Outcomes of I-125 Low-Dose-Rate Brachytherapy in Patients with Localized Prostate Cancer: A Comprehensive Analysis from a Specialized Tertiary Referral Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Treatment Characteristics
2.3. Follow-Up/Statistics
3. Results
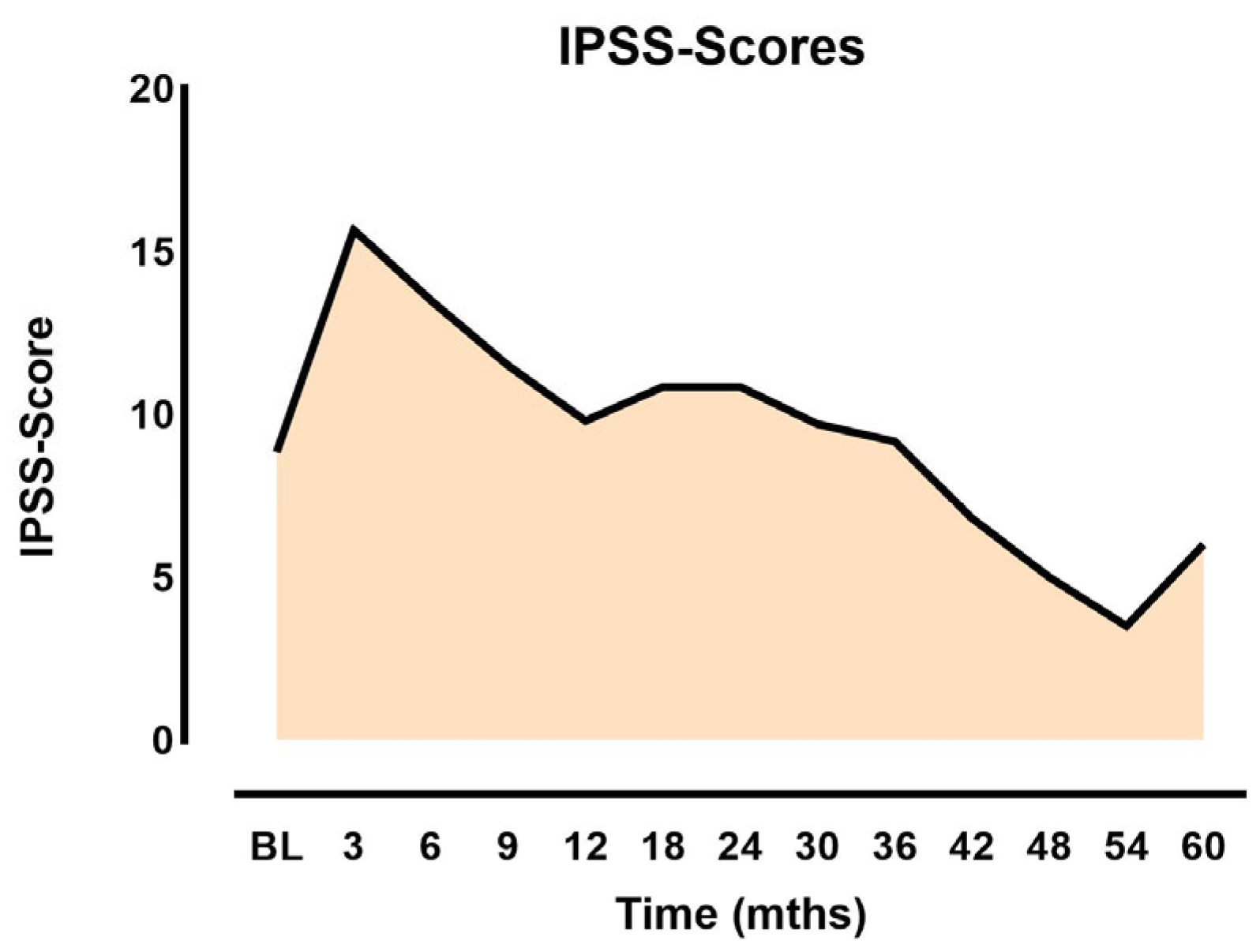
3.1. Side Effects and Quality of Life
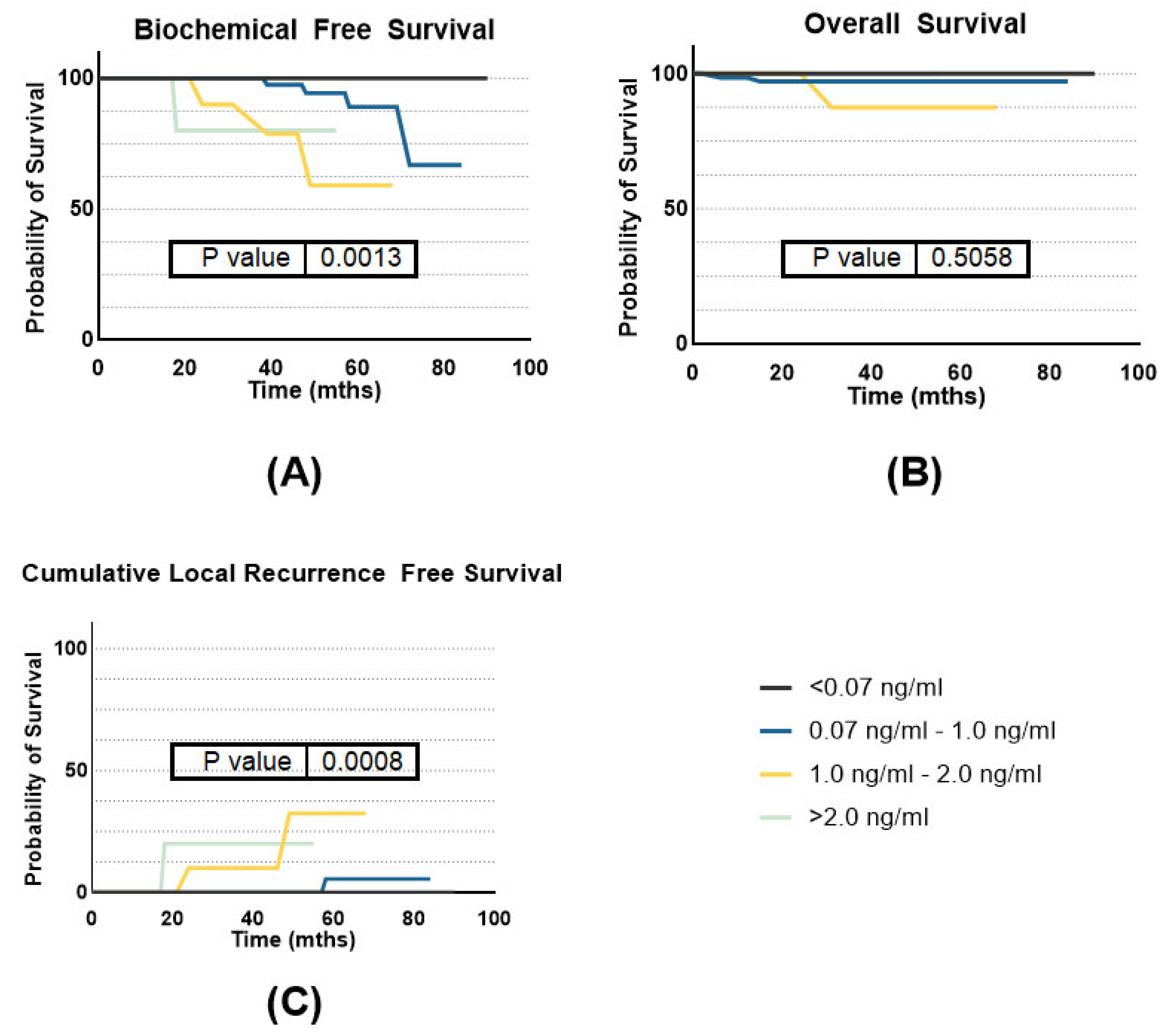
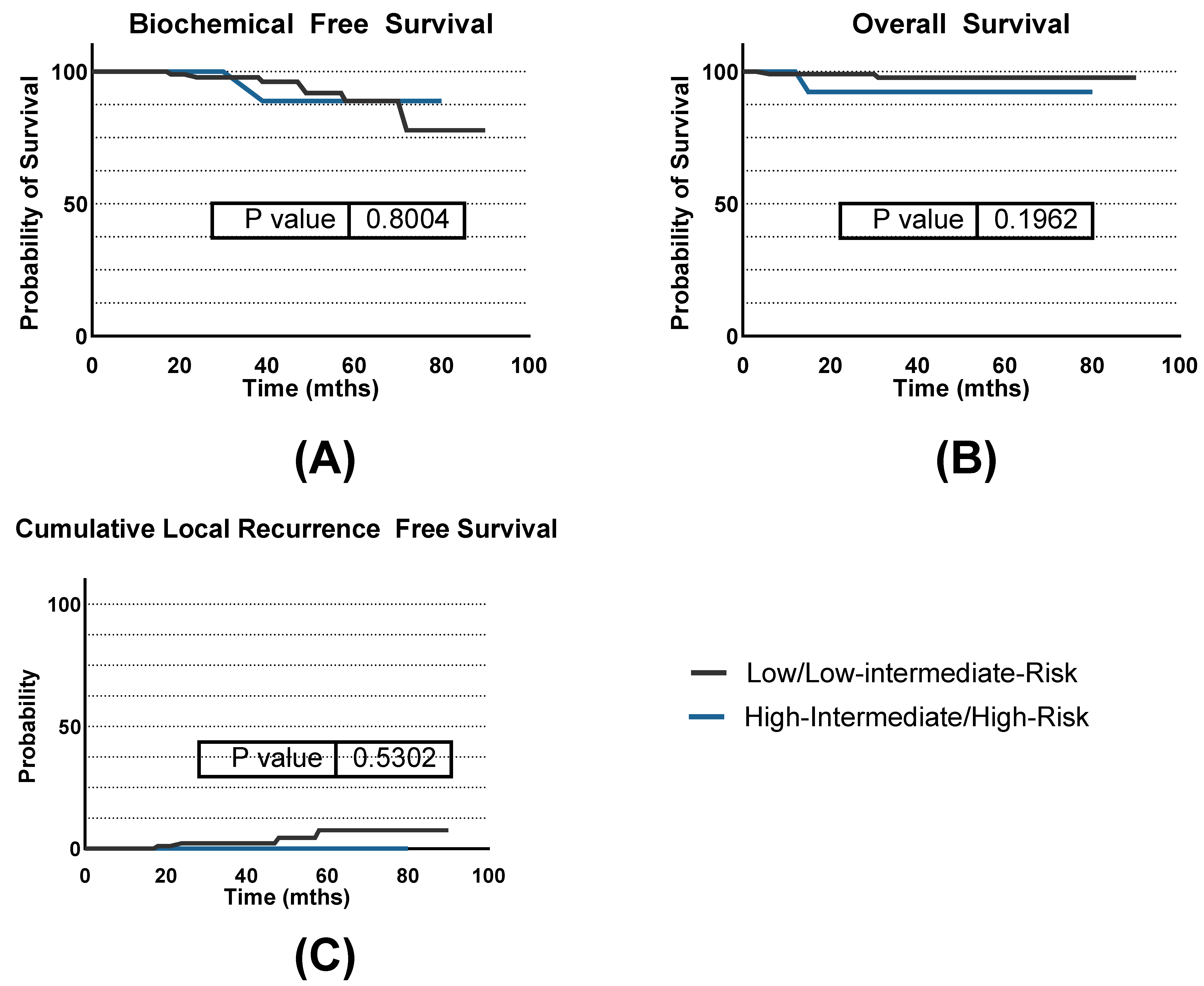
3.2. Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar]
- Henry, A.; Pieters, B.R.; Andre Siebert, F.; Hoskin, P. GEC-ESTRO ACROP prostate brachytherapy guidelines. Radiother. Oncol. 2022, 167, 244–251. [Google Scholar]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar]
- Rosenthal, S.A.; Bittner, N.H.; Beyer, D.C.; Demanes, D.J.; Goldsmith, B.J.; Horwitz, E.M.; Ibbott, G.S.; Lee, W.R.; Nag, S.; Suh, W.W.; et al. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the transperineal permanent brachytherapy of prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 335–341. [Google Scholar]
- Armstrong, A.; Ho, H.; Mark Tacey, M.; Bolton, D.; Chan, Y.; Tan, A.; Cham, C.W.; Pham, T.; McMillan, K.; Koufogiannis, G.; et al. Low-dose-rate brachytherapy and long-term treatment outcomes in patients younger than 60 years of age. J. Contemp. Brachyther. 2024, 16, 6–11. [Google Scholar]
- Ueno, Y.; Fukumori, T.; Kusuhara, Y.; Fukawa, T.; Tsuda, M.; Daizumoto, K.; Sasaki, Y.; Tomida, R.; Yamamoto, Y.; Yamaguchi, K.; et al. Prostate-specific Antigen Levels Following Brachytherapy Impact Late Biochemical Recurrence in Japanese Patients With Localized Prostate Cancer. Vivo 2023, 37, 738–746. [Google Scholar]
