Applications of Artificial Intelligence-Based Systems in the Management of Esophageal Varices
Abstract
:1. Introduction
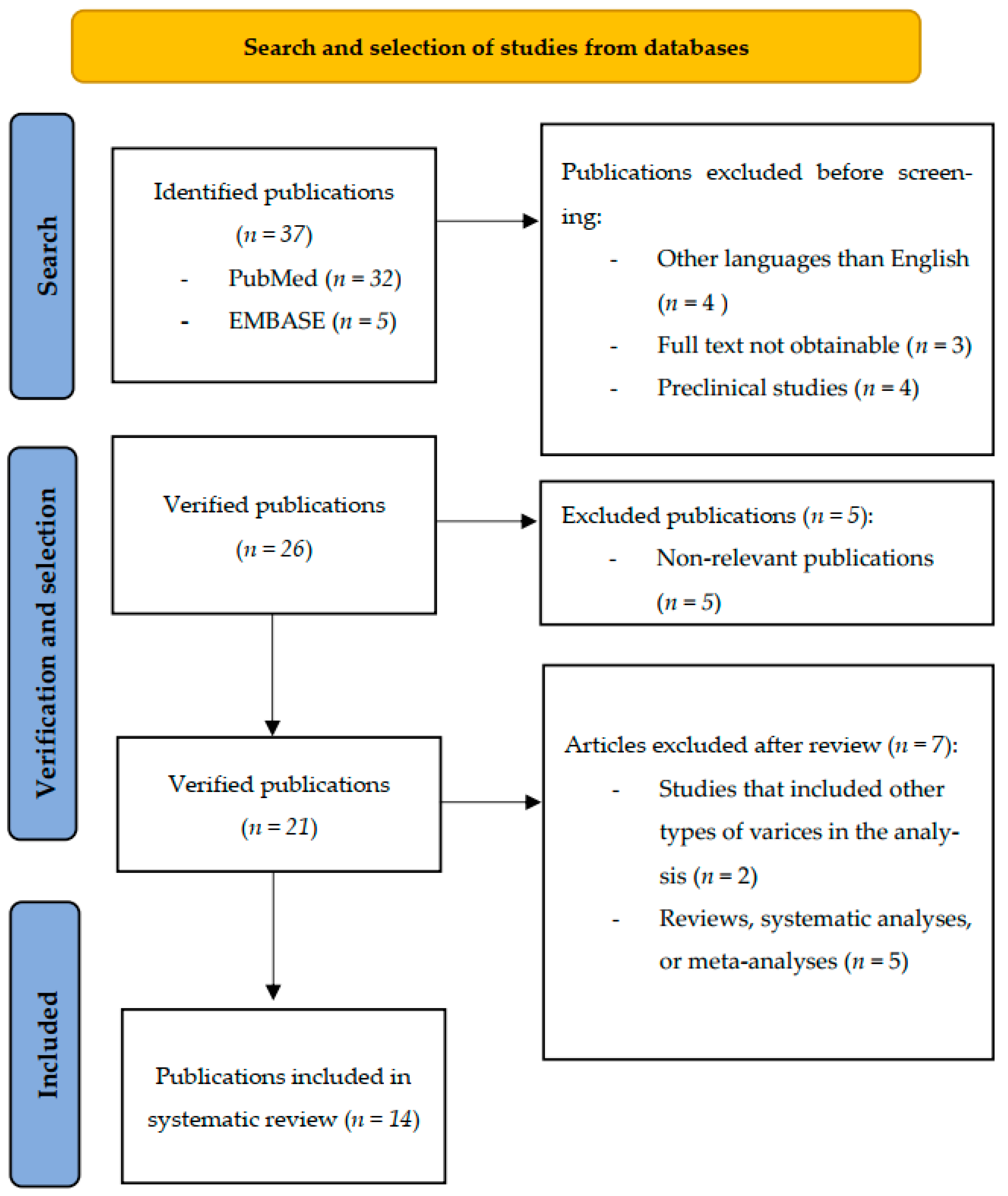
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
3. Results
3.1. AI, Laboratory Parameters, and Clinical Scores
3.2. AI and Endoscopy Images
3.3. AI and CT Scans
3.4. Quality Assessment of the Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamada, T.; Alpers, D.H.; Kalloo, A.N.; Kaplowitz, N.; Owyang, C.; Powell, D.W. Textbook of Gastroenterology, 5th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lesmana, C.R.A.; Raharjo, M.; Gani, R.A. Managing liver cirrhotic complications: Overview of esophageal and gastric varices. Clin. Mol. Hepatol. 2020, 26, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W.; Shuhart, M.C.; Davis, G.L.; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; The Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Duboc, M.C.; Garcia-Pagan, J.C.; Fuccio, L.; Karstensen, J.G.; Hucl, T.; Jovanovic, I.; Awadie, H.; Hernandez-Gea, V.; Tantau, M.; et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 1094–1120. [Google Scholar] [CrossRef] [PubMed]
- Frenette, C.T.; Kuldau, J.G.; Hillebrand, D.J.; Lane, J.; Pockros, P.J. Comparison of esophageal capsule endoscopy and esophagogastroduodenoscopy for diagnosis of esophageal varices. World J. Gastroenterol. 2008, 14, 4480–4485. [Google Scholar] [CrossRef]
- Lipp, M.J.; Broder, A.; Hudesman, D.; Suwandhi, P.; Okon, S.A.; Horowitz, M.; Clain, D.J.; Friedmann, P.; Min, A.D. Detection of esophageal varices using CT and MRI. Dig. Dis. Sci. 2011, 56, 2696–2700. [Google Scholar] [CrossRef]
- Borhani, A.; Luu, H.; Mohseni, A.; Xu, Z.; Shaghaghi, M.; Tolosa, C.; Attari, M.M.A.; Madani, S.P.; Shahbazian, H.; Khoshpouri, P.; et al. Screening for exclusion of high-risk bleeding features of esophageal varices in cirrhosis through CT and MRI. Clin. Imaging 2024, 110, 110168. [Google Scholar] [CrossRef]
- Mifune, H.; Akaki, S.; Ida, K.; Sei, T.; Kanazawa, S.; Okada, H. Evaluation of esophageal varices by multidetector-row CT: Correlation with endoscopic “red color sign”. Acta Med. Okayama 2007, 61, 247–254. [Google Scholar] [CrossRef]
- Meng, D.; Wei, Y.; Feng, X.; Kang, B.; Wang, X.; Qi, J.; Zhao, X.; Zhu, Q. CT-Based Radiomics Score Can Accurately Predict Esophageal Variceal Rebleeding in Cirrhotic Patients. Front. Med. 2021, 8, 745931. [Google Scholar] [CrossRef]
- Paternostro, R.; Reiberger, T.; Bucsics, T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J. Gastroenterol. 2019, 25, 308–329. [Google Scholar] [CrossRef]
- Pateu, E.; Oberti, F.; Calès, P. The noninvasive diagnosis of esophageal varices and its application in clinical practice. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 6–16. [Google Scholar] [CrossRef]
