Sex Differences in Biochemical Analyses, Cardiometabolic Risk Factors and Their Correlation with CRP in Healthy Mexican Individuals
Abstract
1. Introduction
2. Subjects and Methods
2.1. Ethical Considerations and Study Population
2.2. Subjects
2.2.1. Study Design
2.2.2. Procedures
2.3. Personal Variables
2.4. Biochemical Variables Measurement
2.5. Statistical Analysis
3. Results
3.1. Comparison of Abnormal Values between Sexes
3.2. Sex Comparisons in the Correlation between Cardiovascular Risk Factors and CRP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agu, P.; Egbugara, M.; Ogboi, J.; Ajah, L.; Nwagha, U.; Ugwu, E.; Ezugwu, E. Atherogenic index, cardiovascular risk ratio, and atherogenic coefficient as risk factors for cardiovascular disease in pre-eclampsia in Southeast Nigeria: A Cross-Sectional study. Niger. J. Clin. Pract. 2024, 27, 221–227. [Google Scholar] [CrossRef] [PubMed]
- D’Marco, L.; Checa-Ros, A. Exploring the Link between Cardiorenal and Metabolic Diseases. Healthcare 2023, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Flores, Y.N.; Zhang, Z.; Bastani, R.; Leng, M.; Crespi, C.M.; Ramírez-Palacios, P.; Stevens, H.A.; Salmerón, J. Risk factors for liver disease among adults of Mexican descent in the United States and Mexico. World J. Gastroenterol. 2018, 24, 4281–4290. [Google Scholar] [CrossRef]
- Mejía-Rodríguez, F.; Mundo-Rosas, V.; Rodríguez-Ramírez, S.; Hernández-F, M.; García-Guerra, A.; Rangel-Baltazar, E.; Gómez-Acosta, L.M.; Shamah-Levy, T. Alta prevalencia de anemia en mujeres mexicanas en pobreza, Ensanut 100k. Salud Publica De Mexico 2019, 61, 841. [Google Scholar] [CrossRef] [PubMed]
- Flores-Alfaro, E.; Parra-Rojas, I.; Jiménez-Acevedo, A.; Fernández-Tilapa, G. Pruebas presuntivas en el análisis de orina en el diagnóstico de infección de vias urinarias entre diabéticos tipo 2. Salud Pública de México 2005, 47, 376–380. [Google Scholar] [CrossRef][Green Version]
- González-Pedraza Avilés, A.; Dávila-Mendoza, R.; Acevedo-Giles, O.; Ramírez-Martínez, M.E.; Gilbaja-Velázquez, S.; Valencia-Gómez, C.; Cruz-Zamora, L.; Iriarte-Molina, A. Infección de las vías urinarias: Prevalencia, sensibilidad antimicrobiana y factores de riesgo asociados en pacientes con diabetes mellitus tipo 2. Revista Cubana de Endocrinología 2014, 25, 57–65. [Google Scholar]
- McAdams, M.A.; Van Dam, R.M.; Hu, F.B. Comparison of self-reported and measured BMI as correlates of disease markers in U.S. adults. Obesity 2007, 15, 188. [Google Scholar] [CrossRef]
- Kurl, S.; Jae, S.Y.; Voutilainen, A.; Laukkanen, J.A. The combined effect of blood pressure and C-reactive protein with the risk of mortality from coronary heart and cardiovascular diseases. NMCD Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2051–2057. [Google Scholar] [CrossRef]
