Neuroinflammatory Approach to Surgical Trauma: Biomarkers and Mechanisms of Immune and Neuroendocrine Responses
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Systemic Inflammation and Tissue Damage
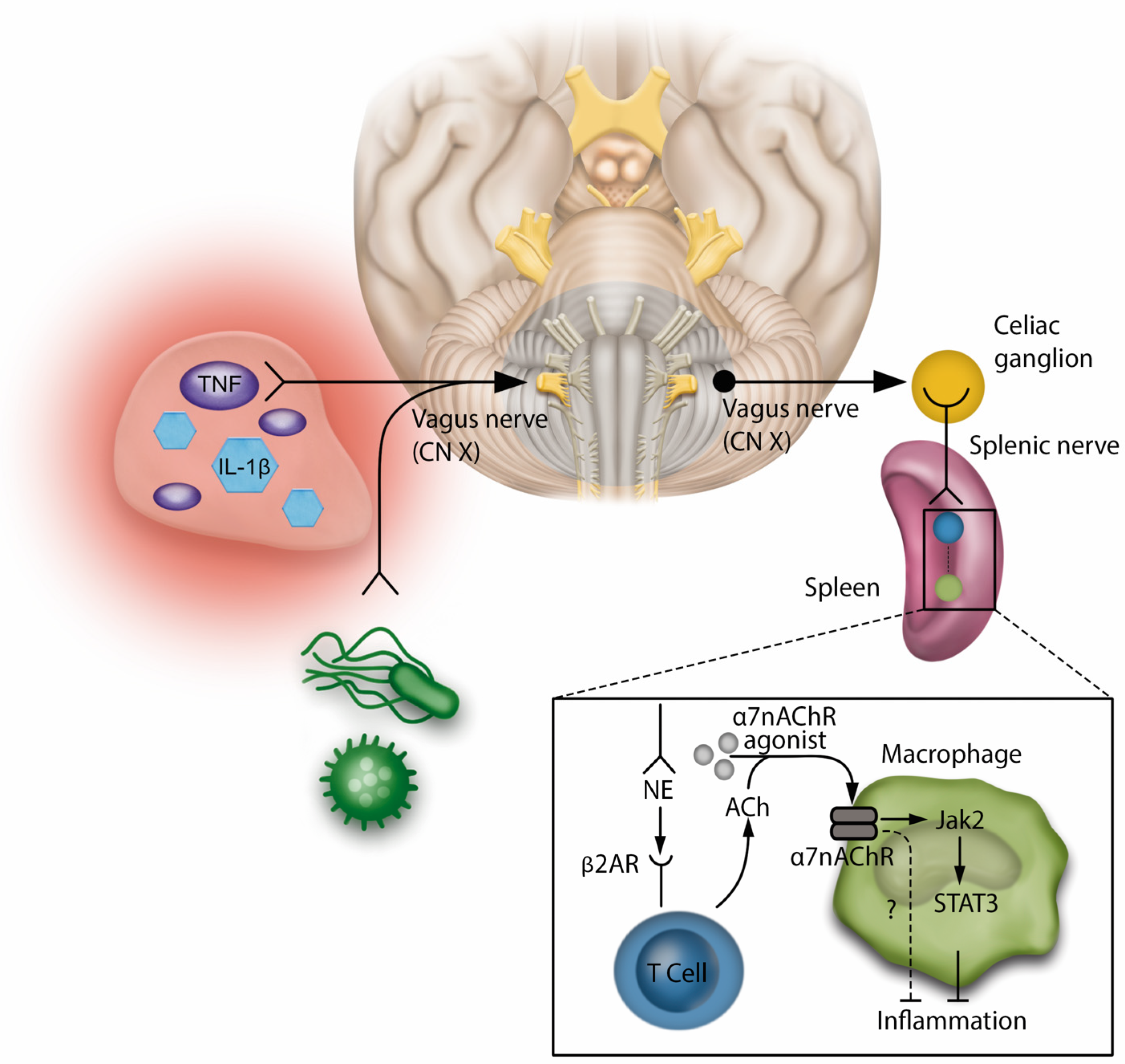
3.1.1. Immune Response
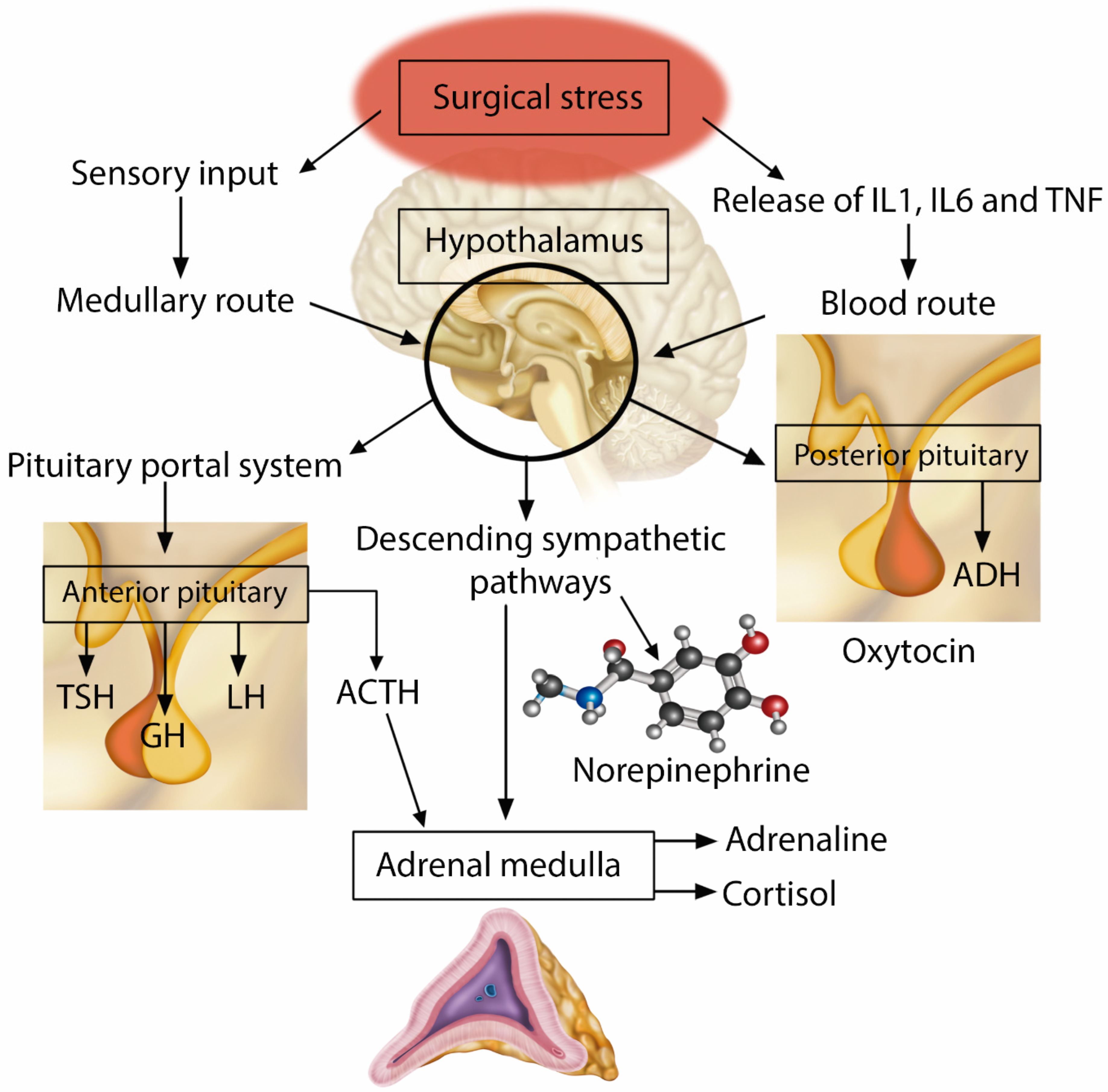
3.1.2. Neuroendocrine and Humoral Response
3.2. Modulation of the Inflammatory Response to Surgical Trauma—Therapeutic Interventions and Protective Strategies
Anesthetic Drugs
- −
- Dexmedetomidine
- −
- ketamine
- −
- Opioids
- −
- Peripheral Regional and Neuraxial Blocks
3.3. Ischemic and Pharmacologic Preconditioning
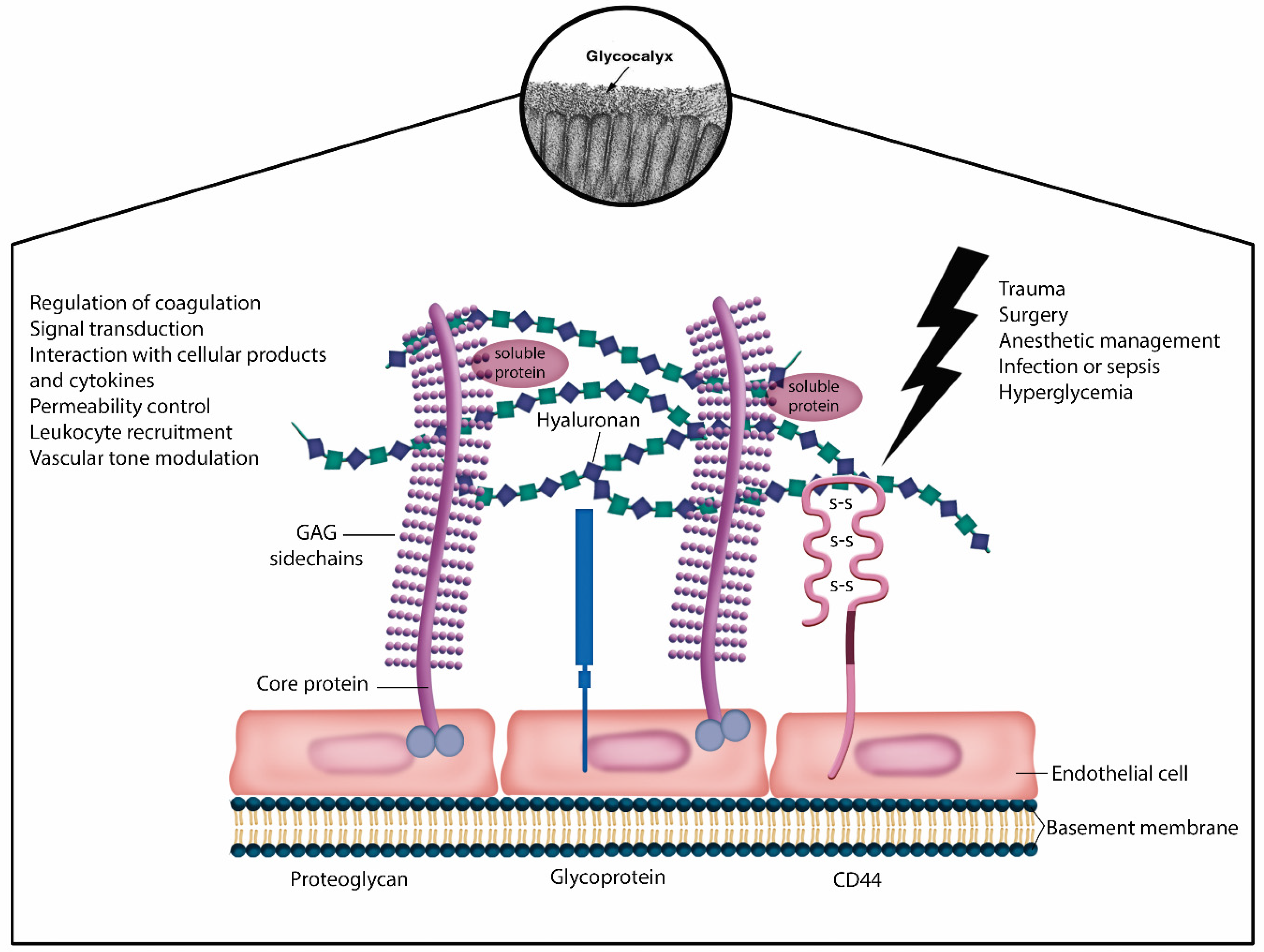
3.4. Endothelial Glycocalyx Protection
4. Biomarkers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, G.N.; Brandão, V.G.; Perez, M.V.; Lewandrowski, K.-U.; Fiorelli, R.K.A. Effects of Dexmedetomidine on Immunomodulation and Pain Control in Videolaparoscopic Cholecystectomies: A Randomized, Two-Arm, Double-Blinded, Placebo-Controlled Trial. J. Pers. Med. 2023, 13, 622. [Google Scholar] [CrossRef]
- Silva, G.N.; Brandão, V.G.; Fiorelli, R.; Perez, M.V.; Mello, C.R.; Negrini, D.; Levandrowski, K.-U.; Martinelli, R.B.; dos Reis, T.P.D.A. Outcomes of dexmedetomidine as adjuvant drug in patients undergoing videolaparoscopic cholecystectomy: A randomized and prospective clinical trial. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231196977. [Google Scholar] [CrossRef] [PubMed]
- Brandão, V.G.A.; Silva, G.N.; Perez, M.V.; Lewandrowski, K.-U.; Fiorelli, R.K.A. Effect of Quadratus Lumborum Block on Pain and Stress Response after Video Laparoscopic Surgeries: A Randomized Clinical Trial. J. Pers. Med. 2023, 13, 586. [Google Scholar] [CrossRef]
- Geng, Z.; Bi, H.; Zhang, D.; Xiao, C.; Song, H.; Feng, Y.; Cao, X.; Li, X. The impact of multimodal analgesia based enhanced recovery protocol on quality of recovery after laparoscopic gynecological surgery: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 179. [Google Scholar] [CrossRef]