- Tanaka, N. The oncologic and safety outcomes of low-dose-rate brachytherapy for the treatment of prostate cancer. Prostate Int. 2023, 11, 127–133. [Google Scholar]
- Sanmamed, N.; Locke, G.; Crook, J.; Liu, A.; Raman, S.; Glicksman, R.; Chung, P.; Berlin, A.; Fleshner, N.; Helou, J. Long-Term Biochemical Control of a Prospective Cohort of Prostate Cancer Patients Treated With Interstitial Brachytherapy Versus Radical Prostatectomy. Clin. Oncol. 2023, 35, 262–268. [Google Scholar]
- Rodda, S.; Tyldesley, S.; Morris, W.J.; Keyes, M.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; et al. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 286–295. [Google Scholar]
- Nakai, Y.; Tanaka, N.; Asakawa, I.; Hori, S.; Miyake, M.; Yamaki, K.; Anai, S.; Torimoto, K.; Inoue, T.; Hasegawa, M.; et al. Quality of life in patients who underwent robot-assisted radical prostatectomy compared with those who underwent low-dose-rate brachytherapy. Prostate 2023, 83, 701–712. [Google Scholar] [PubMed]
- Peinemann, F.; Grouven, U.; Hemkens, L.G.; Bartel, C.; Borchers, H.; Pinkawa, M.; Heidenreich, A.; Sauerland, S. Low-dose rate brachytherapy for men with localized prostate cancer. Cochrane Database Syst. Rev. 2011, 7, CD008871. [Google Scholar]
- Patel, M.; Turchan, W.T.; Morris, C.G.; Augustine, D.; Wu, T.; Oto, A.; Zagaja, G.P.; Liauw, S.L. A Contemporary Report of Low-Dose-Rate Brachytherapy for Prostate Cancer Using MRI for Risk Stratification: Disease Outcomes and Patient-Reported Quality of Life. Cancers 2023, 15, 1336. [Google Scholar] [CrossRef] [PubMed]
