Enhancing Adult Asthma Management: A Review on the Utility of Remote Home Spirometry and Mobile Applications
Abstract
1. Introduction
1.1. Asthma
1.2. Telemedicine
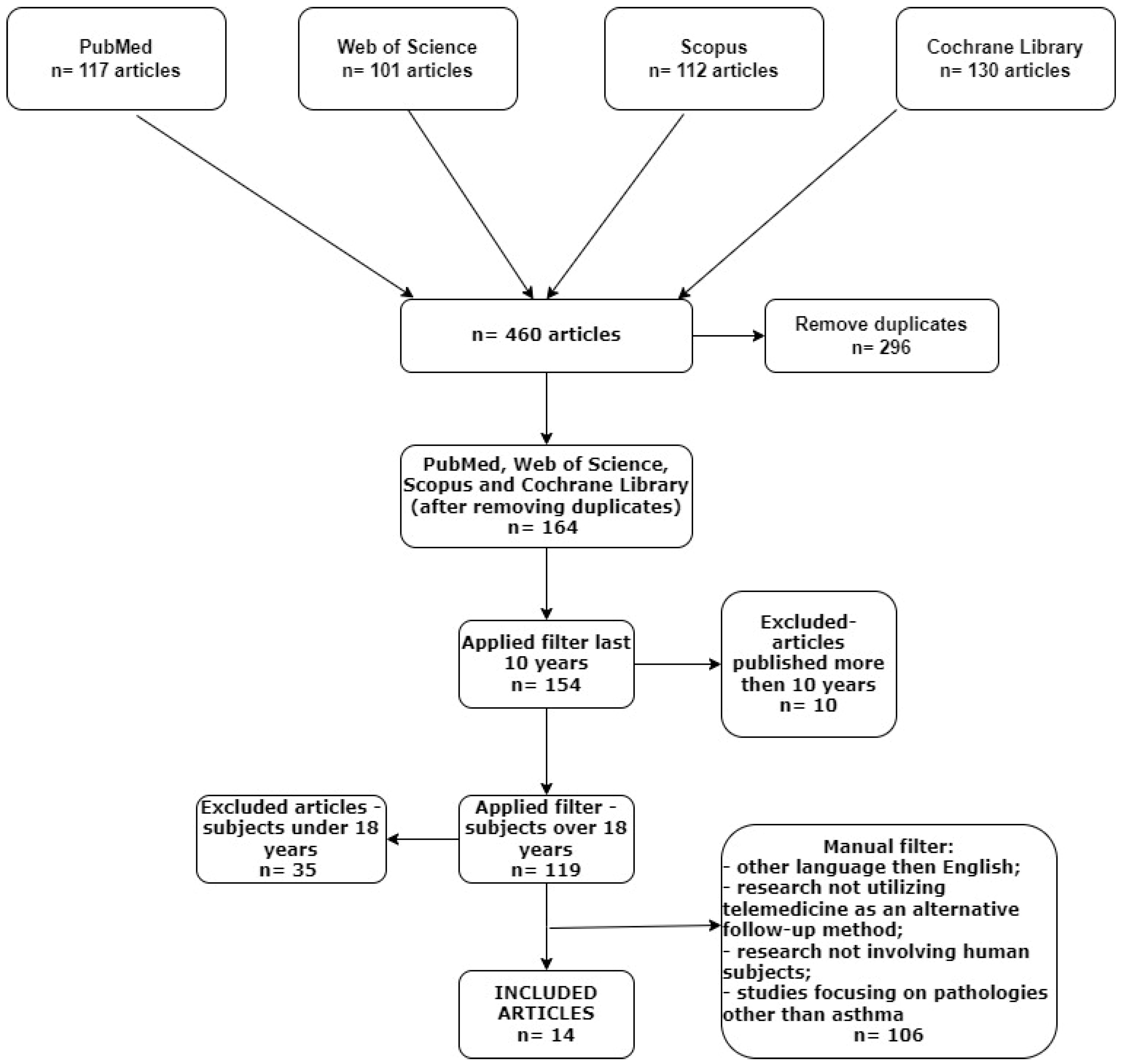
2. Materials and Methods
- (1)
- Studies not in the English language;
- (2)
- Articles published over 10 years ago;
- (3)
- Research not involving human subjects;
- (4)
- Studies conducted on individuals under 18 years old;
- (5)
- Research not utilizing telemedicine as an alternative follow-up method;
- (6)
- Studies focusing on pathologies other than asthma;
- (7)
- Reviews about telemedicine and asthma.