- Bai, W.; Abraldes, J.G. Noninvasive assessment oesophageal varices: Impact of the Baveno VI criteria. Curr. Opin. Gastroenterol. 2022, 38, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Procopet, B.; Cristea, V.M.; Robic, M.A.; Grigorescu, M.; Agachi, P.S.; Metivier, S.; Peron, J.M.; Selves, J.; Stefanescu, H.; Berzigotti, A.; et al. Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig. Liver Dis. 2015, 47, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Mattos, Â.Z.; Schacher, F.C.; John Neto, G.; Mattos, A.A. Screening for esophageal varices in cirrhotic patients—Non-invasive methods. Ann. Hepatol. 2019, 18, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Kröner, P.T.; Engels, M.M.; Glicksberg, B.S.; Johnson, K.W.; Mzaik, O.; van Hooft, J.E.; Krittanawong, C. Artificial intelligence in gastroenterology: A state-of-the-art review. World J. Gastroenterol. 2021, 27, 6794–6824. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Bayani, A.; Hosseini, A.; Asadi, F.; Hatami, B.; Kavousi, K.; Aria, M.; Zali, M.R. Identifying predictors of varices grading in patients with cirrhosis using ensemble learning. Clin. Chem. Lab. Med. 2022, 60, 1938–1945. [Google Scholar] [CrossRef]
- Bayani, A.; Asadi, F.; Hosseini, A.; Hatami, B.; Kavousi, K.; Aria, M.; Zali, M.R. Performance of machine learning techniques on prediction of esophageal varices grades among patients with cirrhosis. Clin. Chem. Lab. Med. 2022, 60, 1955–1962. [Google Scholar] [CrossRef]
- Dong, T.S.; Kalani, A.; Aby, E.S.; Le, L.; Luu, K.; Hauer, M.; Kamath, R.; Lindor, K.D.; Tabibian, J.H. Machine Learning-based Development and Validation of a Scoring System for Screening High-Risk Esophageal Varices. Clin. Gastroenterol. Hepatol. 2019, 17, 1894–1901.e1. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, H.; Zhang, Q.; Yang, Y.; Liu, X.; Wang, X.; Jiang, Y. Machine learning-based model for predicting the esophagogastric variceal bleeding risk in liver cirrhosis patients. Diagn. Pathol. 2023, 18, 29. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Zheng, T.; Ji, D.; Wong, Y.J.; You, H.; Gu, Y.; Li, M.; Zhao, L.; Li, S.; et al. Development and validation of a machine learning-based model for varices screening in compensated cirrhosis (CHESS2001): An international multicenter study. Gastrointest. Endosc. 2023, 97, 435–444.e2. [Google Scholar] [CrossRef]
- Simsek, C.; Sahin, H.; Tekin, I.E.; Sahin, T.K.; Balaban, H.Y.; Sivri, B. Artificial intelligence to predict overall survivals of patients with cirrhosis and outcomes of variceal bleeding. Hepatol. Forum 2021, 2, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sharma, S.; Kumar, M.; Venishetty, S.; Bhardwaj, A.; Kaushal, K.; Gopi, S.; Mohta, S.; Gunjan, D.; Saraya, A.; et al. Development of a machine learning model to predict bleed in esophageal varices in compensated advanced chronic liver disease: A proof of concept. J. Gastroenterol. Hepatol. 2021, 36, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, J.; Xiao, Y.; Wu, L.; Hu, S.; Chen, S.; Yi, G.; Hu, W.; Xie, X.; Zhu, Y.; et al. Automated and real-time validation of gastroesophageal varices under esophagogastroduodenoscopy using a deep convolutional neural network: A multicenter retrospective study (with video). Gastrointest. Endosc. 2021, 93, 422–432.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, Y.; Zhou, X.; Gao, X.; Yu, C.; Lin, J.; Liu, L.; Gao, J.; Yin, M.; Xu, G.; et al. Automated Multimodal Machine Learning for Esophageal Variceal Bleeding Prediction Based on Endoscopy and Structured Data. J. Digit. Imaging 2023, 36, 326–338. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, Q.; Mo, F.; Yin, M.; Xu, C.; Zhu, S.; Lin, J.; Xu, G.; Gao, J.; Liu, L.; et al. Deep learning to predict esophageal variceal bleeding based on endoscopic images. J. Int. Med. Res. 2023, 51, 03000605231200371. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, S.S.; Choi, W.M.; Kim, K.M.; Sung, Y.S.; Lee, S.; Suk, H.I. An index based on deep learning-measured spleen volume on CT for the assessment of high-risk varix in B-viral compensated cirrhosis. Eur. Radiol. 2021, 31, 3355–3365. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Fan, C.; Zhang, Y.; Zhang, S.; Wang, Z.; Huang, T.; Ding, Z.; Hu, K.; Li, L.; et al. A novel machine learning-based radiomic model for diagnosing high bleeding risk esophageal varices in cirrhotic patients. Hepatol. Int. 2022, 16, 423–432. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, Q.; Li, X.; Xia, C.; Zhou, J.; Xia, T.; Zhao, B.; Qiu, Y.; Zha, J.-H.; Wang, Y.; et al. An imaging-based machine learning model outperforms clinical risk scores for prognosis of cirrhotic variceal bleeding. Eur. Radiol. 2023, 33, 8965–8973. [Google Scholar] [CrossRef]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Koh, F.H.; Ladlad, J.; Teo, E.-K.; Lin, C.-L.; Foo, F.-J. Real-time artificial intelligence (AI)-aided endoscopy improves adenoma detection rates even in experienced endoscopists: A cohort study in Singapore. Surg. Endosc. 2023, 37, 165–171. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Liu, M.; Lai, Y.; Liu, P.; Wang, Z.; Xing, T.; Huang, Y.; Li, Y.; Li, A.; et al. Artificial Intelligence-Assisted Colonoscopy for Detection of Colon Polyps: A Prospective, Randomized Cohort Study. J. Gastrointest. Surg. 2021, 25, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Meinikheim, M.; Mendel, R.; Palm, C.; Probst, A.; Muzalyova, A.; Scheppach, M.W.; Ebigbo, A. Effect of AI on performance of endoscopists to detect Barrett neoplasia: A Randomized Tandem Trial. Endoscopy 2023, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Ainechi, D.; Misawa, M.; Barua, I.; Larsen, S.L.V.; Paulsen, V.; Garborg, K.K.; Aabakken, L.; Tønnesen, C.J.; Løberg, M.; Kalager, M.; et al. Impact of artificial intelligence on colorectal polyp detection for early-career endoscopists: An international comparative study. Scand. J. Gastroenterol. 2022, 57, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Bhutani, M.S.; Sun, S. Artificial intelligence: The new wave of innovation in EUS. Endosc. Ultrasound 2021, 10, 79–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agudo Castillo, B.; Mascarenhas, M.; Martins, M.; Mendes, F.; de la Iglesia, D.; Costa, A.M.M.P.D.; Esteban Fernández-Zarza, C.; González-Haba Ruiz, M. Advancements in biliopancreatic endoscopy: A comprehensive review of artificial intelligence in EUS and ERCP. Rev. Esp. Enferm. Dig. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fan, X.; Liu, W. Applications and Prospects of Artificial Intelligence-Assisted Endoscopic Ultrasound in Digestive System Diseases. Diagnostics 2023, 13, 2815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.Y.; Song, W.; Mao, T.; Zhang, Q.; Zhang, C.; Li, X.Y. Application of artificial intelligence in the diagnosis of subepithelial lesions using endoscopic ultrasonography: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 915481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasoppokakorn, T.; Tiyarattanachai, T.; Chaiteerakij, R.; Decharatanachart, P.; Mekaroonkamol, P.; Ridtitid, W.; Kongkam, P.; Rerknimitr, R. Application of artificial intelligence for diagnosis of pancreatic ductal adenocarcinoma by EUS: A systematic review and meta-analysis. Endosc. Ultrasound 2022, 11, 17–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akhai, S. From black boxes to transparent machines: The quest for explainable AI. SSRN 2023. [Google Scholar] [CrossRef]
