie, Y.; Sheng, W.; Nguyen, H.T.; He, X. A Bayesian method for rare variant analysis using functional annotations and its application to Autism. bioRxiv 2021, 828061. [Google Scholar] [CrossRef]
- Wu, M.C.; Lee, S.; Cai, T.; Li, Y.; Boehnke, M.; Lin, X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 2011, 89, 82–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Li, Z.; Zhou, H.; Gaynor, S.M.; Liu, Y.; Chen, H.; Sun, R.; Dey, R.; Arnett, D.K.; Aslibekyan, S.; et al. Dynamic incorporation of multiple in silico functional annotations empowers rare variant association analysis of large whole-genome sequencing studies at scale. Nat. Genet. 2020, 52, 969–983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.; Emond, M.J.; Bamshad, M.J.; Barnes, K.C.; Rieder, M.J.; Nickerson, D.A.; NHLBI GO Exome Sequencing Project—ESP Lung Project Team; Christiani, D.C.; Wurfel, M.M.; Lin, X. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am. J. Hum. Genet. 2012, 91, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Samocha, K.E.; Kosmicki, J.A.; Karczewski, K.J.; O’Donnell-Luria, A.H.; Pierce-Hoffman, E.; MacArthur, D.G.; Neale, B.M.; Daly, M.J. Regional missense constraint improves variant deleteriousness prediction. bioRxiv 2022, 148353. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020, 581, 434–443, Erratum in Nature 2021, 590, E53; Erratum in Nature 2021, 597, E3–E4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, H.T.; Bryois, J.; Kim, A.; Dobbyn, A.; Huckins, L.M.; Munoz-Manchado, A.B.; Ruderfer, D.M.; Genovese, G.; Fromer, M.; Xu, X.; et al. Integrated Bayesian analysis of rare exonic variants to identify risk genes for schizophrenia and neurodevelopmental disorders. Genome Med. 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, X.; Sanders, S.J.; Liu, L.; De Rubeis, S.; Lim, E.T.; Sutcliffe, J.S.; Schellenberg, G.D.; Gibbs, R.A.; Daly, M.J.; Buxbaum, J.D.; et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS Genet. 2013, 9, e1003671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deciphering Developmental Disorders Study. Large-scale discovery of novel genetic causes of developmental disorders. Nature 2015, 519, 223–228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassett, A.S.; Lowther, C.; Merico, D.; Costain, G.; Chow, E.W.C.; van Amelsvoort, T.; McDonald-McGinn, D.; Gur, R.E.; Swillen, A.; Van den Bree, M.; et al. Rare Genome-Wide Copy Number Variation and Expression of Schizophrenia in 22q11.2 Deletion Syndrome. Am. J. Psychiatry 2017, 174, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gauthier, J.; Champagne, N.; Lafrenière, R.G.; Xiong, L.; Spiegelman, D.; Brustein, E.; Lapointe, M.; Peng, H.; Côté, M.; Noreau, A.; et al. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc. Natl. Acad. Sci. USA 2010, 107, 7863–7868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moskowitz, A.M.; Belnap, N.; Siniard, A.L.; Szelinger, S.; Claasen, A.M.; Richholt, R.F.; De Both, M.; Corneveaux, J.J.; Balak, C.; Piras, I.S.; et al. A de novo missense mutation in ZMYND11 is associated with global developmental delay, seizures, and hypotonia. Cold Spring Harb. Mol. Case Stud. 2016, 2, a000851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwartz, S.; Wilson, S.J.; Hale, T.K.; Fitzsimons, H.L. Ankyrin2 is essential for neuronal morphogenesis and long-term courtship memory in Drosophila. Mol. Brain. 2023, 16, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirov, G.; Gumus, D.; Chen, W.; Norton, N.; Georgieva, L.; Sari, M.; O’Donovan, M.C.; Erdogan, F.; Owen, M.J.; Ropers, H.H.; et al. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum. Mol. Genet. 2008, 17, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Rujescu, D.; Ingason, A.; Cichon, S.; Pietiläinen, O.P.; Barnes, M.R.; Toulopoulou, T.; Picchioni, M.; Vassos, E.; Ettinger, U.; Bramon, E.; et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum. Mol. Genet. 2009, 18, 988–996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 2008, 455, 237–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sebastian, R.; Jin, K.; Pavon, N.; Bansal, R.; Potter, A.; Song, Y.; Babu, J.; Gabriel, R.; Sun, Y.; Aronow, B.; et al. Schizophrenia-associated NRXN1 deletions induce developmental-timing- and cell-type-specific vulnerabilities in human brain organoids. Nat. Commun. 