3. Results
3.1. Home Spirometry
3.2. Clinic Versus Home Spirometry
3.3. Self-Management
3.4. Remote Digital Coaching—Follow Up
4. Complementary Telemedicine Tools
5. Ensuring Privacy and Data Protection in Telemedicine
6. Discussion
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma—GINA. GINA Main Report. 2023. Available online: https://ginasthma.org/2023-gina-main-report/ (accessed on 2 May 2024).
- Nurmagambetov, T.; Kuwahara, R.; Garbe, P. The Economic Burden of Asthma in the United States, 2008–2013. Ann. ATS 2018, 15, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhat, G.; Pianosi, P. What is New in the Management of Childhood Asthma? Indian J. Pediatr. 2018, 85, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A. Editorial: Advancements in asthma diagnosis and management: What’s new? Curr. Opin. Pulm. Med. 2022, 28, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, J.R. Spirometry: Key to the diagnosis of respiratory disorders. Med. J. Aust. 2017, 207, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.; Odenthal, D. The uses of telemedicine to improve asthma control. J. Allergy Clin. Immunol. Pract. 2015, 3, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Kew, K.M.; Cates, C.J. Remote versus face-to-face check-ups for asthma. Cochrane Database Syst. Rev. 2016, 4, CD011715. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R. Telemedicine and Telehealth: The Potential to Improve Rural Access to Care. Am. J. Nurs. 2017, 117, 17–18. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.; Sheikh, A.; Cresswell, K.; Nurmatov, U.; Mukherjee, M.; Hemmi, A.; Pagliari, C. The Impact of Telehealthcare on the Quality and Safety of Care: A Systematic Overview. PLoS ONE 2013, 8, e71238. [Google Scholar] [CrossRef]
- Pinnock, H.; Hui, C.Y.; Van Boven, J.F.M. Implementation of digital home monitoring and management of respiratory disease. Curr. Opin. Pulm. Med. 2023, 29, 302–312. [Google Scholar] [CrossRef]
- Persaud, Y.K. Using Telemedicine to Care for the Asthma Patient. Curr. Allergy Asthma Rep. 2022, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Persaud, Y.K.; Portnoy, J.M. Ten Rules for Implementation of a Telemedicine Program to Care for Patients with Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Mobile devices and apps for health care professionals: Uses and benefits. Pharm. Ther. 2014, 39, 356–364. [Google Scholar]
- Misra, S.; Lewis, T.L.; Aungst, T.D. Medical Application Use and the Need for Further Research and Assessment for Clinical Practice: Creation and Integration of Standards for Best Practice to Alleviate Poor Application Design. JAMA Dermatol. 2013, 149, 661. [Google Scholar] [CrossRef]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.; Wallin, M.T.; Sloan, A.; Maloni, H.; Kane, R.; Martz, L.; Haselkorn, J.K. Clinical Management of Multiple Sclerosis Through Home Telehealth Monitoring. Int. J. MS Care 2013, 15, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jaglal, S.B.; Haroun, V.A.; Salbach, N.M.; Hawker, G.; Voth, J.; Lou, W.; Kontos, P.; Cameron, J.E.; Cockerill, R.; Bereket, T. Increasing Access to Chronic Disease Self-Management Programs in Rural and Remote Communities Using Telehealth. Telemed. E-Health 2013, 19, 467–473. [Google Scholar] [CrossRef]
- Bond, C.S. Telehealth as a tool for independent self-management by people living with long term conditions. Stud. Health Technol. Inf. 2014, 206, 1–6. [Google Scholar]
- Rixon, L.; Hirani, S.P.; Cartwright, M.; Beynon, M.; Doll, H.; Steventon, A.; Henderson, C.; Newman, S.P. A RCT of telehealth for COPD patient’s quality of life: The whole system demonstrator evaluation. Clin. Respir. J. 2017, 11, 459–469. [Google Scholar] [CrossRef]
- Potter, A.J.; Ward, M.M.; Natafgi, N.; Ullrich, F.; Clinton, A.; Bell, A.L.; Mueller, K.J. Perceptions of the Benefits of Telemedicine in Rural Communities. 2016. Available online: https://www.proquest.com/openview/a1bec3f367e3222b4b6e3b07a71177c1/1?pq-origsite=gscholar&cbl=51400 (accessed on 2 May 2024).