- Moll, M.; Nechvile, E.; Kirisits, C.; Komina, O.; Pajer, T.; Kohl, B.; Miszczyk, M.; Widder, J.; Knocke-Abulesz, T.-H.; Goldner, G. Radiotherapy in localized prostate cancer: A multicenter analysis evaluating tumor control and late toxicity after brachytherapy and external beam radiotherapy in 1293 patients. Strahlenther. Onkol. 2024, 200, 698–705. [Google Scholar] [PubMed]
- Tsuchiya, K.; Kawase, M.; Nakane, K.; Nakano, M.; Iinuma, K.; Kato, D.; Takai, M.; Tobisawa, Y.; Mori, T.; Takano, H.; et al. Chronological Changes of Lower Urinary Tract Symptoms in Elderly Patients with Prostate Cancer after Low-Dose-Rate Prostate Brachytherapy. Life 2023, 13, 1507. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.; Laing, R.W.; Langley, S.E. Quality of life following treatment for early prostate cancer: Does low dose rate (LDR) brachytherapy offer a better outcome? A review. Eur. Urol. 2004, 45, 134–141. [Google Scholar] [PubMed]
- Nakai, Y.; Tanaka, N.; Asakawa, I.; Miyake, M.; Anai, S.; Yamaki, K.; Hasegawa, M.; Fujimoto, K. Erectile dysfunction and sexual quality of life in patients who underwent low-dose-rate brachytherapy alone for prostate cancer. Andrologia 2022, 54, e14288. [Google Scholar] [PubMed]
- Geara, F.B.; Bulbul, M.; Khauli, R.B.; Andraos, T.Y.; Abboud, M.; Al Mousa, A.; Sarhan, N.; Salem, A.; Ghatasheh, H.; Alnsour, A.; et al. Nadir PSA is a strong predictor of treatment outcome in intermediate and high risk localized prostate cancer patients treated by definitive external beam radiotherapy and androgen deprivation. Radiat. Oncol. 2017, 12, 149. [Google Scholar] [PubMed]