- Li, N.; Wu, S.; Shu, R.; Song, H.; Wang, J.; Chen, S.; Yang, W.; Wang, G.; Yang, J.; Yang, X.; et al. The combination of high uric acid and high C-reactive protein increased the risk of cardiovascular disease: A 15-year prospective cohort study. NMCD Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1508–1517. [Google Scholar] [CrossRef]
- Song, Y.; Yang, S.K.; Kim, J.; Lee, D. Association between C-Reactive Protein and Metabolic Syndrome in Korean Adults. Korean J. Fam. Med. 2019, 40, 116–123. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Shoaibinobarian, N.; Noormohammadi, M.; Mousavi, A.F.; Rakhsh, A.S.; Salari, A.; Ghorbani, Z. Inflammatory markers and atherogenic coefficient: Early markers of metabolic syndrome. Int. J. Endocrinol. Metab. 2022, 20, e127445. [Google Scholar] [CrossRef] [PubMed]
- Koziarska-Rościszewska, M.; Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. High-Sensitivity C-Reactive Protein Relationship with Metabolic Disorders and Cardiovascular Diseases Risk Factors. Life 2021, 11, 742. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, S.; Huang, Y.; Huang, H.; Zhong, V. Severity of abdominal obesity and cardiometabolic diseases in US adults. Public Health 2024, 227, 154–162. [Google Scholar] [CrossRef]
- Lioy, B.; Webb, R.J.; Amirabdollahian, F. The Association between the Atherogenic Index of Plasma and Cardiometabolic Risk Factors: A Review. Healthcare 2023, 11, 966. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Li, S.; Ma, Y.; Lin, J.; Wan, J.; Zhao, M. The atherogenic index of plasma (AIP) is a predictor for the severity of coronary artery disease. Front. Cardiovasc. Med. 2023, 10, 1140215. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Mosquera-Rojas, M.D.; Campos-Aspajo, A.; Salazar-Valdivia, F.E.; Valdez-Cornejo, V.A.; Benites-Zapata, V.A.; Herrera-Añazco, P.; Valenzuela-Rodríguez, G.; et al. Atherogenic index of plasma and coronary artery disease: A systematic review. Open Med. 2022, 17, 1915–1926. [Google Scholar] [CrossRef]
- Lear, S.A.; James, P.T.; Ko, G.T.; Kumanyika, S.K. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur. J. Clin. Nutr. 2010, 64, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Miranda, C.; Sánchez-Barrera, R.G.; Hernández-Saavedra, D.; Cruz-López, M. Prevalencia de dislipidemias en una población de sujetos en apariencia sanos y su relación con la resistencia a la insulina. Salud Publica De Mexico 2008, 50, 375–382. [Google Scholar] [CrossRef][Green Version]
- Tran, N.L.; Blizzard, C.L.; Luong, K.N.; Van Truong, N.L.; Tran, B.; Otahal, P.; Nelson, M.T.; Magnussen, C.G.; Van Bui, T.; Srikanth, V.; et al. Sex differences in total cholesterol of Vietnamese adults. PLoS ONE 2001, 16, e0256589. [Google Scholar] [CrossRef]