- Ambrosino, N.; Vitacca, M.; Dreher, M.; Isetta, V.; Montserrat, J.M.; Tonia, T.; Turchetti, G.; Winck, J.C.; Burgos, F.; Kampelmacher, M.; et al. Tele-monitoring of ventilator-dependent patients: A European Respiratory Society Statement. Eur. Respir. J. 2016, 48, 648–663. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, n71, 372. [Google Scholar] [CrossRef]
- Honkoop, P.J.; Simpson, A.; Bonini, M.; Snoeck-Stroband, J.B.; Meah, S.; Fan Chung, K.; Usmani, O.S.; Fowler, S.; Sont, J.K. MyAirCoach: The use of home-monitoring and mHealth systems to predict deterioration in asthma control and the occurrence of asthma exacerbations; study protocol of an observational study. BMJ Open 2017, 7, e013935. [Google Scholar] [CrossRef] [PubMed]
- Rasulnia, M.; Burton, B.S.; Ginter, R.P.; Wang, T.Y.; Pleasants, R.A.; Green, C.L.; Lugogo, N. Assessing the impact of a remote digital coaching engagement program on patient-reported outcomes in asthma. J. Asthma 2018, 55, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-F.Y.; Wang, P.; Rogers, L.; Tignor, N.; Zweig, M.; Hershman, S.G.; Genes, N.; Scott, E.R.; Krock, E.; Badgeley, M.; et al. The Asthma Mobile Health Study, a large-scale clinical observational study using ResearchKit. Nat. Biotechnol. 2017, 35, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Nemanic, T.; Sarc, I.; Skrgat, S.; Flezar, M.; Cukjati, I.; Marc Malovrh, M. Telemonitoring in asthma control: A randomized controlled trial. J. Asthma 2019, 56, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.; Poot, C.C.; Wensing, M.; Chavannes, N.H.; De Smet, P.A.; Teichert, M. Self-Management Maintenance Inhalation Therapy With eHealth (SELFIE): Observational Study on the Use of an Electronic Monitoring Device in Respiratory Patient Care and Research. J. Med. Internet Res. 2019, 21, e13551. [Google Scholar] [CrossRef] [PubMed]
- Rudin, R.S.; Fanta, C.H.; Qureshi, N.; Duffy, E.; Edelen, M.O.; Dalal, A.K.; Bates, D.W. A Clinically Integrated mHealth App and Practice Model for Collecting Patient-Reported Outcomes between Visits for Asthma Patients: Implementation and Feasibility. Appl. Clin. Inf. 2019, 10, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, L.; Chun Wei, Y. Effectiveness of the eCARE programme: A short message service for asthma monitoring. BMJ Health Care Inf. 2019, 26, e100007. [Google Scholar] [CrossRef] [PubMed]
- Kupczyk, M.; Hofman, A.; Kołtowski, Ł.; Kuna, P.; Łukaszyk, M.; Buczyłko, K.; Bodzenta-Łukaszyk, A.; Nastałek, P.; Soliński, M.; Dąbrowiecki, P. Home self-monitoring in patients with asthma using a mobile spirometry system. J. Asthma 2021, 58, 505–511. [Google Scholar] [CrossRef]
- Huang, C.; Izmailova, E.S.; Jackson, N.; Ellis, R.; Bhatia, G.; Ruddy, M.; Singh, D. Remote FEV1 Monitoring in Asthma Patients: A Pilot Study. Clin. Transl. Sci. 2021, 14, 529–535. [Google Scholar] [CrossRef]
- Guarnieri, G.; Caminati, M.; Achille, A.; Vaia, R.; Chieco Bianchi, F.; Senna, G.; Vianello, A. Severe Asthma, Telemedicine, and Self-Administered Therapy: Listening First to the Patient. J. Clin. Med. 2022, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Furci, F.; Caminati, M.; Genovese, S.; Gangemi, S.; Senna, G. Upsides and downsides of a telecounselling model of integrated asthma management between general practitioners and specialists. Clin. Transl. Allergy 2022, 12, e12088. [Google Scholar] [CrossRef] [PubMed]
