The Usability and Feasibility of a Dietary Intake Self-Monitoring Application in a Population with Varying Literacy Levels
Abstract
1. Introduction
2. Background
2.1. Overview of Design Studies
- Match food categories with users’ mental models: Hemodialysis patients think of food items in terms of categories [22]. In particular, they are familiar with USFDA food groups through diet recall sessions with dietitians. They also think of categorizing food items as “what to eat” and “what not to eat”. Moreover, they frequently consume certain items to avoid overshooting their dietary restrictions.
- Incorporate meal building functionality: hemodialysis patients record their meals by recalling items one at a time [22], which allows for a more accurate tracking of their intake.
- Utilize a linear navigation style: The target users prefer to record their meals using a linear guide, which provides a clear, step-by-step pathway through tasks [19]. This approach reduces the cognitive load and makes the application easier to use for patients, especially those who may not be technically savvy.
- Offer real-time and personalized feedback: Healthcare professionals believe that offering immediate feedback on nutrient intake allows patients to make informed dietary choices on the fly [23]. The feedback should be customized to each patient’s dietary needs, displaying crucial information, such as fluid, sodium, and potassium levels.
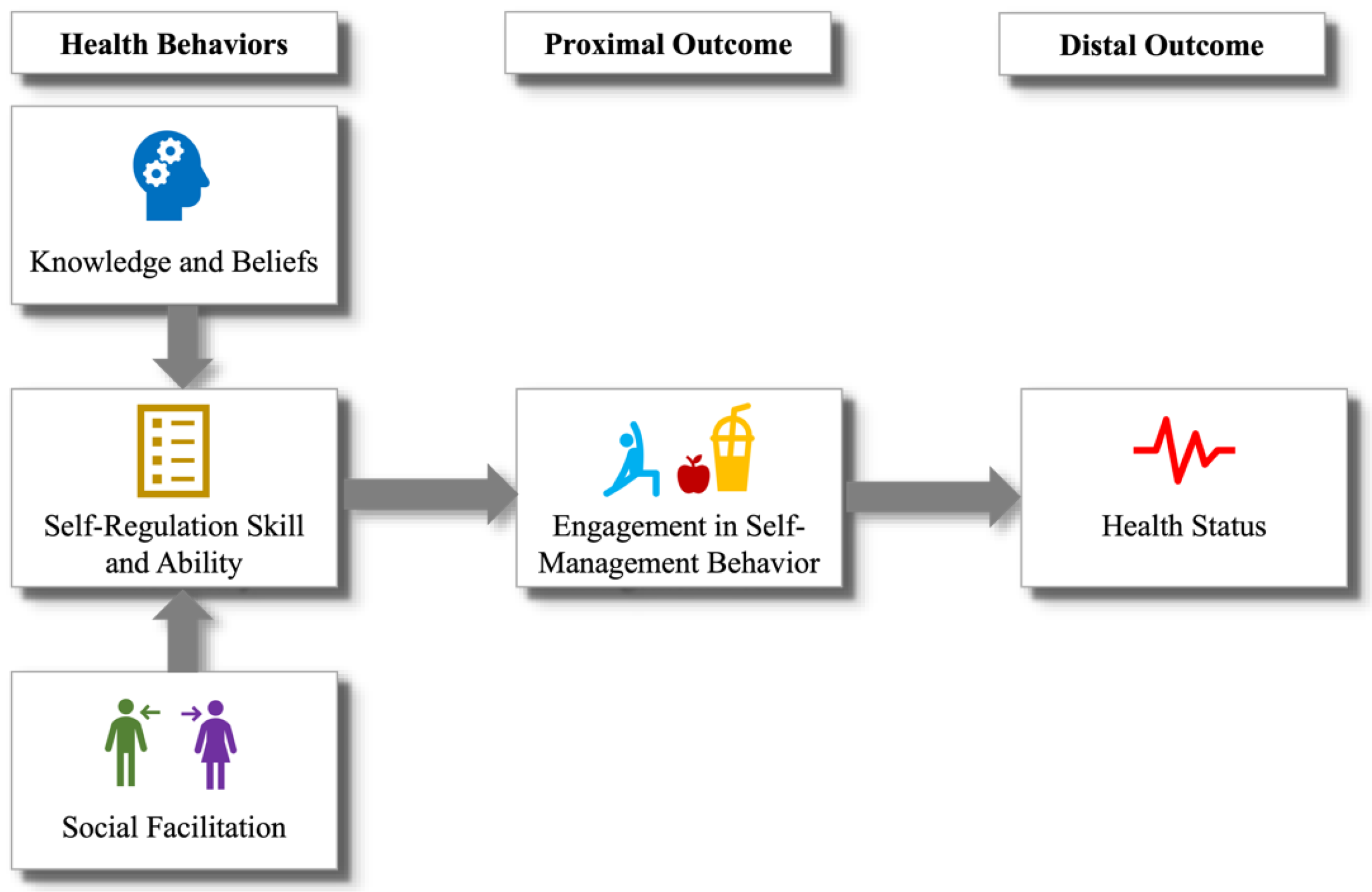
- Conduct a pilot study: We conducted a six-week, in situ evaluation where the target population used the DIMA, enhanced with an interface for estimating fluid portion sizes [18]. Beyond validating the appropriateness of the icons and establishing portion estimation requirements, the feedback from the participants enabled us to identify behavioral health constructs that could help people accurately track and effectively monitor their portion intake. These constructs aligned with those specified by the Integrated Theory of Health Behavior Change (ITHBC) [30], which suggests that health behavior change can be facilitated by fostering knowledge and beliefs, increasing self-regulation skills, and promoting social facilitation.
2.2. Theory behind DIMA-P
- i.
- Knowledge and belief enhancement: The theory posits that increasing a person’s knowledge about their health condition and related behaviors can positively influence their beliefs about health and illness. This can involve educating individuals about the causes, consequences, and management of their health conditions, thereby fostering a better understanding of and belief in the efficacy of health behaviors.
- ii.
- Self-regulation skills and abilities: ITHBC emphasizes the importance of self-regulation in health behavior change. This includes behavioral aspects such as goal setting, self-monitoring, problem-solving, and decision-making skills. The theory suggests that, by enhancing these skills, individuals are better equipped to initiate and maintain health-promoting behaviors. For example, goal setting might involve defining specific, measurable, achievable, relevant, and time-bound (SMART) objectives for health behavior, while self-monitoring could include keeping a diary of dietary intake or physical activity.
- iii.
- Social facilitation: The model emphasizes the role of social support and interaction in facilitating health behavior change. This includes support from healthcare providers, family, friends, and community. Effective communication, encouragement, and practical assistance from these social networks can significantly influence an individual’s motivation and ability to engage in and sustain health behaviors.
3. Materials and Methods
3.1. Intervention Design
3.2. ITHBC Implementation
3.2.1. Knowledge and Belief Enhancement
- (a)
- Nutritional feedback: The DIMA-P provides immediate, visible feedback on the nutritional values of foods as users log their meals. This feedback consists of a glanceable display (Figure 2h), featuring representative icons (e.g., water tap, saltshaker, and heart) for the three critical nutrients that hemodialysis patients need to monitor: fluid, sodium, and potassium [22]. Each icon is linked to a bar chart that fills up as that nutrient is consumed. This helps to educate users about their dietary intake in real time, which enhances their knowledge about how different foods affect their health.
- (b)
- Detailed nutritional displays and feedback pages: By offering detailed information on nutrient intake (Figure 2j) and how it aligns with dietary limits, the app helps reinforce beliefs about the importance of diet management. This ongoing educational component is crucial for modifying users’ dietary habits and beliefs about their health management.
- (c)
- Portions reference: The DIMA-P clarifies how to interpret food portion sizes with the physical sizes of visual aids for specific food groups (Figure 2i). This content was sourced from online educational resources on portion size estimation and verified with a dietitian.
3.2.2. Self-Regulation Skills and Abilities
- (a)
- Portion Estimator Interfaces (PSEI): These interfaces (e.g., Figure 2e,f) allow users to adjust the portion sizes based on visual aids, which helps them to manage their dietary intake more accurately. The ability to adjust portion sizes directly fosters self-regulation skills, as users learn to align their dietary intake with their health goals.