- Dobson, G.P. Trauma of major surgery: A global problem that is not going away. Int. J. Surg. 2020, 81, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-pituitary-adrenal axis modulation of glucocorticoids in the cardiovascular system. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic inflammatory response syndrome after surgery: Mechanisms and protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.N.; Brandão, V.G.; Perez, M.V.; Sobrinho, S.L.; Villardi, J.G.d.C.C.; Sacramento, P.M.D.; Ribeiro, L.C.P.; Fiorelli, R.K.A. Immunotherapeutic Properties of Dexmedetomidine on Pain Management and Cardiovascular Function in Videolaparoscopic Cholecystectomies: A randomized, two-arm, double-blinded, placebo-controlled trial. Surg. Innov. 2024, 31, 137–147. [Google Scholar] [CrossRef]
- Watt, D.G.; Horgan, P.G.; McMillan, D.C. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: A systematic review. Surgery 2015, 157, 362–380. [Google Scholar] [CrossRef]
- Floros, T.; Philippou, A.; Bardakostas, D.; Mantas, D.; Koutsilieris, M. The growth endocrine axis and inflammatory responses after laparoscopic cholecystectomy. Hormones 2016, 15, 73–80. [Google Scholar] [CrossRef]
- Page, A.J.; Ejaz, A.; Spolverato, G.; Zavadsky, T.; Grant, M.C.; Galante, D.J.; Wick, E.C.; Weiss, M.; Makary, M.A.; Wu, C.L.; et al. Enhanced recovery after surgery protocols for open hepatectomy--physiology, immunomodulation, and implementation. J. Gastrointest. Surg. 2015, 19, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Curry, N.; Brohi, K. Surgery in Traumatic Injury and Perioperative Considerations. Semin. Thromb. Hemost. 2020, 46, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect. Biol. 2016, 8, a006072. [Google Scholar] [CrossRef] [PubMed]