- Bindler, R.; Haverkamp, H.C.; O’Flanagan, H.; Whicker, J.; Rappold, A.G.; Walden, V.; Postma, J. Feasibility and acceptability of home monitoring with portable spirometry in young adults with asthma. J. Asthma 2023, 60, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Potonos, D.; Exarchos, K.; Assioura, A.; Sioutkou, A.; Tatsis, K.; Kyriakopoulos, C.; Gogali, A.; Kostikas, K. Achievement of high-quality home spirometry inpatients with asthma: The NuvoAir platform. Pneumon 2023, 36, A4536. [Google Scholar] [CrossRef]
- Izmailova, E.S.; Kilian, R.; Bakker, J.P.; Evans, S.; Scotina, A.D.; Reiss, T.F.; Singh, D.; Wagner, J.A. Study protocol: A comparison of mobile and clinic-based spirometry for capturing the treatment effect in moderate asthma. Clin. Transl. Sci. 2023, 16, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, J.; Hanania, N.A.; Chaudhuri, R.; Sagara, H.; Bailes, Z.; Fowler, A.; Peachey, G.; Pizzichini, E.; Slade, D. Clinic vs Home Spirometry for Monitoring Lung Function in Patients With Asthma. Chest 2023, 164, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Jacox, C.; A Guide to At-Home Lung Monitoring Devices. Health Care Originals. 2022. Available online: https://healthcareoriginals.com/a-guide-to-at-home-lung-monitoring-devices/ (accessed on 21 May 2024).
- Vrijdag, X.C.; Van Waart, H.; Sames, C.; Sleigh, J.W.; Mitchell, S.J. Comparing the EMMA capnograph with sidestream capnography and arterial carbon dioxide pressure at 284 kPa. Diving Hyperb. Med. 2023, 53, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Van De Hei, S.J.; Stoker, N.; Flokstra-de Blok, B.M.J.; Poot, C.C.; Meijer, E.; Postma, M.J.; Chavannes, N.H.; Kocks, J.W.H.; Van Boven, J.F.M. Anticipated barriers and facilitators for implementing smart inhalers in asthma medication adherence management. npj Prim. Care Respir. Med. 2023, 33, 22. [Google Scholar] [CrossRef] [PubMed]
- Su, J.G.; Barrett, M.A.; Combs, V.; Henderson, K.; Van Sickle, D.; Hogg, C.; Simrall, G.; Moyer, S.S.; Tarini, P.; Wojcik, O.; et al. Identifying impacts of air pollution on subacute asthma symptoms using digital medication sensors. Int. J. Epidemiol. 2022, 51, 213–224. [Google Scholar] [CrossRef]
- Kuipers, E.; Wensing, M.; De Smet, P.; Teichert, M. Self-management research of asthma and good drug use (SMARAGD study): A pilot trial. Int. J. Clin. Pharm. 2017, 39, 888–896. [Google Scholar] [CrossRef]
- Sulaiman, I.; Greene, G.; MacHale, E.; Seheult, J.; Mokoka, M.; D’Arcy, S.; Taylor, T.; Murphy, D.M.; Hunt, E.; Lane, S.J.; et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur. Respir. J. 2018, 51, 1701126. [Google Scholar] [CrossRef] [PubMed]