- Brambila-Tapia, A.J.L.; Dávalos-Rodríguez, I.P.; Méndez-García, C.A.; Bárcenas-Robles, F.I.; Gutiérrez-Hurtado, I.A. Sex Differences in the Atherogenic Risk Index in Healthy Mexican Population and Its Relationship with Anthropometric and Psychological Factors. J. Pers. Med. 2023, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kuk, J.L.; Caprio, S.; Buchanan, T.A. Race and Gender Differences in the Relationships Between Anthropometrics and Abdominal Fat in Youth. Obesity 2008, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, W.M.M.; Kromhout, D. Total cholesterol concentration and mortality at a relatively young age: Do men and women differ? BMJ 1995, 311, 779–783. [Google Scholar] [CrossRef][Green Version]
- Mederico, M.; Paoli, M.; Zerpa, Y.; Briceño, Y.; Gómez-Pérez, R.; Martínez, J.L.; Camacho, N.; Cichetti, R.; Molina, Z.; Mora, Y.; et al. Valores de referencia de la circunferencia de la cintura e índice de la cintura/cadera en escolares y adolescentes de Mérida, Venezuela: Comparación con referencias internacionales. Endocrinol. Nutr. 2013, 60, 235–242. [Google Scholar] [CrossRef]
- Zhang, S.; Hong, F.; Ma, C.; Yang, S. Hepatic Lipid Metabolism Disorder and Atherosclerosis. Endocr. Metab. Immune Disord. 2021, 22, 590–600. [Google Scholar] [CrossRef]
- Ríos-González, B.E.; Saldaña-Cruz, A.M.; Gallardo-Moya, S.G.; Brambila-Tapia, A.J.L. Sex Differences in the Relationship between Personal, Psychological and Biochemical Factors with Blood Pressure in a Healthy Adult Mexican Population: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Galeano, I.O.; Franquelo-Morales, P.; Notario-Pacheco, B.; Rodríguez, J.A.N.; Canñete, M.U.; Martínez-Vizcaíno, V. Prehipertensión arterial en adultos jóvenes. Rev. Clin. Esp. 2012, 212, 287–291. [Google Scholar] [CrossRef]
- Dong, G.; Wang, D.H.; Liu, M.; Liu, Y.; Zhao, Y.; Yang, M.; Meng, X.; Tian, S.; Meng, X.; Zhang, H. Sex difference of the prevalence and risk factors associated with prehypertension among urban Chinese adults from 33 communities of China. J. Hypertens. 2012, 30, 485–491. [Google Scholar] [CrossRef]
- Yanes, L.L.; Romero, D.G.; Iliescu, R.; Zhang, H.; Davis, D.; Reckelhoff, J.F. Postmenopausal hypertension: Role of the renin- angiotensin system. Hypertension 2010, 56, 359–363. [Google Scholar] [CrossRef]
- Kringeland, E.; Tell, G.S.; Midtbo, H.; Igland, J.; Haugsgjerd, T.R.; Gerdts, E. Stage 1 hypertension, sex, and acute coronary syndromes during midlife: The Hordaland health study. Eur. J. Prev. Cardiol. 2022, 29, 147–154. [Google Scholar] [CrossRef]
- Ji, H.; Niiranen, T.J.; Rader, F.; Henglin, M.; Kim, A.; Ebinger, J.E.; Claggett, B.; Merz, C.N.B.; Cheng, S. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation 2021, 143, 761–763. [Google Scholar] [CrossRef]