- Combes, A.D.; Palma, C.A.; Calopedos, R.; Wen, L.; Woo, H.; Fulham, M.; Leslie, S. PSMA PET-CT in the Diagnosis and Staging of Prostate Cancer. Diagnostics 2022, 12, 2594. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Moideen, N.; Arbour, G.; Castro, F.; Araujo, C.; Batchelar, D.; Halperin, R.; Hilts, M.; Kim, D.; Petrik, D.; et al. A Randomized Trial Comparing Quality of Life After Low-Dose Rate or High-Dose Rate Prostate Brachytherapy Boost With Pelvic External Beam Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 59–68. [Google Scholar]
- Hoskin, P.J.; Motohashi, K.; Bownes, P.; Bryant, L.; Ostler, P. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: Initial results of a randomised phase three trial. Radiother. Oncol. 2007, 84, 114–120. [Google Scholar]
- Rosenbrock, J.; Baues, C.; Kreis, M.; Fouassi, R.; Celik, E.; Paffenholz, P.; Pfister, D.; Heidenreich, A.; Marnitz, S. Toxicity of dose-escalated radiotherapy up to 84 Gy for prostate cancer. Strahlenther. Onkol. 2023, 199, 574–584. [Google Scholar] [PubMed]
- Moll, M.; Magrowski, L.; Mittlbock, M.; Heinzl, H.; Kirisits, C.; Ciepal, J.; Masri, O.; Heilemann, G.; Stando, R.; Krzysztofiak, T.; et al. Biochemical control in intermediate- and high-risk prostate cancer after EBRT with and without brachytherapy boost. Strahlenther. Onkol. 2024. [Google Scholar] [CrossRef]
- Light, A.; Kanthabalan, A.; Otieno, M.; Pavlou, M.; Omar, R.; Adeleke, S.; Giganti, F.; Brew-Graves, C.; Williams, N.R.; Emara, A.; et al. The Role of Multiparametric MRI and MRI-targeted Biopsy in the Diagnosis of Radiorecurrent Prostate Cancer: An Analysis from the FORECAST Trial. Eur. Urol. 2024, 85, 35–46. [Google Scholar]