- Broască, L.; Trușculescu, A.A.; Ancușa, V.M.; Ciocârlie, H.; Oancea, C.-I.; Stoicescu, E.-R.; Manolescu, D.L. A Novel Method for Lung Image Processing Using Complex Networks. Tomography 2022, 8, 1928–1946. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Miner, S.; Sterling, M.; Halterman, J.S.; Fairbanks, E. The Development of an Automated Device for Asthma Monitoring for Adolescents: Methodologic Approach and User Acceptability. JMIR Mhealth Uhealth 2014, 2, e27. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R.; on behalf of the American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (Fe) for Clinical Applications. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (F E NO ) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Bjermer, L.; Alving, K.; Diamant, Z.; Magnussen, H.; Pavord, I.; Piacentini, G.; Price, D.; Roche, N.; Sastre, J.; Thomas, M.; et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir. Med. 2014, 108, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, K.; Li, W.; Peng, Y.; Tang, X.; Yang, T. Validation of a new portable system containing both FeNO analysis and spirometry measurement. Front. Med. 2023, 10, 1210329. [Google Scholar] [CrossRef] [PubMed]
- Nittari, G.; Khuman, R.; Baldoni, S.; Pallotta, G.; Battineni, G.; Sirignano, A.; Amenta, F.; Ricci, G. Telemedicine Practice: Review of the Current Ethical and Legal Challenges. Telemed. E-Health 2020, 26, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Kahn, E.N.; La Marca, F.; Mazzola, C.A. Neurosurgery and Telemedicine in the United States: Assessment of the Risks and Opportunities. World Neurosurg. 2016, 89, 133–138. [Google Scholar] [CrossRef] [PubMed]
- GDPR.eu. What is GDPR, the EU’s New Data Protection Law? 2018. Available online: https://gdpr.eu/what-is-gdpr/ (accessed on 25 July 2024).
- Botrugno, C. Telemedicine in daily practice: Addressing legal challenges while waiting for an EU regulatory framework. Health Policy Technol. 2018, 7, 131–136. [Google Scholar] [CrossRef]
- Parimbelli, E.; Bottalico, B.; Losiouk, E.; Tomasi, M.; Santosuosso, A.; Lanzola, G.; Quaglini, S.; Bellazzi, R. Trusting telemedicine: A discussion on risks, safety, legal implications and liability of involved stakeholders. Int. J. Med. Inform. 2018, 112, 90–98. [Google Scholar] [CrossRef]
- Ramos Hernández, C.; Núñez Fernández, M.; Pallares Sanmartín, A.; Mouronte Roibas, C.; Cerdeira Domínguez, L.; Botana Rial, M.I.; Blanco Cid, N.; Fernández Villar, A. Validation of the portable Air-Smart Spirometer. PLoS ONE 2018, 13, e0192789. [Google Scholar] [CrossRef] [PubMed]
- Exarchos, K.P.; Gogali, A.; Sioutkou, A.; Chronis, C.; Peristeri, S.; Kostikas, K. Validation of the portable Bluetooth® Air Next spirometer in patients with different respiratory diseases. Respir. Res. 2020, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.; Jeong, I.C. Machine learning approaches to personalize early prediction of asthma exacerbations. Ann. N. Y. Acad. Sci. 2017, 1387, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Morita, P.P.; Yeung, M.S.; Ferrone, M.; Taite, A.K.; Madeley, C.; Stevens Lavigne, A.; To, T.; Lougheed, M.D.; Gupta, S.; Day, A.G.; et al. A Patient-Centered Mobile Health System That Supports Asthma Self-Management (breathe): Design, Development, and Utilization. JMIR Mhealth Uhealth 2019, 7, e10956. [Google Scholar] [CrossRef] [PubMed]