2023, 14, 3770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moncayo, J.A.; Ayala, I.N.; Argudo, J.M.; Aguirre, A.S.; Parwani, J.; Pachano, A.; Ojeda, D.; Cordova, S.; Mora, M.G.; Tapia, C.M.; et al. Understanding Protein Protocadherin-19 (PCDH19) Syndrome: A Literature Review of the Pathophysiology. Cureus 2022, 14, e25808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lenge, M.; Balestrini, S.; Napolitano, A.; Mei, D.; Conti, V.; Baldassarri, G.; Trivisano, M.; Pellacani, S.; Macconi, L.; Longo, D.; et al. Morphometric network-based abnormalities correlate with psychiatric comorbidities and gene expression in PCDH19-related developmental and epileptic encephalopathy. Transl. Psychiatry 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alkelai, A.; Greenbaum, L.; Shohat, S.; Povysil, G.; Malakar, A.; Ren, Z.; Motelow, J.E.; Schechter, T.; Draiman, B.; Chitrit-Raveh, E.; et al. Genetic insights into childhood-onset schizophrenia: The yield of clinical exome sequencing. Schizophr. Res. 2023, 252, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yin, J.; Liang, C.; Luo, X.; Lv, D.; Dai, Z.; Xiong, S.; Fu, J.; Li, Y.; Lin, J.; et al. CACNA1C (rs1006737) may be a susceptibility gene for schizophrenia: An updated meta-analysis. Brain Behav. 2019, 9, e01292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, A.L.; Haan, N.; Wilkinson, L.S.; Thomas, K.L.; Hall, J. CACNA1C: Association With Psychiatric Disorders, Behavior, and Neurogenesis. Schizophr. Bull. 2018, 44, 958–965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stevenson, R.A.; Park, S.; Cochran, C.; McIntosh, L.G.; Noel, J.P.; Barense, M.D.; Ferber, S.; Wallace, M.T. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr. Res. 2017, 179, 97–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalal, T.C.; Muller, A.M.; Stevenson, R.A. The Relationship Between Multisensory Temporal Processing and Schizotypal Traits. Multisens. Res. 2021, 34, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Lai, I.Y.S.; Hung, K.S.Y.; Chan, M.K.M.; Ho, Z.T.Y.; Lam, J.P.H.; Lui, S.S.Y.; Chan, R.C.K. Audiovisual temporal processing in adult patients with first-episode schizophrenia and high-functioning autism. Schizophrenia 2022, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Sweet, R.A.; Bergen, S.E.; Sun, Z.; Sampson, A.R.; Pierri, J.N.; Lewis, D.A. Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol. Psychiatry 2004, 55, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bertelsen, A.B.; Holm, I.E.; Nyengaard, J.R.; Rosenberg, R.; Dorph-Petersen, K.A. Hippocampal volume and cell number in depression, schizophrenia, and suicide subjects. Brain Res. 2020, 1727, 146546. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Arion, D.; Unger, T.; Maldonado-Avilés, J.G.; Morris, H.M.; Volk, D.W.; Mirnics, K.; Lewis, D.A. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 2008, 13, 147–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashimoto, T.; Bazmi, H.H.; Mirnics, K.; Wu, Q.; Sampson, A.R.; Lewis, D.A. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am. J. Psychiatry 2008, 165, 479–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Hashimoto, T. Deciphering the disease process of schizophrenia: The contribution of cortical GABA neurons. Int. Rev. Neurobiol. 2007, 78, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F.; Glen, A.I.; Vaddadi, K. The membrane hypothesis of schizophrenia. Schizophr. Res. 1994, 13, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Tessier, C.; Sweers, K.; Frajerman, A.; Bergaoui, H.; Ferreri, F.; Delva, C.; Lapidus, N.; Lamaziere, A.; Roiser, J.P.; De Hert, M.; et al. Membrane lipidomics in schizophrenia patients: A correlational study with clinical and cognitive manifestations. Transl. Psychiatry 2016, 6, e906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrade, A.; Brennecke, A.; Mallat, S.; Brown, J.; Gomez-Rivadeneira, J.; Czepiel, N.; Londrigan, L. Genetic Associations between Voltage-Gated Calcium Channels and Psychiatric Disorders. Int. J. Mol. Sci. 2019, 20, 3537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heyes, S.; Pratt, W.S.; Rees, E.; Dahimene, S.; Ferron, L.; Owen, M.J.; Dolphin, A.C. Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog. Neurobiol. 