- Tomsett, R.; Preece, A.; Braines, D.; Cerutti, F.; Chakraborty, S.; Srivastava, M.; Pearson, G.; Kaplan, L. Rapid trust calibration through interpretable and uncertainty-aware AI. Patterns 2020, 1, 100049. [Google Scholar] [CrossRef]
- Yonazu, S.; Ozawa, T.; Nakanishi, T.; Ochiai, K.; Shibata, J.; Osawa, H.; Hirasawa, T.; Kato, Y.; Tajiri, H.; Tada, T. Cost-effectiveness analysis of the artificial intelligence diagnosis support system for early gastric cancers. DEN Open 2024, 4, e289. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Repici, A.; Mori, Y. Cost of artificial intelligence: Elephant in the room and its cage. Dig. Endosc. 2023, 35, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.-E.; Wan, F.-T.; Ladlad, J.; Chue, K.-M.; Centre, S.E.; Teo, E.-K.; Lin, C.-L.; Foo, F.-J.; Koh, F.H. One-year review of real-time artificial intelligence (AI)-aided endoscopy performance. Surg. Endosc. 2023, 37, 6402–6407. [Google Scholar] [CrossRef] [PubMed]
- Tokat, M.; Van Tilburg, L.; Koch, A.D.; Spaander, M.C.W. Artificial Intelligence in Upper Gastrointestinal Endoscopy. Dig. Dis. 2022, 40, 395–408. [Google Scholar] [CrossRef]
| Author (Year) | Type of Algorithm | Number of Patients | Main Findings |
|---|---|---|---|
| Bayani et al. (2022) [17] | EL | 490 patients 236 with EVs 5-fold cross-validation | CatBoost: Precision: 1 XGB classifier: Precision: 0.84 Accuracy: 0.92 |
| Bayani et al. (2022) [18] | ML: RF ANN SVM LR | 490 patients 236 with EVs 5-fold cross-validation | RF: Best results and best AUC Recall: 1 for grade 0, 2, and 3 EVs Precision: 1 for grade 1, 2, and 3 EVs F1-score: 1 for grade 2 and 3 EVs ANN: Precision: 1 for grade 0 and 3 EVs SVM: Recall: 1 for grade 1 and 3 EVs Precision: 1 for grade 0 and 2 EVs |
| Dong et al. (2019) [19] | RF | 347 patients 238 in the training cohort 109 in the validation cohort | For the score determined (≤3.90): In the training cohort: AUC: 0.84 (0.79–0.89) for EVs AUC: 0.74 (0.67–0.82) for VNT In the validation cohort: AUC: 0.82 (0.74–0.91) for EVs AUC: 0.75 (0.66–0.84) for VNT |
| Hou et al. (2023) [20] | ANN | 1100 patients 999 in the training cohort 101 in the validation cohort | AUC: 0.959 Outperformed: NIEC index (AUC: 0.669) Rev-NIEC index (AUC: 0.725) |
| Huang et al. (2023) [21] | ML | 2794 patients 1283 patients in the real-world cohort 1154 in the training cohort 129 in the internal validation cohort 1511 patients in the external validation cohort 966 test cohort 1 545 test cohort 2 | In the training cohort: 52.6% spared EGDs 3.6% missed HRVs In the validation cohort: 58.1% spared EGDs 1.4% missed HRVs In the test cohorts: 52.4% spared EGDs 2.8% missed HRVs 41.1% spared EGDs 3.1% missed HRVs Better performance than Baveno IV criteria in all cohorts. |