- (b)
- Nutrient displays and portions review: The visual representation of nutrient consumption (Figure 2h,j) (e.g., bar charts that fill up with nutrient consumption) allows users to actively monitor their diet throughout the day. This supports self-monitoring—one of the key self-regulation skills. If a nutrient’s intake exceeds a safe threshold (indicated by the bar turning red), the user can immediately adjust their portions using the portions review feature (Figure 2k), effectively utilizing problem-solving and decision-making skills to manage their diet.
- (c)
- Saved meals: By accessing historical meal data (the checked plate icon in Figure 2a), users can review their dietary patterns over time, fostering reflective learning and better planning for future meals, which enhances their ability to regulate their dietary behavior.
3.2.3. Social Facilitation
- (a)
- Sharing of dietary data: Users can potentially show their dietary logs or nutritional status to healthcare providers or family members, who can then provide encouragement, advice, or even direct intervention. This sharing process can enhance the social support system, which is crucial for sustained behavioral change.
- (b)
- Symbolic social support [31]: features like compliance indicators and real-time feedback (e.g., icons that react to dietary adherence) can serve as forms of symbolic social support, motivating users through positive reinforcement.
3.3. Study Design and Evaluations
3.3.1. Baseline Phase
3.3.2. Self-Monitoring
3.3.3. End of Self-Monitoring Phase
3.4. Participants
3.5. Analysis
- (i)
- Usage frequency, defined as the number of application use days per two weeks of this study;