- Thurairajah, K.; Briggs, G.D.; Balogh, Z.J. The source of cell-free mitochondrial DNA in trauma and potential therapeutic strategies. Eur. J. Trauma Emerg. Surg. 2018, 44, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Cortez, V.S.; Robinette, M.L.; Colonna, M. Innate lymphoid cells: New insights into function and development. Curr. Opin. Immunol. 2015, 32, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G.; Colonna, M.; Di Santo, J.P.; McKenzie, A.N.J. Innate lymphoid cells. Innate lymphoid cells: A new paradigm in immunology. Science 2015, 348, aaa6566. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Faisal, M.; Schäfer, C.N.; Myrelid, P.; Winberg, M.E.; Söderholm, J.D.; Keita, V.; Eintrei, C. Effects of analgesic and surgical modality on immune response in colorectal cancer surgery. Surg. Oncol. 2021, 38, 101602. [Google Scholar] [CrossRef]
- Steinberg, B.E.; Sundman, E.; Terrando, N.; Eriksson, L.I.; Olofsson, P.S. Neural Control of Inflammation: Implications for Perioperative and Critical Care. Anesthesiology 2016, 124, 1174–1189. [Google Scholar] [CrossRef]
- Barman, S.M. 2019 Ludwig Lecture: Rhythms in sympathetic nerve activity are a key to understanding neural control of the cardiovascular system. Am. J. Physiol. Integr. Comp. Physiol. 2020, 318, R191–R205. [Google Scholar] [CrossRef]
- Cusack, B.; Buggy, D.J. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Karasu, E.; Huber-Lang, M. Complement After Trauma: Suturing Innate and Adaptive Immunity. Front. Immunol. 2018, 9, 2050. [Google Scholar] [CrossRef]
- Cruz, F.F.; Rocco, P.R.M.; Pelosi, P. Anti-inflammatory properties of anesthetic agents. Crit. Care 2017, 21, 67. [Google Scholar] [CrossRef]
- Kaye, A.D.; Chernobylsky, D.J.; Thakur, P.; Siddaiah, H.; Kaye, R.J.; Eng, L.K.; Harbell, M.W.; Lajaunie, J.; Cornett, E.M. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) Protocols for Postoperative Pain. Curr. Pain Headache Rep. 2020, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Hao, C.; Luan, H.; Zhang, X.; Zhao, Z. Effects of anesthetic depth on perioperative T lymphocyte subsets in patients undergoing laparoscopic colorectal cancer surgery: A prospective, parallel-controlled randomized trial. BMC Anesthesiol. 