| Procopet et al. (2015) [13] | ANN | 202 patients 69 with EVs 158 in the training cohort 44 in the validation cohort | High diagnostic accuracy (>0.8). Not statistically significant compared to only liver stiffness. Liver stiffness was the best non-invasive test. Fibrosis-4 and Lok scores were the most accurate out of the serum tests/scores. |
| Simsek et al. (2021) [22] | ML | 124 patients 80 with EVs | For the entire population: AUC: 0.87 at 1 month AUC: 0.85 at 3 months AUC: 0.76 at 12 months For bleeding patients: AUC: 0.91 at 1 month AUC: 0.88 at 3 months AUC: 0.91 at 12 months Better performance than: CTP score (AUCs: 0.75, 0.77, 0.69) MELD-Na score (AUCs: 0.74, 0.73, 0.68) |
| Author (Year) | Type of Algorithm | Number of Patients | Main Findings |
|---|---|---|---|
| Agarwal et al. (2021) [23] | ML | 828 patients 497 in the training cohort 149 patients in the internal validation cohort 182 patients in the external validation cohort | On the training cohort: Accuracy: 0.987 On the internal validation cohort: Accuracy: 0.937 On the external validation cohort: Accuracy: 0.857 Better performance than endoscopic classification alone (Accuracy: 0.589) |
| Chen et al. (2020) [24] | DCNNs | 3021 patients 8566 endoscopic gastroesophageal varices images 3168 patients 6152 normal esophagus/stomach images | On the test dataset for EVs: Accuracy: 0.995 Sensitivity: 0.995 Specificity: 0.994 Compared to endoscopists: Statistically significant difference in: EV detection (0.97 vs. 0.939) RC (0.842 vs. 0.735) Mucosal findings (0.615 vs. 0.461) Red spots (0.853 vs. 0.775) Average time/image (0.13 s vs. 18.75 s) Treatment follow-up (EVs) Total treatment suggestion (EVs) No statistically significant difference in: Size (EVs) Form (EVs) Bleeding signs (EVs) Prophylactic therapy (EVs) EVs that do not require treatment |
| Wang et al. (2022) [25] | MMML | Pretraining dataset: 4000 normal/esophagitis cardia endoscopic images Initially: 810 images of EVs from 341 patients After image augmentation: Training dataset: 2000 images 1000 control 1000 bleeding Validation dataset: 400 images 200 control 200 bleeding | EfficientNet was the highest-performing DL model with: Accuracy: 0.868 Recall: 0.845 Specificity: 0.885 F1-score: 0.864 Stacking model was the highest-performing MMML model in the test dataset with: AUC: 0.975 Accuracy: 0.932 Sensitivity: 0.952 Specificity: 0.924 Recall: 0.952 Precision: 0.816 F1-score: 0.879 It performed better than all clinical indexes: AUC: 0.686 (CTP) AUC: 0.680 (MELD) AUC: 0.739 (APRI) AUC: 0.703 (FIB-4) |
| Hong et al. (2023) [26] | DL | Initially: 675 images of EVs After image augmentation: Training dataset: 2000 images 1000 control 1000 bleeding Validation dataset: 400 images 200 control 200 bleeding | EfficientNet was the highest-performing DL model and performed better than the 2 endoscopists with: On the training dataset: Accuracy: 0.992 Recall: 0.99 Precision: 0.993 F1-score: 0.991 On the validation dataset: Accuracy: 0.91 Recall: 0.9 Precision: 0.918 F1-score: 0.909 On the test dataset: Accuracy: 0.893 Recall: 0.87 Precision: 0.911 F1-score: 0.89 Highest performance achieved by combining the 2 endoscopists and EfficientNet with: Accuracy: 0.938 and 0.908 Recall: 0.92 and 0.875 Precision: 0.953 and 0.936 F1-score: 0.936 and 0.904 |
| Author (Year) | Type of Algorithm | Number of Patients | Main Findings |