- (ii)
- Self-monitoring frequency, defined as the average of the daily usage ratings per two weeks of this study. A maximum rating of 2 was assigned to a day with more than one recorded meal, and a minimum rating of 0 was assigned to days with no recorded meals.
- (a)
- Behavioral changes were identified using a qualitative analysis of the COU questionnaire and interview responses.
- (b)
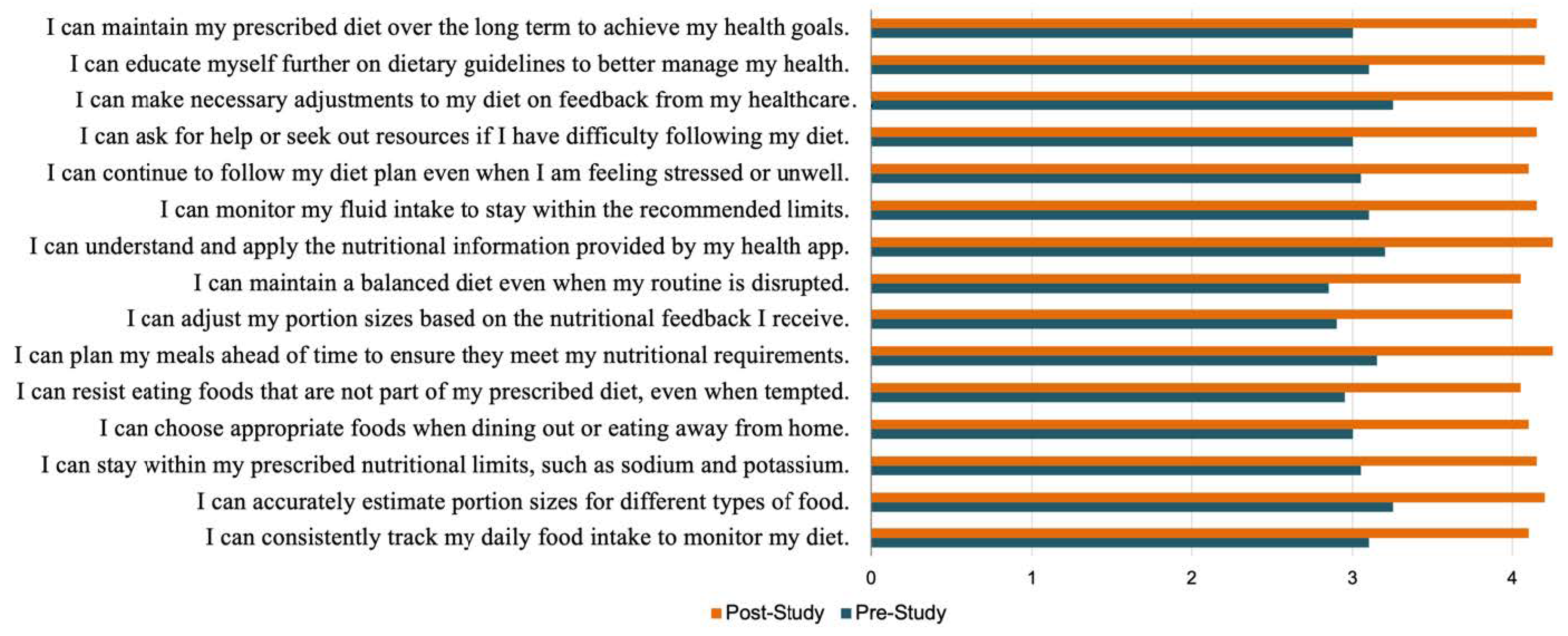
- Self-regulation skills were assessed by calculating the change in the Portion Size Estimation Test scores over the course of this study, obtained by subtracting the pre-study test scores from the post-study test scores.
- (c)
- Self-regulation self-efficacy was evaluated by comparing the pre- and post-study DeSReSES scores.
4. Results
- RQ1: How would participants use the DIMA-P to track and monitor their diet?
- RQ2: How would participants evaluate the usability of the DIMA-P?
- RQ3: What kind of behavioral changes would be instigated through the DIMA-P usage?
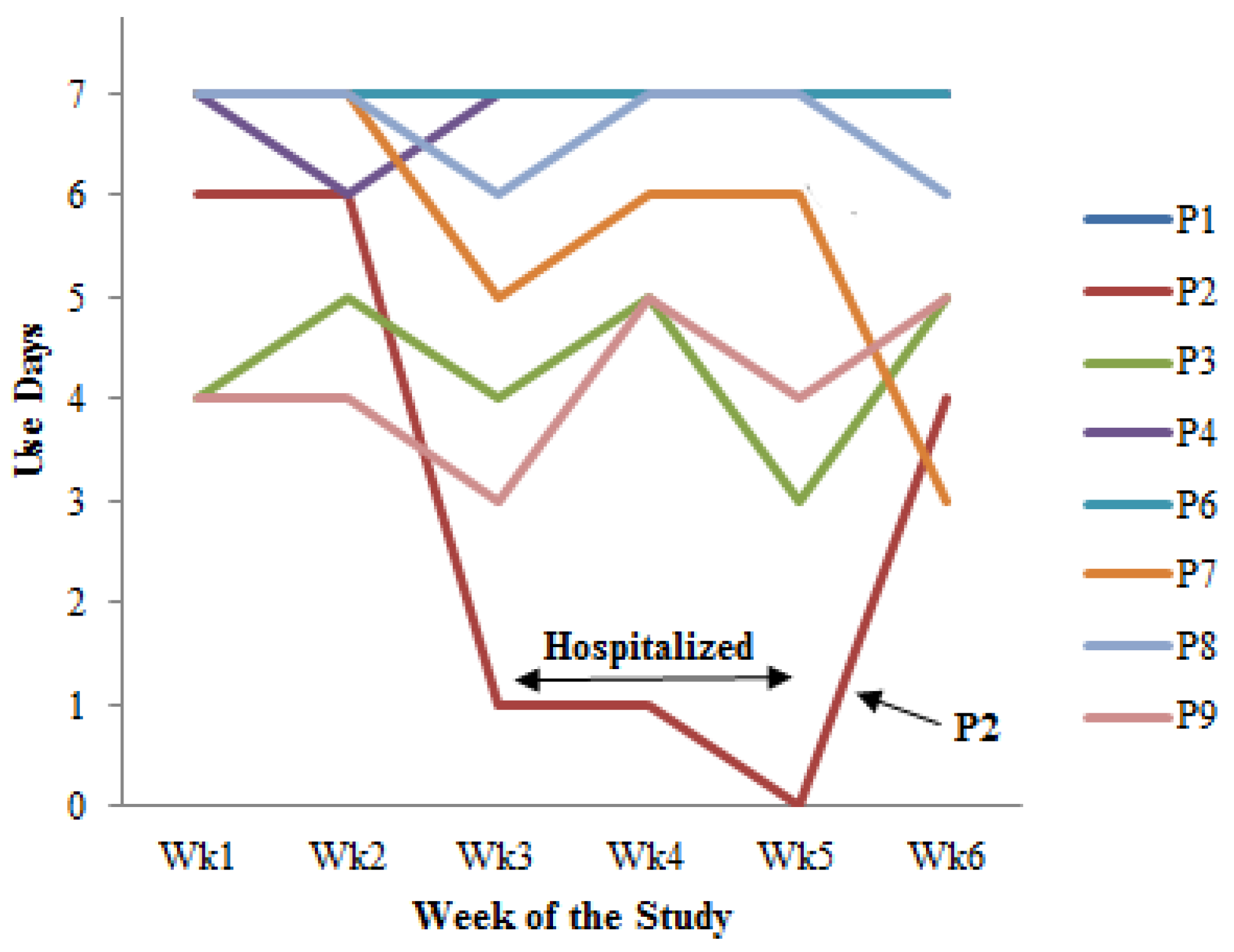
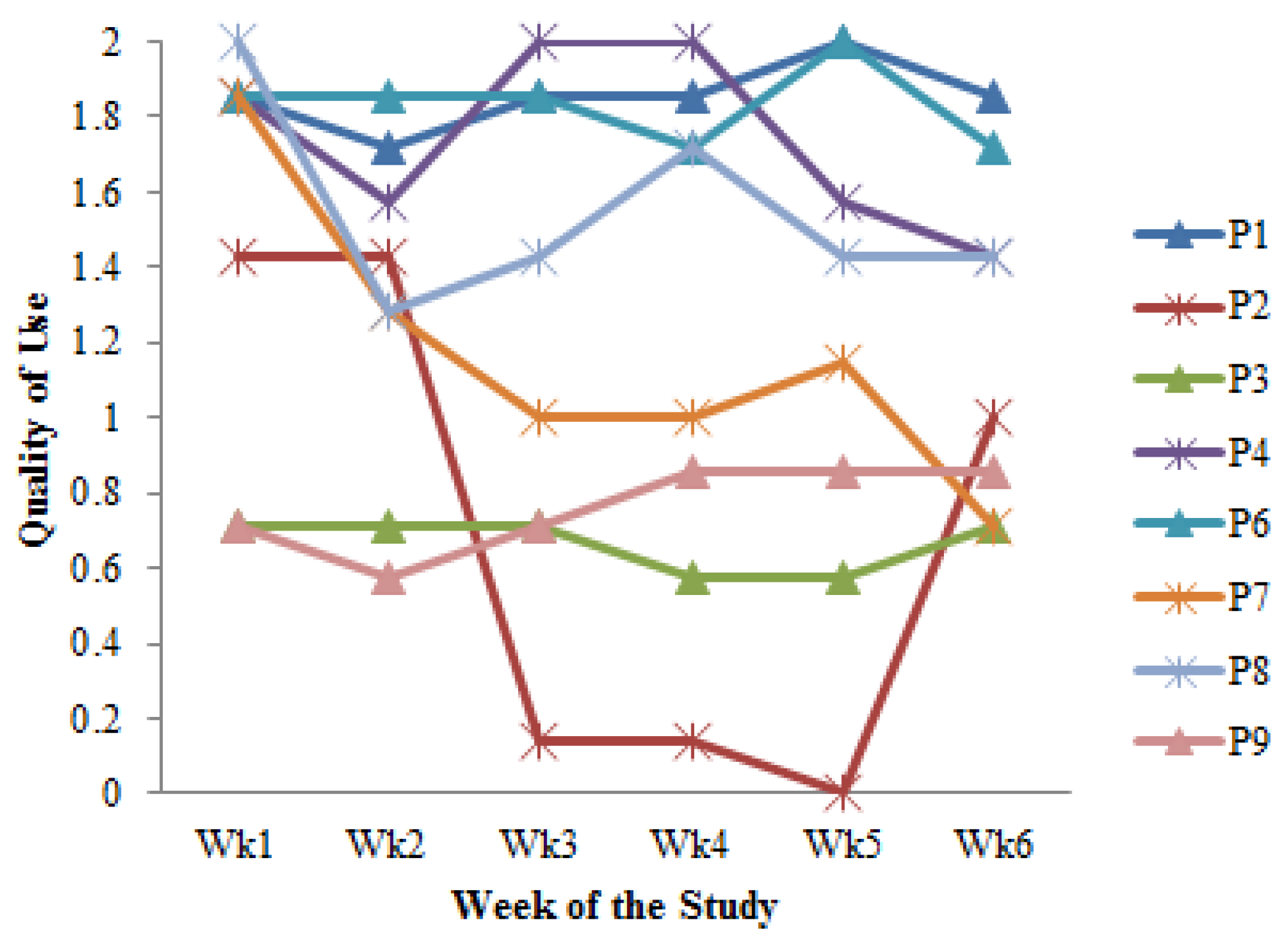
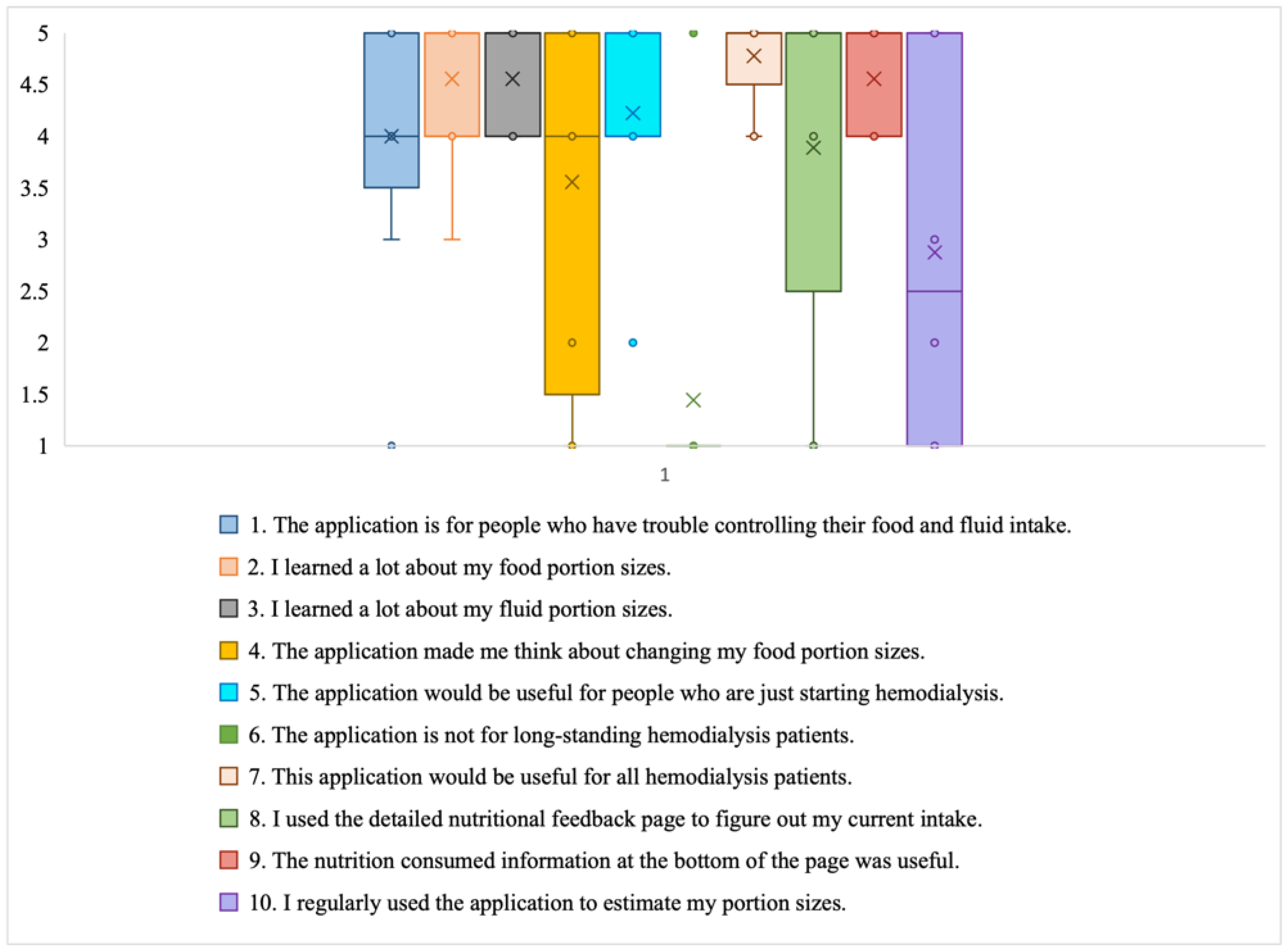
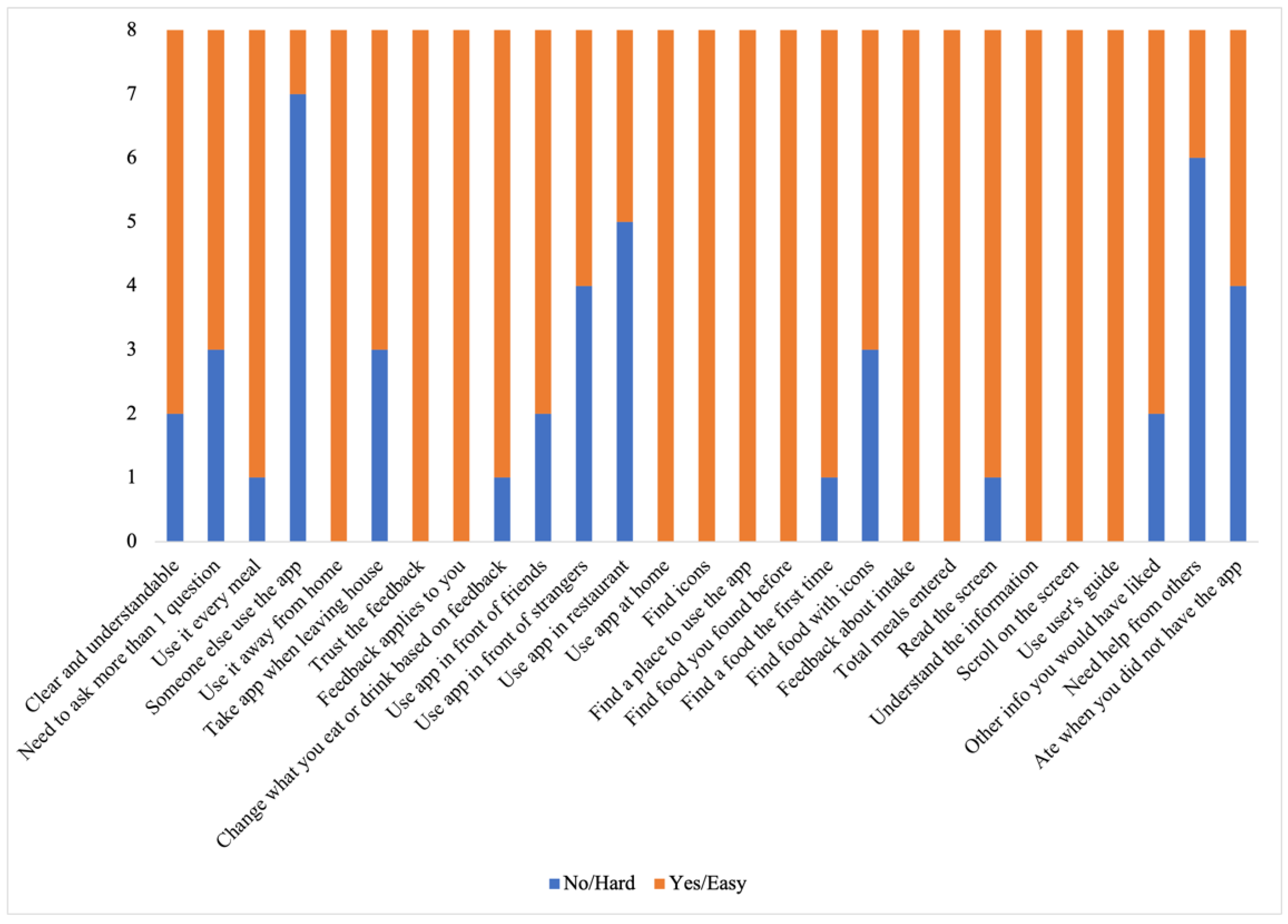
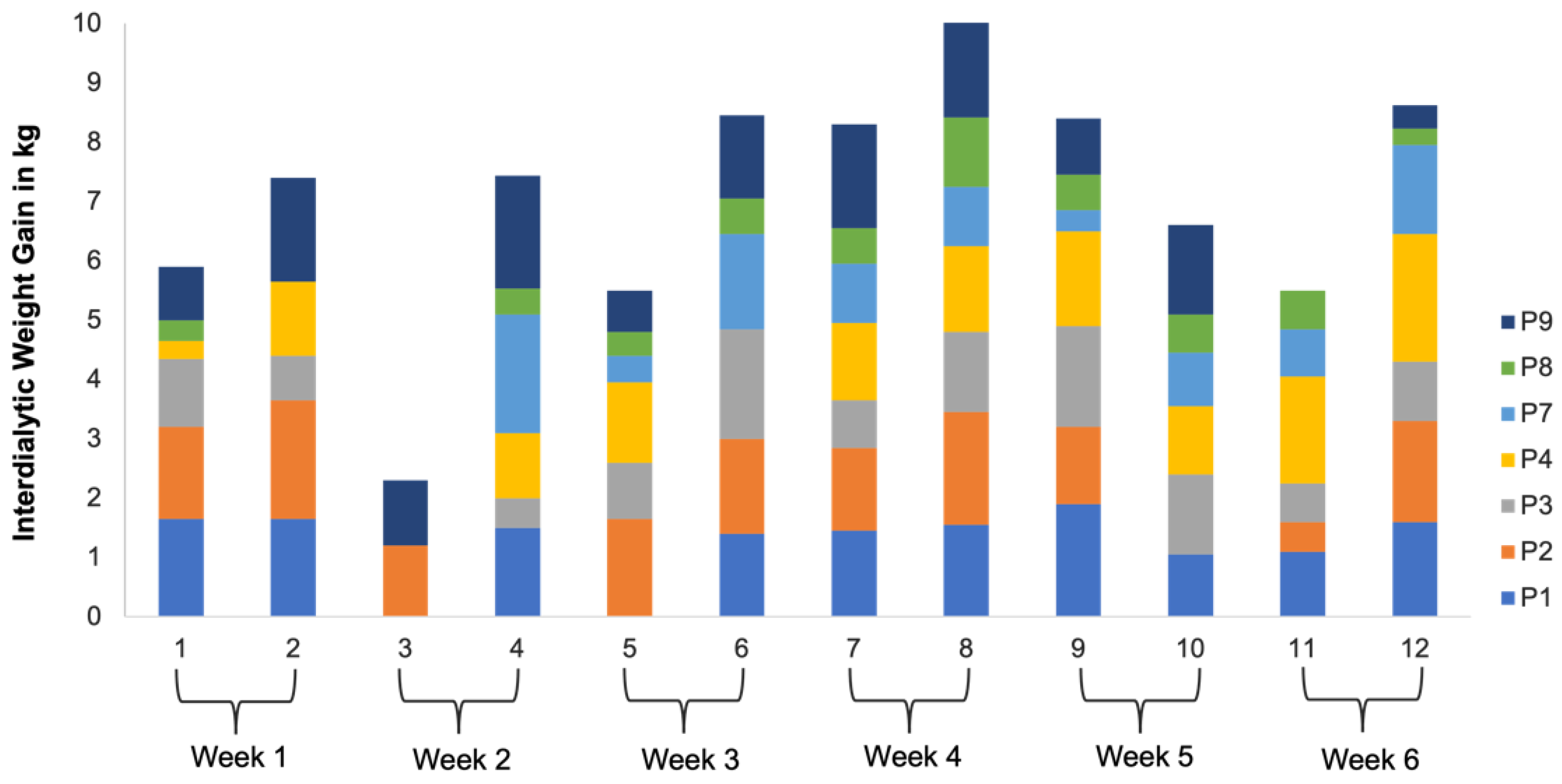
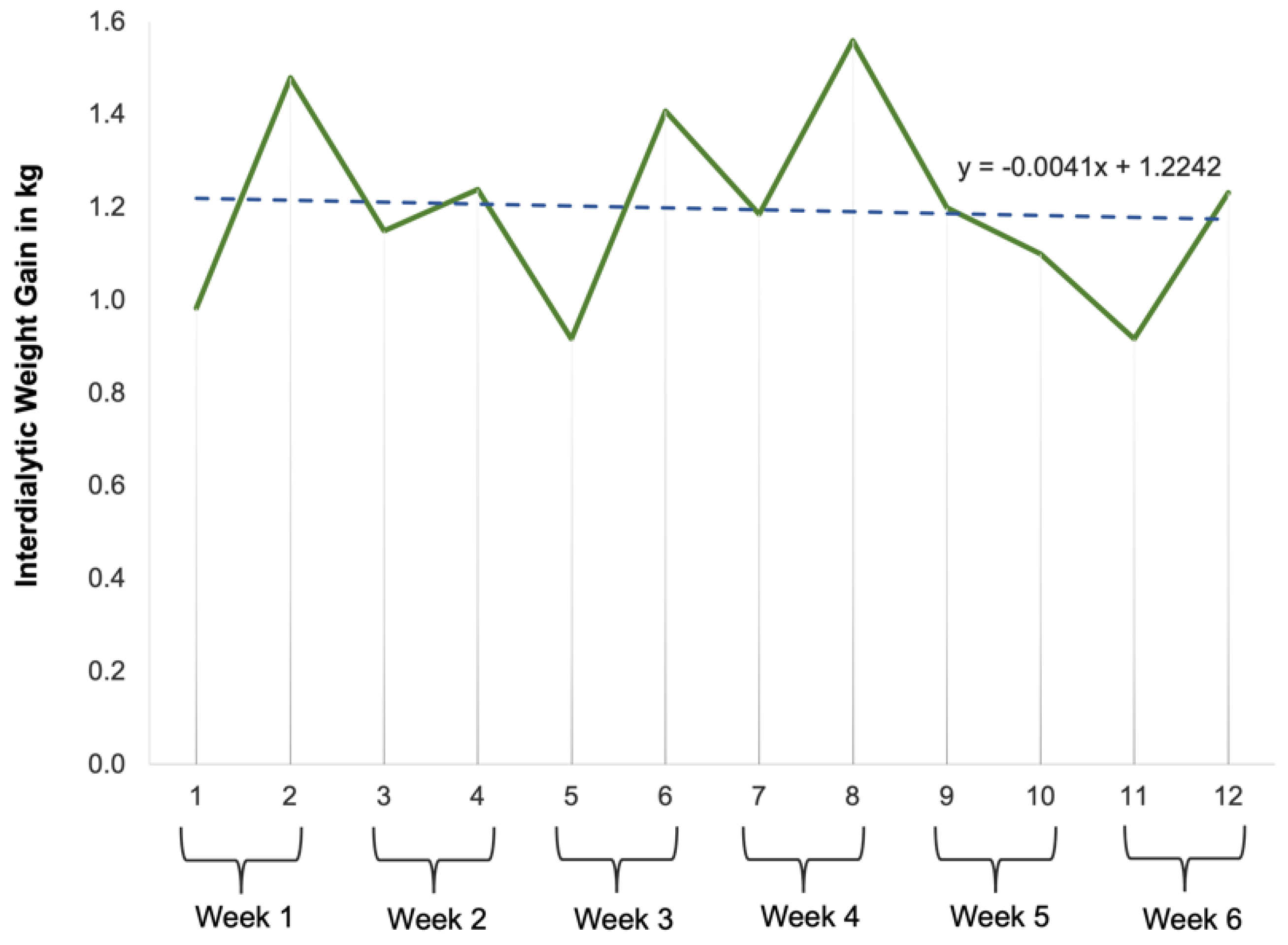
- Cultivating a meal tracking habit: The participants unanimously acknowledged the utility of using the DIMA-P for meal tracking, and, as indicated by the context-of-use (COU) questionnaires, they reported using it consistently with each meal, whether at home or away from home. However, a more detailed examination of their click log data (Figure 3) revealed that not all the participants