- Mammen, J.R.; Java, J.J.; Halterman, J.; Berliant, M.N.; Crowley, A.; Frey, S.M.; Reznik, M.; Feldman, J.M.; Schoonmaker, J.D.; Arcoleo, K. Development and preliminary results of an Electronic Medical Record (EMR)-integrated smartphone telemedicine program to deliver asthma care remotely. J. Telemed. Telecare 2021, 27, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Ghulam Nabi, F.; Sundaraj, K.; Iqbal, M.S.; Shafiq, M.; Palaniappan, R. A telemedicine software application for asthma severity levels identification using wheeze sounds classification. Biocybern. Biomed. Eng. 2022, 42, 1236–1247. [Google Scholar] [CrossRef]
- Harada, N.; Harada, S.; Ito, J.; Atsuta, R.; Hori, S.; Takahashi, K. Mobile Health App for Japanese Adult Patients With Asthma: Clinical Observational Study. J. Med. Internet Res. 2020, 22, e19006. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.E.; Leszinsky, L.; Walsh, R.; Hepner, H.; Wu, A.C. Mobile Health and Inhaler-Based Monitoring Devices for Asthma Management. J. Allergy Clin. Immunol. Pract. 2019, 7, 2535–2543. [Google Scholar] [CrossRef]
- Sousa, C.S.; Trigueiro-Barbosa, M.; Aguiar, R.; Benito-Garcia, F.; Morais-Almeida, M. What do asthmatic patients think about telemedicine visits? Eur. Ann. Allergy Clin. Immunol. 2021, 53, 138. [Google Scholar] [CrossRef]
| Authors (Publication Year) | Study Location | No. of Patients | Age of Patients (Mean-SD) | Duration of Study | Telemedicine Tools | Outcome Category | Results |
|---|---|---|---|---|---|---|---|
| Honkoop et al. (2017) [24] | UK Netherlands | 150 | ≥18 | 1 year | Portable spirometer; Portable FeNo; Device for exhale breath temperature, RR, physical activity, HR; Smartinhaler device; Pollen forecast | Asthma control; Self-management Quality of life | Positive impact of telemedicine on asthma control, self-management, and quality of life. |
| Rasulnia et al. (2017) [25] | US | 51 | ≥18 | 12 weeks | E-mail; Phone call; SMS | Quality of life; Asthma control | The digital coaching program, using experienced health coaches and digital tools, significantly improved mental health, and decreased outpatient exacerbations, body weight, and ASUI. |
| Chan et al. (2018) [26] | US | 7593 | ≥18 | 6 months | Mobile application | Feasibility; Asthma control | There is a rise in the number of reported asthma symptoms in areas impacted by heat, pollen, and wildfires. |
| Nemanic et al. (2018) [27] | Slovenia | 100 | ≥18 | 1 year | Web-based; SMS | Asthma control; Self-monitoring | The success of a newly developed home monitoring program, utilizing technology, in aiding asthma patients manage their disease effectively. |
| Kuipers et al. (2019) [28] | Netherlands | 32 | ≥18 | 5 weeks | E-inhalation; Phone call; Mobile app; Web-based | Self-management | Bluetooth connectivity and data synchronization are necessary. Tailoring features to individual needs could enhance the program’s acceptability. |
| Rudin et al. (2019) [29] | US | 26 | 54 (16) | 25 weeks | Mobile application; Web-based; Phone call | Symptom monitoring | Robust patient involvement and commitment to adhering to guidelines for monitoring asthma symptoms, while keeping the workload on clinicians to a minimum. |
| Prabhakaran et al. (2019) [30] | Singapore | 424 | ≥18 | 2 years | SMS | Asthma control; Home monitoring | Telemedicine through SMS did not have a positive effect on those in the eCARE program. |