- Ong, W.L.; Loblaw, A. The march toward single-fraction stereotactic body radiotherapy for localized prostate cancer-Quo Vadimus? World J. Urol. 2023, 41, 3485–3491. [Google Scholar] [PubMed]
- Zamboglou, C.; Spohn, S.K.B.; Adebahr, S.; Huber, M.; Kirste, S.; Sprave, T.; Gratzke, C.; Chen, R.C.; Carl, E.G.; Weber, W.A.; et al. PSMA-PET/MRI-Based Focal Dose Escalation in Patients with Primary Prostate Cancer Treated with Stereotactic Body Radiation Therapy (HypoFocal-SBRT): Study Protocol of a Randomized, Multicentric Phase III Trial. Cancers 2021, 13, 5795. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Value |
|---|---|
| Median Age (years) | 69.0 (48–83) |
| Gleason Score 6 | 79 |
| Gleason Score 7a | 37 |
| Gleason Score 7b | 6 |
| Mean PSA at Diagnosis (ng/mL) | 7.09 |
| PSA Range (ng/mL) | 0.4–19.42 |
| cT1a | 4 |
| cT1b | 1 |
| cT1c | 67 |
| cT2a | 1 |
| cT2b | 6 |
| Neoadjuvant Androgen deprivation (yes) | 11 |
| Characteristic | Value (Range) |
|---|---|
| Median Number of Seeds Implanted | 53 (49–74) |
| Median Prostate Volume (cc) | 27.4 (9.9–69.5) |
| Median Dose to 30cc of Urethra (Gy) | 129.3 (106.3–148.6) |
| Median Percentage of Prostate Volume Receiving 100% of the Prescribed Dose (V100) | 96.7% (79.68–99.39%) |
| Median Percentage of Prostate Volume Receiving 100% of the Prescribed Dose (V100) in POST-PLAN | 94.0% (57.2–99.71%) |
| Median Percentage of Prostate Volume Receiving 150% of the Prescribed Dose (V150) | 60.4% (42.4–73.06%) |
| Median Dose to 90% of Prostate Volume (Gy) | 116.15 (105.3–127.59) |
| Median Dose to 2cc of Rectum (Gy) | 75.1 (29.5–91.29) |
| Median Dose to 2cc of Rectum (Gy) in POST-PLAN | 58.3 (28.54–91.39) |