recorded their meals every single day throughout this study. Additionally, some participants mentioned in the interviews that they occasionally deferred meal recording and inputted their dietary information retrospectively. For instance, one participant explained, “There were days when I added everything at the end of the day” (P3).Furthermore, the participants disclosed variations in their daily eating patterns. Some individuals revealed that they consumed only one meal a day or occasionally skipped meals, especially on dialysis days when they were not feeling well. As one participant noted, “When I am not feeling too good especially on dialysis days, I tend to eat less” (P9). Consequently, the lower frequency of use or quality metrics should not be interpreted as a lack of meal tracking; rather, it reflects the flexible and adaptive nature of how the participants engaged with the application to suit their unique dietary routines and health conditions.
- Increased interest in self-monitoring: The users reported that they were easily able to find all the meals they had entered into the application, which supports the self-monitoring aspect of behavior change. The participants further explained that the app helped them track and review their meals, helping them decide what to eat later: “Documenting helps you make better choices and how much to eat” (P3).
- Using the application as a problem-solving tool: The participants, particularly those who were infrequent users, tended to use the application as a problem-solving tool rather than for tracking every meal. Many of these participants reported that they did not vary their diet much (typically, consuming items they had experimented with before). They preferred to stick with familiar, safe choices. As a result, using the app daily did not seem necessary to them. Instead, they primarily relied on the app to make informed decisions about unfamiliar foods or those they did not consume regularly. They often used the app’s reference feature to determine the correct portion sizes or experimented by trying out different meal options as exemplified by P3, “I’m pretty consistent with my diet, so I didn’t use the app every day. I kept it handy for those times when I needed to confirm if something was okay to eat”.
- Adherence to application feedback: All the participants reported changing what they were eating and drinking based on the feedback from the application, indicating that the DIMA-P was effective in helping the participants adhere to their dietary restrictions: “I slowed down when it [nutrient feedback icon] was red and I tried to watch what I was eating” (P2).
- Improvement in nutritional intake: Several participants highlighted the positive impact of the app on their dietary habits. One participant expressed, “It made me realize I was not eating enough protein, so I started incorporating more protein into my diet” (P7*). This observation suggests that the application not only facilitated portion size estimation but also raised awareness about nutritional aspects, encouraging users to make healthier dietary choices. One participant also reported that the app helped him lose weight. “I lost 3 kg; it helped me see how much I was eating. It will help me even more after that I am done losing more weight” (P8†).