- Nwankwo, T.; Yoon, S.S.; Burt, V.; Gu, Q. Hypertension among Adults in the United States: National Health and Nutrition Examination Survey, 2011–2012; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2013; pp. 1–8.
- Asghar, A.; Talha, K.M.; Waqar, E.; Sperling, L.; DiNino, E.; Sharafkhaneh, A.; Virani, S.S.; Ballantyne, C.M.; Nambi, V.; Minhas, A.M.K. Trends in sleep apnea and heart failure related mortality in the United States from 1999 to 2019. Curr. Probl. Cardiol. 2024, 49, 102342. [Google Scholar] [CrossRef]
- Robles-Rivera, K.; Argoty-Pantoja, A.D.; Hidalgo-Bravo, A.; Quezada-Sánchez, A.D.; León-Reyes, G.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R.; Rivera-Paredez, B. Uric Acid Levels Are Associated with Bone Mineral Density in Mexican Populations: A Longitudinal Study. Nutrients 2022, 14, 4245. [Google Scholar] [CrossRef] [PubMed]
- Бикбoв, M.; Kazakbaeva, G.; Зайнуллин, P.; Salavatova, V.F.; Gilmanshin, T.R.; Yakupova, D.F.; Uzianbaeva, Y.V.; Arslangareeva, I.I.; Panda-Jonas, S.; Mukhamadieva, S.R.; et al. Prevalence and associated factors of anemia in a Russian population: The Ural eye and medical study. BMC Public Health 2019, 19, 762. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Jaimes, E.; Casanova-Román, G.; Galindo-Fraga, A.; Esecto-Gutiérrez, P.; Gutiérrez-Escoto, P.; Landa-Juárez, S.; Espinosa-Moreno, S.; Rodríguez-Covarrubias, F.; Simón-Pereira, L.; Valdez-Vázquez, R. Diagnóstico y tratamiento de las infecciones de vías urinarias: Un enfoque multidisciplinario para casos no complicados. Boletín Médico del Hospital Infantil de México 2013, 70, 3–10. [Google Scholar]
- Brambila-Tapia, A.J.L.; Jacquez-Castañeda, A.L.; Carrillo-Delgadillo, L.A.; Dávila-Flores, J.N.; Macías-Espinoza, F.; Santos, S.R.L.; Gutiérrez-Hurtado, I.A. Association between Psychological, Biochemical and Personal Factors with the Inflammatory Marker High-Sensitive C Reactive Protein (Hs-CRP) in Mexican Healthy Population. J. Pers. Med. 2023, 13, 876. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Razak, F.; Davis, A.D.; Jacobs, R.; Vuksan, V.; Teo, K.; Yusuf, S. Social disadvantage and cardiovascular disease: Development of an index and analysis of age, sex, and ethnicity effects. Int. J. Epidemiol. 2006, 35, 1239–1245. [Google Scholar] [CrossRef]
- Varghese, T.P. Genetic biomarkers of cardiovascular disease. Curr. Probl. Cardiol. 2024, 49, 102588. [Google Scholar] [CrossRef]
- Kim, J.Y.; Seo, C.; Pak, H.; Lim, H.; Chang, T.I. Uric acid and risk of cardiovascular disease and mortality: A longitudinal cohort study. J. Korean Med. Sci. 2023, 38, e302. [Google Scholar] [CrossRef]