| Dose to 2cc of Bladder (Gy) | 70.28 (33.9–112.83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, P.; Strnad, V.; Höfler, D.; Schweizer, C.; Putz, F.; Lotter, M.; Kreppner, S.; Karius, A.; Fietkau, R.; Merten, R. Outcomes of I-125 Low-Dose-Rate Brachytherapy in Patients with Localized Prostate Cancer: A Comprehensive Analysis from a Specialized Tertiary Referral Center. J. Pers. Med. 2024, 14, 882. https://doi.org/10.3390/jpm14080882
Schubert P, Strnad V, Höfler D, Schweizer C, Putz F, Lotter M, Kreppner S, Karius A, Fietkau R, Merten R. Outcomes of I-125 Low-Dose-Rate Brachytherapy in Patients with Localized Prostate Cancer: A Comprehensive Analysis from a Specialized Tertiary Referral Center. Journal of Personalized Medicine. 2024; 14(8):882. https://doi.org/10.3390/jpm14080882
Chicago/Turabian StyleSchubert, Philipp, Vratislav Strnad, Daniel Höfler, Claudia Schweizer, Florian Putz, Michael Lotter, Stephan Kreppner, Andre Karius, Rainer Fietkau, and Ricarda Merten. 2024. "Outcomes of I-125 Low-Dose-Rate Brachytherapy in Patients with Localized Prostate Cancer: A Comprehensive Analysis from a Specialized Tertiary Referral Center" Journal of Personalized Medicine 14, no. 8: 882. https://doi.org/10.3390/jpm14080882
APA StyleSchubert, P., Strnad, V., Höfler, D., Schweizer, C., Putz, F., Lotter, M., Kreppner, S., Karius, A., Fietkau, R., & Merten, R. (2024). Outcomes of I-125 Low-Dose-Rate Brachytherapy in Patients with Localized Prostate Cancer: A Comprehensive Analysis from a Specialized Tertiary Referral Center. Journal of Personalized Medicine, 14(8), 882. https://doi.org/10.3390/jpm14080882