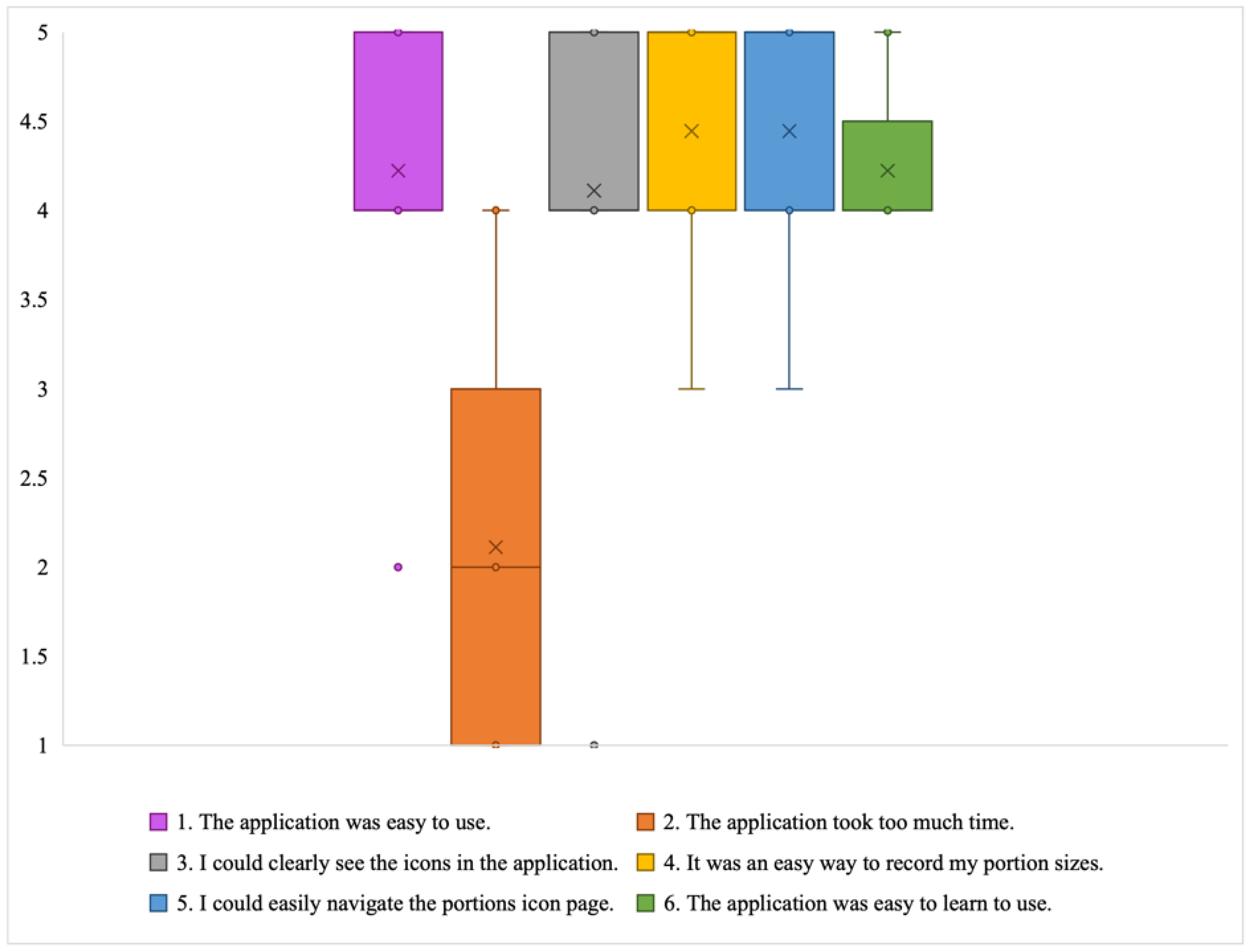
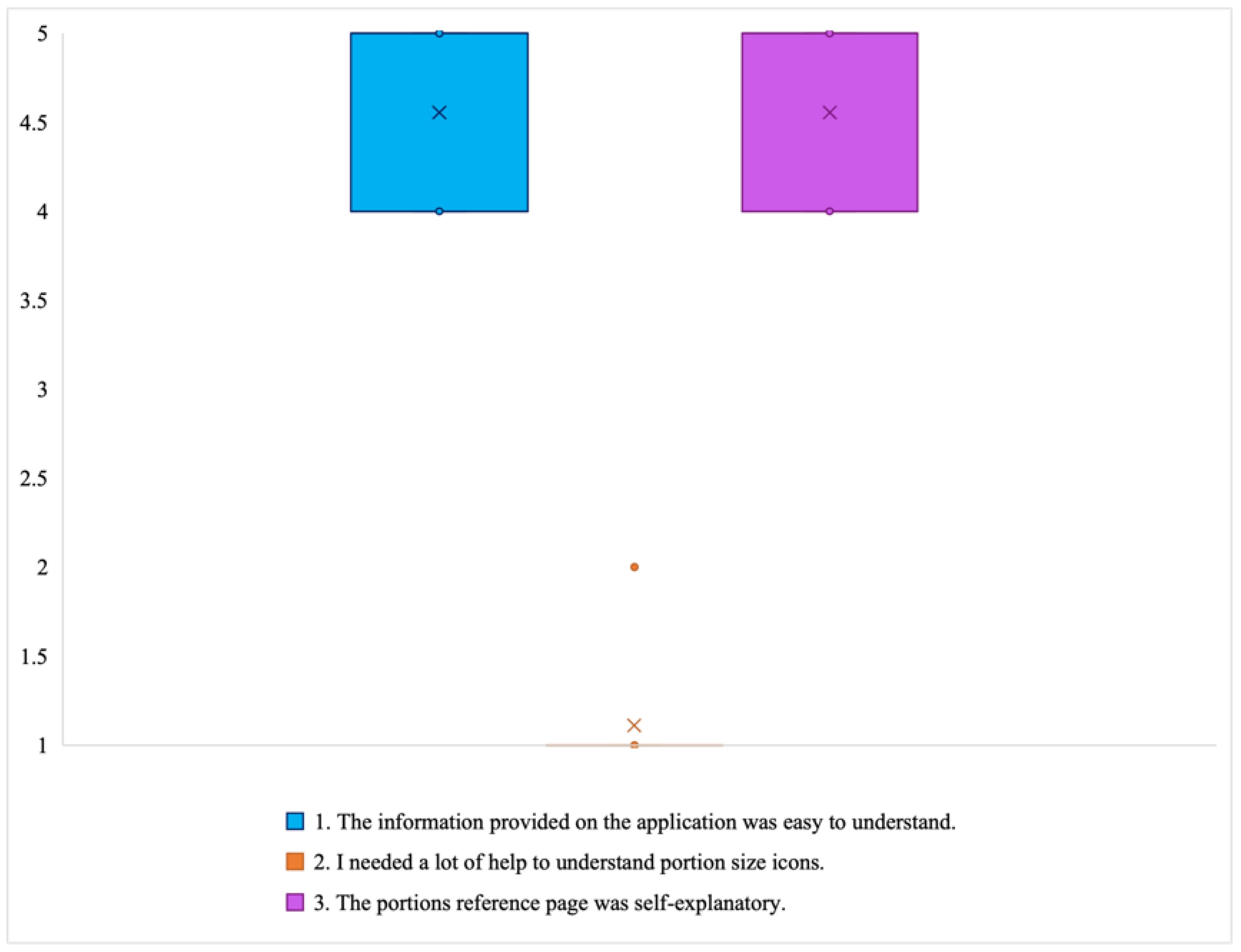
- Learning to trust the application: Some participants stated that initially they had a hard time believing some of the information presented in the application, such as some portion size images being hard to believe. These participants engaged in experimentation, which ultimately led them to believe the application and correct their own misperceptions: “Some of the portion sizes are not believable, but when you look at the actual size of the object, it becomes believable” (P2). In particular, according to the end-of-study COU questionnaires, most of the participants stated that they trusted the nutritional information in the DIMA-P. This is important for users to act on the information and advice provided.
- RQ4: How would the participants’ interdialytic weight gains vary over the course of this study?