2023, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Han, H.-J.; Choi, W.-K.; Yoo, S.; Baek, S.; Lee, J. Immunomodulatory effects of intraoperative dexmedetomidine on T helper 1, T helper 2, T helper 17 and regulatory T cells cytokine levels and their balance: A prospective, randomised, double-blind, dose-response clinical study. BMC Anesthesiol. 2018, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Vorobeichik, L.; Brull, R.; Abdallah, F.W. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: A systematic review and meta-analysis of randomized controlled trials. Br. J. Anaesth. 2017, 118, 167–181. [Google Scholar] [CrossRef]
- Yazdi, B.; Modir, H.; Piri, M.; Almasi-Hashiani, A. An investigation of the effects of dexmedetomidine and fentanyl as an adjuvant to ropivacaine on pain scores and hemodynamic changes following laparoscopic cholecystectomy. Med. Gas Res. 2021, 11, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.M.; Prashanth, K.; Bhatia, N.; Goel, N.; Kaman, L. Paravertebral block using levobupivacaine or dexmedetomidine-levobupivacaine for analgesia after cholecystectomy: A randomized double-blind trial. Braz. J. Anesthesiol. 2021, 71, 358–366. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A., Jr.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Lan, X.; Li, H.; Chao, Z.; Ning, Y. Plasma inflammatory cytokines and treatment-resistant depression with comorbid pain: Improvement by ketamine. J. Neuroinflamm. 2021, 18, 200. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, S.-C.; Chang, P.-C.; Chen, I.-W.; Hsing, C.-H.; Lin, C.-M.; Chen, J.-Y.; Chu, C.-C.; Sun, C.-K. Impact of Intraoperative Ketamine on Postoperative Analgesic Requirement Following Bariatric Surgery: A Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2021, 31, 5446–5457. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Nazir, N.; Mustafi, S. Preemptive low-dose intravenous ketamine in the management of acute and chronic postoperative pain following laparoscopic cholecystectomy: A prospective randomized control study. Med. Gas Res. 2022, 12, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Plein, L.M.; Rittner, H.L. Opioids and the immune system—Friend or foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef]
- Butelman, E.R.; Goldstein, R.Z.; Nwaneshiudu, C.A.; Girdhar, K.; Roussos, P.; Russo, S.J.; Alia-Klein, N. Neuroimmune Mechanisms of Opioid Use Disorder and Recovery: Translatability to Human Studies, and Future Research Directions. Neuroscience 2023, 528, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Y.; Li, Z.-P.; Yao, M.; Zhou, Q.-H. Standard opioid-containing versus opioid-sparing anesthesia on early postoperative recovery after video-assisted thoracic surgery: A propensity-weighted analysis. Front. Surg. 2022, 9, 1015467. [Google Scholar] [CrossRef] [PubMed]
- Brandão, V.G.A.; Silva, G.N.; Fiorelli, R.K.A.; Perez, M.V. Outcome of Ultrasound Guided Anterior Quadratus Lumborum Block after Video Laparoscopic Cholecystectomies: A Prospective Randomized Clinical Trial. Surg. Innov. 2023, 30, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Negrini, D.; Ihsan, M.; Freitas, K.; Pollazzon, C.; Graaf, J.; Andre, J.; Linhares, T.; Brandao, V.; Silva, G.; Fiorelli, R.; et al. The clinical impact of the perioperative epidural anesthesia on surgical outcomes after pancreaticoduodenectomy: A retrospective cohort study. Surg. Open Sci. 2022, 10, 91–96. [Google Scholar] [CrossRef]