| Kupczyk et al. (2020) [31] | Poland | 86 | 37.4 (11.4) | 3 weeks | Portable spirometer; Mobile application | Symptom control; Self-monitoring | A positive impact of telemedicine on self-monitoring and symptom control. |
| Huang et al. (2021) [32] | UK | 12 | 41.1 (9.9) | 28 days | Mobile spirometer; Mobile application | Monitoring; Compliance | A positive impact of telemedicine on monitoring and compliance of the patient. |
| Guarnieri et al. (2022) [33] | Italy | 180 | ≥18 | Not specified | E-mail; Mobile spirometer; Oxyhemoglobin saturation meter | Self-injection therapy; Self-measurements | The importance of extending telemedicine to general patients with chronic respiratory diseases. |
| Furci et al. (2022) [34] | Italy | 302 | 56 | 2 months | Portable spirometer; Web-based | Collaboration between hospitals | The importance of implementing integrated management across hospital and community settings to attain effective asthma control. |
| Bindler et al. (2023) [35] | USA | 67 | ≥18 | 2 months | Portable spirometer; Mobile application | Self-measurements; Self-monitoring | Self-administered spirometry is a feasible and acceptable method for young adults. |
| Potonos et al. (2023) [36] | Greece | 10 | 42.0 (10.7) | 382 days | Portable spirometer; Mobile application | Follow up | Home spirometry performed without supervision can yield results of high quality. |
| Izmailova et al. (2023) [37] | US | 60 | ≥18 | 24 weeks | Mobile spirometer; Mobile application; Wrist-worn | Treatment effect; Adherence; Accuracy; Asthma control | A positive impact of telemedicine on the early detection of exacerbations, decreased expenses linked to clinic appointments, and improved safety for patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wellmann, N.; Marc, M.S.; Stoicescu, E.R.; Pescaru, C.C.; Trusculescu, A.A.; Martis, F.G.; Ciortea, I.; Crisan, A.F.; Balica, M.A.; Velescu, D.R.; et al. Enhancing Adult Asthma Management: A Review on the Utility of Remote Home Spirometry and Mobile Applications. J. Pers. Med. 2024, 14, 852. https://doi.org/10.3390/jpm14080852
Wellmann N, Marc MS, Stoicescu ER, Pescaru CC, Trusculescu AA, Martis FG, Ciortea I, Crisan AF, Balica MA, Velescu DR, et al. Enhancing Adult Asthma Management: A Review on the Utility of Remote Home Spirometry and Mobile Applications. Journal of Personalized Medicine. 2024; 14(8):852. https://doi.org/10.3390/jpm14080852
Chicago/Turabian StyleWellmann, Norbert, Monica Steluta Marc, Emil Robert Stoicescu, Camelia Corina Pescaru, Ana Adriana Trusculescu, Flavia Gabriela Martis, Ioana Ciortea, Alexandru Florian Crisan, Madalina Alexandra Balica, Diana Raluca Velescu, and et al. 2024. "Enhancing Adult Asthma Management: A Review on the Utility of Remote Home Spirometry and Mobile Applications" Journal of Personalized Medicine 14, no. 8: 852. https://doi.org/10.3390/jpm14080852
APA StyleWellmann, N., Marc, M. S., Stoicescu, E. R., Pescaru, C. C., Trusculescu, A. A., Martis, F. G., Ciortea, I., Crisan, A. F., Balica, M. A., Velescu, D. R., & Fira-Mladinescu, O. (2024). Enhancing Adult Asthma Management: A Review on the Utility of Remote Home Spirometry and Mobile Applications. Journal of Personalized Medicine, 14(8), 852. https://doi.org/10.3390/jpm14080852