5. Discussion
5.1. Principal Findings
5.2. Theoretical Contributions
5.2.1. Knowledge and Beliefs
5.2.2. Self-Regulation Skills and Abilities
5.2.3. Social Facilitation
6. Limitations
7. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozkan, I.; Taylan, S. Diet and fluid restriction experiences of patients on hemodialysis: A meta-synthesis study. Rev. De Nefrol. Diálisis Y Traspl. 2022, 42, 22–40. [Google Scholar]
- Vijay, V.; Kang, H.K. The worldwide prevalence of nonadherence to diet and fluid restrictions among hemodialysis patients: A systematic review and meta-analysis. J. Ren. Nutr. 2022, 32, 658–669. [Google Scholar] [CrossRef]
- Silva, P.; Araújo, R.; Lopes, F.; Ray, S. Nutrition and food literacy: Framing the challenges to health communication. Nutrients 2023, 15, 4708. [Google Scholar] [CrossRef] [PubMed]
- Madilo, F.K.; Owusu-Kwarteng, J.; Kunadu, A.P.-H.; Tano-Debrah, K. Self-reported use and understanding of food label information among tertiary education students in Ghana. Food Control 2020, 108, 106841. [Google Scholar] [CrossRef]
- Ginde, A.A.; Clark, S.; Goldstein, J.N.; Camargo, C.A. Demographic Disparities in Numeracy among Emergency Department Patients: Evidence from Two Multicenter Studies. Patient Educ. Couns. 2008, 72, 350–356. [Google Scholar] [CrossRef]
- Wijayaratne, S.P. The Role of the Dietary Gatekeeper’s Food Literacy in the Home Environment. Doctoral Dissertation, RMIT University, Melbourne, Australia, 2020. [Google Scholar]
- Karimi, N.; Opie, R.; Crawford, D.; O’Connell, S.; Hamblin, P.S.; Steele, C.; Ball, K. Participants’ and Health Care Providers’ Insights Regarding a Web-Based and Mobile-Delivered Healthy Eating Program for Disadvantaged People With Type 2 Diabetes: Descriptive Qualitative Study. JMIR Form. Res. 2023, 7, e37429. [Google Scholar] [CrossRef]
- Raab, R.; Geyer, K.; Zagar, S.; Hauner, H. App-supported lifestyle interventions in pregnancy to manage gestational weight gain and prevent gestational diabetes: Scoping review. J. Med. Internet Res. 2023, 25, e48853. [Google Scholar] [CrossRef]
- O’Leary, T.; Torous, J. Smartphone apps for eating disorders: An overview of the marketplace and research trends. Int. J. Eat. Disord. 2022, 55, 625–632. [Google Scholar] [CrossRef]
- Scarry, A.; Rice, J.; O’connor, E.M.; Tierney, A.C. Usage of mobile applications or mobile health technology to improve diet quality in adults. Nutrients 2022, 14, 2437. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Martinez-Vizcaino, V.; Fernandez-Rodriguez, R.; Saz-Lara, A.; Pascual-Morena, C.; Álvarez-Bueno, C. Effect of behavioral weight management interventions using lifestyle mhealth self-monitoring on weight loss: A systematic review and meta-analysis. Nutrients 2020, 12, 1977. [Google Scholar] [CrossRef]
- Sandborg, J.; Söderström, E.; Henriksson, P.; Bendtsen, M.; Henström, M.; Leppänen, M.H.; Maddison, R.; Migueles, J.H.; Blomberg, M.; Löf, M. Effectiveness of a smartphone app to promote healthy weight gain, diet, and physical activity during pregnancy (healthymoms): Randomized controlled trial. JMIR Mhealth Uhealth 2021, 9, e26091. [Google Scholar] [CrossRef] [PubMed]
- Schrauben, S.J.; Appel, L.; Rivera, E.; Lora, C.M.; Lash, J.P.; Chen, J.; Hamm, L.L.; Fink, J.C.; Go, A.S.; Townsend, R.R.; et al. Mobile health (mhealth) technology: Assessment of availability, acceptability, and use in CKD. Am. J. Kidney Dis. 2021, 77, 941–950.e1. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Peine, A.; Östlund, B. The importance of user involvement: A systematic review of involving older users in technology Design. Gerontologist 2020, 60, e513–e523. [Google Scholar] [CrossRef] [PubMed]
- Harmon, S.M.; Heitkemper, E.M.; Mamykina, L.; Hwang, M.L. Are We Healthier Together? Two Strategies for Supporting Macronutrient Assessment Skills and How the Crowd Can Help (or Not). Proc. ACM Hum.-Comput. Interact. 2022, 6, 1–25. [Google Scholar] [CrossRef]
- Jih, J.; Nguyen, A.; Woo, J.; Tran, W.C.; Wang, A.; Gonzales, N.; Fung, J.; Callejas, J.; Nguyen, T.T.; Ritchie, C.S. A photo-based communication intervention to promote diet-related discussions among older adults with multi-morbidity. J. Am. Geriatr. Soc. 2023, 71, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.F.; Wang, Q.; Schroeder, J.; Cole, A.; Zia, J.; Fogarty, J.; Munson, S.A. Identifying and planning for individualized change: Patient-provider collaboration using lightweight food diaries in healthy eating and irritable bowel syndrome. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2019, 3, 1–27. [Google Scholar] [CrossRef]
- Connelly, K.; Siek, K.A.; Chaudry, B.; Jones, J.; Astroth, K.; Welch, J.L. An offline mobile nutrition monitoring intervention for varying-literacy patients receiving hemodialysis: A pilot study examining usage and usability. J. Am. Med. Inform. Assoc. 2012, 19, 705–712. [Google Scholar] [CrossRef]
- Chaudry, B.M.; Connelly, K.H.; Siek, K.A.; Welch, J.L. Mobile interface design for low literacy populations. In Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium 2012 (IHI 2012), Miami, FL, USA, 28–30 January 2012; pp. 91–100. [Google Scholar]
- Chaudry, B.M.; Connelly, K.; Siek, K.A.; Welch, J.L. Formative evaluation of a mobile liquid portion size estimation interface for people with varying literacy skills. J. Ambient. Intell. Humaniz. Comput. 2013, 4, 779–789. [Google Scholar] [CrossRef]
- Chaudry, B.; Connelly, K.; Siek, K.A.; Welch, J.L. The design of a mobile portion size estimation interface for a low literacy population. In Proceedings of the Pervasive Computing Technologies for Healthcare 2011 (PervasiveHealth 2011), Dublin, Ireland, 23–26 May 2011; pp. 160–167. [Google Scholar]
- Siek, K.A.; Connelly, K.H.; Rogers, Y. Pride and prejudice: Learning how chronically ill people think about food. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems 2006 (CHI 2006), Montréal, QC, Canada, 22–27 April 2006; pp. 947–950. [Google Scholar]
- Siek, K.A.; Connelly, K.H.; Chaudry, B.M.; Lambert, D.; Welch, J.L. Evaluation of two mobile nutrition tracking applications for chronically ill populations with low literacy skills. In Mobile Health Solutions for Biomedical Applications; IGI Global: Hershey, PA, USA, 2009. [Google Scholar]
- Siek, K.A.; Connelly, K.H.; Rogers, Y.; Rowher, P.; Lambert, D.; Welch, J.L. When do we eat? An evaluation of food items input into an electronic food monitoring application. In Proceedings of the Ist International ICST Conference on Pervasive Computing Technologies for Healthcare 2006 (PervasiveHealth 2006), Innsbruck, Austria, 29 November–1 December 2006; pp. 1–10. [Google Scholar]
- Chaudhry, B.M.; Schaefbauer, C.; Jelen, B.; Siek, K.A.; Connelly, K.H. Evaluation of a Food Portion Size Estimation Interface for a Varying Literacy Population. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems 2016 (CHI 2016), San Jose, CA, USA, 7–12 May 2016. [Google Scholar]
- Da Silva, D.C.G.; de Almeida, J.N.M.; dos Santos, T.C.; de Souza Almeida, E.; de Souza, J.V.; de Oliveira Santana, A. Interdialytic weight gain and associated factors in patients undergoing hemodialysis treatment. Demetra Food Nutr. Health/Aliment. Nutr. Saúde 2021, 16, e55333. [Google Scholar]
- Harte, R.; Glynn, L.; Rodríguez-Molinero, A.; Baker, P.M.; Scharf, T.; Quinlan, L.R.; ÓLaighin, G. A human-centered design methodology to enhance the usability, human factors, and user experience of connected health systems: A three-phase methodology. JMIR Hum. Factors 2017, 4, e8. [Google Scholar] [CrossRef]
- Hertzum, M. Usability Testing: A Practitioner’s Guide to Evaluating the User Experience; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Ahmadi, M.; Shahrokhi, S.N.; Khavaninzadeh, M.; Alipour, J. Development of a Mobile-Based Self-care Application for Patients with Breast Cancer-Related Lymphedema in Iran. Appl. Clin. Inform. 2022, 13, 935–948. [Google Scholar] [CrossRef]
- Ryan, P. Integrated Theory of Health Behavior Change. Clin. Nurse Spéc. 2009, 23, 161–170. [Google Scholar] [CrossRef]
- Kahn, R.L.; Antonucci, T.C. Convoys over the life course: Attachment roles and social support. In Life-Span Development and Behavior; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Weiss, B.D.; Mays, M.Z.; Martz, W.; Castro, K.M.; DeWalt, D.A.; Pignone, M.P.; Mockbee, J.; Hale, F.A. Quick assessment of literacy in primary care: The newest vital sign. Ann. Fam. Med. 2005, 3, 514–522. [Google Scholar] [CrossRef]
- Ibrahim, S.Y.; Reid, F.; Shaw, A.; Rowlands, G.; Gomez, G.B.; Chesnokov, M.; Ussher, M. Validation of a health literacy screening tool (REALM) in a UK Population with coronary heart disease. J. Public Health 2008, 30, 449–455. [Google Scholar] [CrossRef]
- Ayala, G.X. An experimental evaluation of a group- versus computer-based intervention to improve food portion size estimation skills. Health Educ. Res. 2005, 21, 133–145. [Google Scholar] [CrossRef]
- Chang, M.-W.; Nitzke, S.; Brown, R.L.; Baumann, L.C.; Oakley, L. Development and Validation of a Self-Efficacy Measure for Fat Intake Behaviors of Low-Income Women. J. Nutr. Educ. Behav. 2003, 35, 302–307. [Google Scholar] [CrossRef]
- Lin, H.X.; Choong, Y.-Y.; Salvendy, G. A proposed index of usability: A method for comparing the relative usability of different software systems. Behav. Inf. Technol. 1997, 16, 267–277. [Google Scholar] [CrossRef]
- Liu, P.; Astudillo, K.; Velez, D.; Kelley, L.; Cobbs-Lomax, D.; Spatz, E.S. Use of mobile health applications in low-income populations a prospective study of facilitators and barriers. Circ. Cardiovasc. Qual. Outcomes 2020, 13, 687–691. [Google Scholar] [CrossRef]
- OpenAI. DALL·E: Creating Images from Text. Online. 2024. Available online: https://openai.com/blog/dall-e (accessed on 13 August 2023).