- Hou, Y.-Y.; Li, Y.; He, S.-F.; Song, J.; Yu, D.-X.; Wong, G.T.; Zhang, Y. Effects of differential-phase remote ischemic preconditioning intervention in laparoscopic partial nephrectomy: A single blinded, randomized controlled trial in a parallel group design. J. Clin. Anesth. 2017, 41, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Wu, Y.; Li, M.; Zhang, T.; Chen, Y. Effect of remote ischemic preconditioning on postoperative gastrointestinal function in patients undergoing laparoscopic colorectal cancer resection. Int. J. Color. Dis. 2023, 38, 68. [Google Scholar] [CrossRef]
- Chakravarty, D.; Ratnani, P.; Huang, L.; Dovey, Z.; Sobotka, S.; Berryhill, R.; Merisaari, H.; Al Shaarani, M.; Rai, R.; Jambor, I.; et al. Association between Incidental Pelvic Inflammation and Aggressive Prostate Cancer. Cancers 2022, 14, 2734. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Bosch, D.J.; Leuvenink, H.G. Molecular Aspects of Volatile Anesthetic-Induced Organ Protection and Its Potential in Kidney Transplantation. Int. J. Mol. Sci. 2021, 22, 2727. [Google Scholar] [CrossRef] [PubMed]
- Bunte, S.; Lill, T.; Falk, M.; Stroethoff, M.; Raupach, A.; Mathes, A.; Heinen, A.; Hollmann, M.W.; Huhn, R. Impact of Anesthetics on Cardioprotection Induced by Pharmacological Preconditioning. J. Clin. Med. 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Astapenko, D.; Benes, J.; Pouska, J.; Lehmann, C.; Islam, S.; Cerny, V. Endothelial glycocalyx in acute care surgery—What anaesthesiologists need to know for clinical practice. BMC Anesthesiol. 2019, 19, 238. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Maier, C.L.; Helms, J.; Ferrer, R.; Thachil, J.; Levy, J.H. Managing sepsis and septic shock in an endothelial glycocalyx-friendly way: From the viewpoint of surviving sepsis campaign guidelines. Ann. Intensive Care 2024, 14, 64. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, Y.S.; Park, B.J.; Shin, H.J.; Jeon, S.Y.; Kim, D.J.; Kim, S.Y. Immediate Postoperative High Syndecan-1 is Associated with Short-Term Morbidity and Mortality After Robot-Assisted Esophagectomy: A Prospective Observational Study. Ann. Surg. Oncol. 2023, 30, 5870–5880. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, M.S.; Kattouf, N.; Stewart, I.J.; Ginde, A.A.; Schmidt, E.P.; Shapiro, N.I. Plasma for prevention and treatment of glycocalyx degradation in trauma and sepsis. Critical Care 2024, 28, 1–8. [Google Scholar] [CrossRef]