2015, 134, 36–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Chana, G.; Landau, S.; Beasley, C.; Everall, I.P.; Cotter, D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: Evidence for decreased neuronal somal size and increased neuronal density. Biol. Psychiatry 2003, 53, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Stogios, N.; Gdanski, A.; Gerretsen, P.; Chintoh, A.F.; Graff-Guerrero, A.; Rajji, T.K.; Remington, G.; Hahn, M.K.; Agarwal, S.M. Autonomic nervous system dysfunction in schizophrenia: Impact on cognitive and metabolic health. NPJ Schizophr. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selemon, L.D.; Rajkowska, G.; Goldman-Rakic, P.S. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry 1995, 52, 805–818; discussion 819–820. [Google Scholar] [CrossRef] [PubMed]
- Kaplanis, J.; Samocha, K.E.; Wiel, L.; Zhang, Z.; Arvai, K.J.; Eberhardt, R.Y.; Gallone, G.; Lelieveld, S.H.; Martin, H.C.; McRae, J.F.; et al. Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature 2020, 586, 757–762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-temporal transcriptome of the human brain. Nature 2011, 478, 483–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Autism Genes | DD/ID Genes | SCZ GWAS Genes |
|---|---|---|
| ITSN1; DSCAM; ; SHANK3; ; ZMYND11; ; ANK2; ; CAMTA2; NRXN1; ADCY5; PSMD11 | ; SHANK3; ; ; ZMYND11; ; PCDH19; HIVEP2; ; ; ; ; ; PPP2R1A; BMPR2; CLTC; ADCY5; CACNA1C; SATB1 | ; ; CACNA1C |
| MIRAGE FDR (110) Genes | MIRAGE New (88) Genes | ||
|---|---|---|---|
| Complete GO biological process | Membrane depolarization during cardiac muscle cell action potential (GO:0086012) | 53.13 (0.0011) | - |
| Mechanosensory behavior (GO:0007638) | 51.61 (0.0233) | 64.65 (0.0094) | |
| Membrane depolarization during action potential (GO:0086010) | 47.53 (0.0017) | - | |
| Cell–cell signaling involved in cardiac conduction (GO:0086019) | 33.45 (0.0077) | - | |
| Regulation of heart rate by cardiac conduction (GO:0086091) | 22.03 (0.0491) | - | |
| Regulation of cell size (GO:0008361) | - | 9.28 (0.0292) | |
| Neuron projection morphogenesis (GO:0048812) | - | 5.38 (0.0253) | |
| Plasma-membrane-bounded cell projection morphogenesis (GO:0120039) | - | 5.32 (0.0279) | |
| Cell projection morphogenesis (GO:0048858) | - | 5.27 (0.0308) | |
| Cell part morphogenesis (GO:0032990) | - | 5.08 (0.045) | |
| Complete GO molecular function | Voltage-gated calcium channel activity involved in cardiac muscle cell action potential (GO:0086007) | >100 (0.0272) | - |
| RNA binding (GO:0003723) | 2.59 (0.0436) | 2.83 (0.0365) | |
| Complete GO cellular component | Pre-synaptic active zone (GO:0048786) | 13.06 (0.0152) | - |
| GABA-ergic synapse (GO:0098982) | 12.18 (0.0221) | - | |
| Postsynaptic density (GO:0014069) | 6.28 (0.0010) | - | |
| Asymmetric synapse (GO:0032279) | 6.04 (0.0015) | - | |
| Neuron to neuron synapse (GO:0098984) | 6.02 (0.0015) | - | |
| Postsynaptic specialization (GO:0099572) | 5.87 (0.0020) | - | |
| Postsynapse (GO:0098794) | 5.16 (<0.0001) | ||
| Glutamatergic synapse (GO:0098978) | 5.03 (0.0095) | - | |
| Neuronal cell body (GO:0043025) | - | 4.98 (0.0234) | |
| Synaptic membrane (GO:0097060) | 4.97 (0.0264) | - | |
| Dendrite (GO:0030425) | 4.87 (0.0001) | - | |
| Dendritic tree (GO:0097447) | 4.86 (0.0002) | - | |
| Cell body (GO:0044297) | - | 4.79 (0.0136) | |
| Somatodendritic compartment (GO:0036477) | 4.38 (<0.0001) | 4.25 (0.0017) | |
| Pre-synapse (GO:0098793) | 4.11 (0.0311) | - | |
| PANTHER GO-Slim Biological Process | Import into cell (GO:0098657) | 6.18 (0.0362) | 6.88 (0.0474) |
| Reactome pathways | Neuronal System (R-HSA-112316) | 4.94 (0.0409) | - |
| Nervous system development (R-HSA-9675108) | 4.4 (0.0103) | 4.72 (0.023) | |
| Axon guidance (R-HSA-422475) | 4.27 (0.0314) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S. Bayesian Rare Variant Analysis Identifies Novel Schizophrenia Putative Risk Genes. J. Pers. Med. 2024, 14, 822. https://doi.org/10.3390/jpm14080822
Han S. Bayesian Rare Variant Analysis Identifies Novel Schizophrenia Putative Risk Genes. Journal of Personalized Medicine. 2024; 14(8):822. https://doi.org/10.3390/jpm14080822
Chicago/Turabian StyleHan, Shengtong. 2024. "Bayesian Rare Variant Analysis Identifies Novel Schizophrenia Putative Risk Genes" Journal of Personalized Medicine 14, no. 8: 822. https://doi.org/10.3390/jpm14080822