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Hughes, L.; Ismagilova, E.; Aarts, G.; Coombs, C.; Crick, T.; Duan, Y.; Dwivedi, R.; Edwards, J.; Eirug, A.; et al. Artificial Intelligence (AI): Multidisciplinary perspectives on emerging challenges, opportunities, and agenda for research, practice and policy. Int. J. Inf. Manag. 2019, 57, 101994. [Google Scholar] [CrossRef]
- Schunk, D.; Berger, E.M.; Hermes, H.; Winkel, K.; Fehr, E. Teaching self-regulation. Nat. Hum. Behav. 2022, 6, 1680–1690. [Google Scholar] [CrossRef]
- McDaniel, M.A.; Einstein, G.O. Training learning strategies to promote self-regulation and transfer: The knowledge, belief, commitment, and planning framework. Perspect. Psychol. Sci. 2020, 15, 1363–1381. [Google Scholar] [CrossRef]
- Klasnja, P.; Consolvo, S.; Pratt, W. How to evaluate technologies for health behavior change in HCI research. In Proceedings of the ACM SIGCHI Conference on Human Factors in Computing Systems 2011 (CHI 2011), Vancouver, BC, Canada, 7–12 May 2011. [Google Scholar]


| Total Participants | 8 |
| Duration | 6 weeks of self-monitoring |
| Time | 1 July 2013–30 September 2013 |
| Contact with Researcher | First two weeks = face-to-face Last four weeks = via phone |
| Baseline Assessments | Demographics, MMSE, REALM, NVS, PSESES, PSETs, DeSReSES, IDWG, and nutrition portion size estimation self-efficacy |
| Self-Monitoring Data | Meal photographs, IDWG, nutrient intake data (DIMA-P), food items and portion sizes (DIMA-P), DIMA-P click logs, and field notes |
| End of Self-Monitoring Assessments | PSESES, PSETs, DeSReSES, custom usability questionnaire, context-of-use (COU) questionnaire, IDWG, and exit interview |
| Participant | Education Years | NVS (Literacy Level) | REALM (Literacy Level) | Tech Familiarity |
|---|---|---|---|---|
| P1 | 14 | 3 (possibly low) | 65 (High school) | Medium |
| P2 | 13 | 4 (adequate) | 59 (7th–8th grade) | Low |
| P3 | 14 | 2 (possibly low) | 66 (High school) | Low |
| P4 * | 12 | 3 (possibly low) | 56 (7th–8th grade) | Low |
| P6 | 12 | 5 (adequate) | 47 (7th–8th grade) | Medium |
| P7 * | 13.5 | 1 (likely low) | 45 (7th–8th grade) | Medium |
| P8 † | 12 | 5 (adequate) | 65 (High school) | High |
| P9 | 11 | 5 (adequate) | 60 (7th–8th grade) | Medium |
| Participant | Usage Group | Total Use Days (Study Days = 42) | Mean Quality Metric (SD) | Total Clicks (Over 6 Weeks) | ||
|---|---|---|---|---|---|---|
| Portions Reference | Detailed Feedback | Portions Review (Delete) | ||||
| P1 | # | 42 | 1.82 (0.10) | 1 | 4 | 2 (0) |
| P2 | Ø | 18 | 0.70 (0.64) | 12 | 3 | 4 (0) |
| P3 | Ø | 26 | 0.67 (0.05) | 0 | 4 | 1 (0) |
| P4 * | # | 41 | 0.65 (0.05) | 31 | 3 | 32 (8) |
| P6 | # | 42 | 1.77 (0.15) | 68 | 13 | 32 (15) |
| P7 * | # | 34 | 1.15 (0.37) | 3 | 1 | 4 (0) |
| P8 † | # | 38 | 1.52 (0.29) | 2 | 1 | 5 (0) |
| P9 | Ø | 25 | 0.78 (0.13) | 13 | 2 | 27 (11) |
| Participant | Pictures (Out of 6) | Measures (Out of 10) | Containers (Out of 6) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| P1 | 3 | 6 | 5 | 6 | 4 | 6 |
| P2 | 2 | 4 | 6 | 9 | 2 | 2 |
| P3 | 2 | 4 | 6 | 9 | 2 | 2 |
| P4 * | 3 | 6 | 6 | 4 | 3 | 6 |
| P6 | 6 | 6 | 5 | 6 | 5 | 5 |
| P7 * | 4 | 5 | 4 | 4 | 4 | 4 |
| P8 † | 2 | 6 | 6 | 5 | 2 | 4 |
| P9 | 2 | 5 | 6 | 5 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhry, B.M.; Siek, K.A.; Connelly, K. The Usability and Feasibility of a Dietary Intake Self-Monitoring Application in a Population with Varying Literacy Levels. J. Pers. Med. 2024, 14, 1001. https://doi.org/10.3390/jpm14091001
Chaudhry BM, Siek KA, Connelly K. The Usability and Feasibility of a Dietary Intake Self-Monitoring Application in a Population with Varying Literacy Levels. Journal of Personalized Medicine. 2024; 14(9):1001. https://doi.org/10.3390/jpm14091001
Chicago/Turabian StyleChaudhry, Beenish Moalla, Katie A. Siek, and Kay Connelly. 2024. "The Usability and Feasibility of a Dietary Intake Self-Monitoring Application in a Population with Varying Literacy Levels" Journal of Personalized Medicine 14, no. 9: 1001. https://doi.org/10.3390/jpm14091001
APA StyleChaudhry, B. M., Siek, K. A., & Connelly, K. (2024). The Usability and Feasibility of a Dietary Intake Self-Monitoring Application in a Population with Varying Literacy Levels. Journal of Personalized Medicine, 14(9), 1001. https://doi.org/10.3390/jpm14091001