- Mathis, S.; Putzer, G.; Schneeberger, S.; Martini, J. The Endothelial Glycocalyx and Organ Preservation-From Physiology to Possible Clinical Implications for Solid Organ Transplantation. Int. J. Mol. Sci. 2021, 22, 4019. [Google Scholar] [CrossRef]
| Drugs and Therapeutic Strategies | Outcomes and Clinical Implications |
|---|---|
| Dexmedetomidine | |
| Ketamine |
|
| Opioids |
|
| Peripheral Regional and Neuraxial Blocks |
|
| Ischemic and Pharmacologic Preconditioning |
|
| Endothelial Glycocalyx Protection |
| Inflammatory Markers | Characteristics Of Its Systemic Effects |
|---|---|
| TNF-α |
|
| IL1-β |
|
| IL-2 |
|
| IL-6 |
|
| IL-10 |
|
| IFN-γ |
|
| CRP |
|
| Cortisol |
|
| TP/ TTP/ TAPT |
|
| Adrenaline/Norepinephrine |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.N.; Brandão, V.G.A.; Perez, M.V.; Blum, K.; Lewandrowski, K.-U.; Fiorelli, R.K.A. Neuroinflammatory Approach to Surgical Trauma: Biomarkers and Mechanisms of Immune and Neuroendocrine Responses. J. Pers. Med. 2024, 14, 829. https://doi.org/10.3390/jpm14080829
Silva GN, Brandão VGA, Perez MV, Blum K, Lewandrowski K-U, Fiorelli RKA. Neuroinflammatory Approach to Surgical Trauma: Biomarkers and Mechanisms of Immune and Neuroendocrine Responses. Journal of Personalized Medicine. 2024; 14(8):829. https://doi.org/10.3390/jpm14080829
Chicago/Turabian StyleSilva, Gustavo N., Virna G. A. Brandão, Marcelo V. Perez, Kenneth Blum, Kai-Uwe Lewandrowski, and Rossano K. A. Fiorelli. 2024. "Neuroinflammatory Approach to Surgical Trauma: Biomarkers and Mechanisms of Immune and Neuroendocrine Responses" Journal of Personalized Medicine 14, no. 8: 829. https://doi.org/10.3390/jpm14080829