|---|---|---|---|
| Lee et al. (2020) [27] | DL | 419 patients | Determined cutoff value for spleen volume/platelet ratio: >3.78 On the derivation cohort: Sensitivity: 0.8 Specificity: 0.744 On the validation cohort: Sensitivity: 0.694 Specificity: 0.785 Cutoff value that detected all high-risk varices: >1.63 Using the two cutoff values, patients categorized as low-, intermediate-, and high-risk with a cumulative 5-year incidence of variceal bleeding of 0%, 1%, and 12%. |
| Yan et al. (2022) [28] | ML | 796 patients 391 in the training and internal validation cohort 405 in the external validation cohort 2358 images | For mild EVs: On the training dataset: AUC: 0.943 Sensitivity: 0.863 Specificity: 0.763 Accuracy: 0.841 On the internal validation dataset: AUC: 0.732 Sensitivity: 0.773 Specificity: 0.763 Accuracy: 0.705 On the external validation dataset: AUC: 0.654 Sensitivity: 0.773 Specificity: 0.632 Accuracy: 0.641 For HREVs: In the training dataset: AUC: 0.983 Sensitivity: 0.948 Specificity: 0.977 Accuracy: 0.965 On the internal validation dataset: AUC: 0.834 Sensitivity: 0.916 Specificity: 0.969 Accuracy: 0.947 On the external validation dataset: AUC: 0.736 Sensitivity: 0.69 Specificity: 0.762 Accuracy: 0.743 Model performed better than Baveno VI and expanded Baveno VI criteria. |
| Gao et al. (2023) [29] | ML | 330 patients 5-fold cross-validation | On the training dataset: AUC: 0.9 Sensitivity: 0.829 Specificity: 0.8 On the internal test dataset: AUC: 0.782 Sensitivity: 0.809 Specificity: 0.625 On the external test dataset: AUC: 0.789 Sensitivity: 0.803 Specificity: 0.5 Model performed better than clinical scores: ARS (AUCs: 0.517, 0.532, 0.534) GBS (AUCs: 0.642, 0.730, 0.631) AIMS65 (AUCs: 0.687, 0.585, 0.582) CTP (AUCs: 0.751, 0.507, 0.589) MELD (AUCs: 0.65, 0.554, 0.515) ALBI (AUCs: 0.753, 0.62, 0.52) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brata, V.D.; Incze, V.; Ismaiel, A.; Turtoi, D.C.; Grad, S.; Popovici, R.; Duse, T.A.; Surdea-Blaga, T.; Padureanu, A.M.; David, L.; et al. Applications of Artificial Intelligence-Based Systems in the Management of Esophageal Varices. J. Pers. Med. 2024, 14, 1012. https://doi.org/10.3390/jpm14091012
Brata VD, Incze V, Ismaiel A, Turtoi DC, Grad S, Popovici R, Duse TA, Surdea-Blaga T, Padureanu AM, David L, et al. Applications of Artificial Intelligence-Based Systems in the Management of Esophageal Varices. Journal of Personalized Medicine. 2024; 14(9):1012. https://doi.org/10.3390/jpm14091012
Chicago/Turabian StyleBrata, Vlad Dumitru, Victor Incze, Abdulrahman Ismaiel, Daria Claudia Turtoi, Simona Grad, Raluca Popovici, Traian Adrian Duse, Teodora Surdea-Blaga, Alexandru Marius Padureanu, Liliana David, and et al. 2024. "Applications of Artificial Intelligence-Based Systems in the Management of Esophageal Varices" Journal of Personalized Medicine 14, no. 9: 1012. https://doi.org/10.3390/jpm14091012
APA StyleBrata, V. D., Incze, V., Ismaiel, A., Turtoi, D. C., Grad, S., Popovici, R., Duse, T. A., Surdea-Blaga, T., Padureanu, A. M., David, L., Dita, M. O., Baldea, C. A., & Popa, S. L. (2024). Applications of Artificial Intelligence-Based Systems in the Management of Esophageal Varices. Journal of Personalized Medicine, 14(9), 1012. https://doi.org/10.3390/jpm14091012