- Brero, M.; Meyer, C.L.; Jackson-Morris, A.; Spencer, G.; Ludwig-Borycz, E.; Wu, D.; Cândido, A.L.; Eguiluz, M.; Bonvecchio, A.; Jewell, J.; et al. Investment Case for the Prevention and Reduction of Childhood and adolescent Overweight and Obesity in Mexico. Obes. Rev. 2023, 24, e13595. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Goodale, H.; Xue, H.; Brey, R.A.; Wang, Y. Racial-Ethnic Disparities in Obesity and biological, behavioral, and Sociocultural Influences in the United States: A Systematic review. Adv. Nutr. 2021, 12, 1137–1148. [Google Scholar] [CrossRef] [PubMed]

| Variable | Women (n = 123) | Men (n = 115) | p Value |
| Age, mean ± SD | 29.34 ± 10.54 | 28.73 ± 11.70 | 0.336 |
| With romantic partner, n (%) | 70 (56.9) | 72 (62.6) | 0.428 |
| With children, n (%) | 38 (30.9) | 22 (19.1) | 0.052 |
| With job, n (%) | 74 (60.2) | 67 (58.3) | 0.793 |
| Schooling, n (%) | 0.768 | ||
| Elementary school | 1 (0.8) | 1 (0.9) | |
| Secondary | 5 (4.1) | 5 (4.3) | |
| Preparatory | 53 (43.1) | 55 (47.9) | |
| University (Bachelor’s degree) | 51 (41.5) | 40 (34.8) | |
| Master’s degree | 11 (8.9) | 9 (7.8) | |
| Ph.D. degree | 2 (1.6) | 5 (4.3) | |
| Socioeconomic level, n (%) | 0.144 | ||
| Very low | 0 (0.0) | 2 (1.7) | |
| Low | 17 (13.8) | 17 (14.8) | |
| Average | 105 (85.4) | 91 (79.2) | |
| High | 1 (0.8) | 5 (4.3) | |
| Very high | 0 (0.0) | 0 (0.0) | |
| Monthly extra money, mean ± SD | 2.82 ± 1.17 | 2.97 ± 1.28 | 0.341 |
| Daily hours of physical activity, median (range) | 1 (0–6.4) | 1 (0–6.4) | 0.942 |
| Daily free hours, median (range) | 4 (0–10) | 4 (1–16) | 0.021 |
| Smoking frequency, median (range) | 0 (0–4) | 0 (0–4) | 0.929 |
| Alcohol consumption frequency, median (range) | 1.47 ± 0.94 | 1.62 ± 0.99 | 0.411 |
| Variable, Units º | High Values, n (%) | Low Values, n (%) | p Value (for High) | p Value (for Low) | Reference Values ª | ||
|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | ||||
| Sample Size | Women (N = 123)/Men (N = 115) | ||||||
| Hemoglobin, g/dL | 2 (1.6) | 25 (21.7) | 5 (4.1) | 0 (0.0) | <0.0001 | 0.06 | W: 12.00–16.00 M: 14.00–17.00 |
| Leukocytes, 103/μL | 4 (3.3) | 5 (4.3) | 11 (8.9) | 18 (15.7) | 0.74 | 0.16 | 5.00–10.00 |
| Monocytes, 103/μL | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0.48 | 1.00 | 0.10–1.00 |
| Lymphocytes, 103/μL | 0 (0.0) | 1 (0.9) | 1 (0.8) | 0 (0.0) | 0.48 | 1.00 | 1.00–4.20 |
| Platelets, 103/μL | 4 (3.3) | 2 (1.6) | 0 (0.0) | 0 (0.0) | 0.68 | 1.00 | 141–400 |
| Serum lipids | |||||||
| Total cholesterol, mg/dL | 19 (15.4) | 29 (25.2) | - | - | 0.07 | - | ≤200.00 |
| Low-density lipoprotein (LDL), mg/dL | 59 (48.0) | 74 (64.3) | - | - | 0.01 | - | ≤100.00 |
| High-density lipoprotein (HDL), mg/dL | - | - | 37 (30.1) | 18 (15.7) | - | 0.009 ** | W > 45.00 M > 35.00 |
| Triglycerides, mg/dL | 15 (12.2) | 34 (29.6) | - | - | 0.001 | - | ≤150.00 |
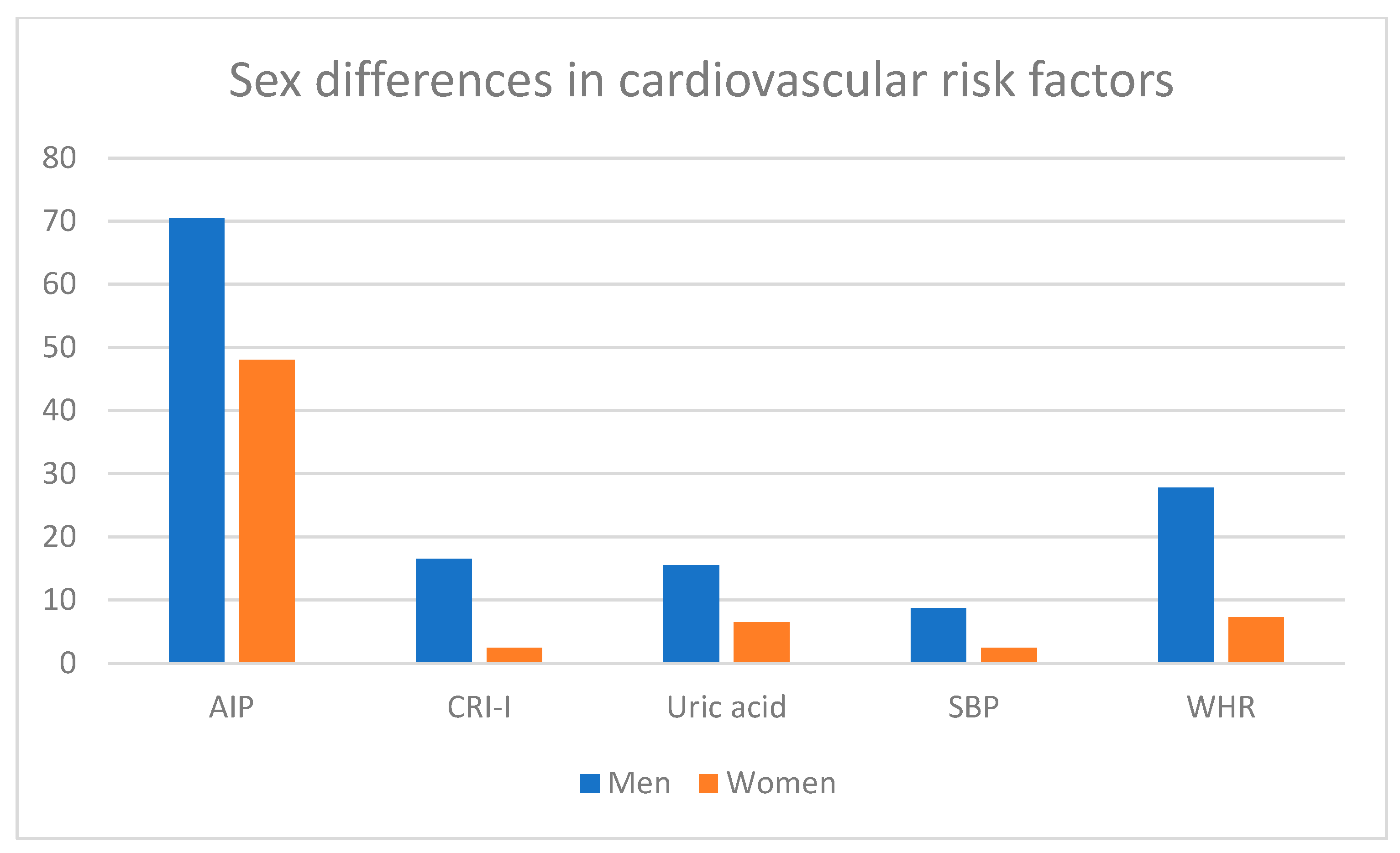
| Castelli’s risk index I (CRI-I) | 3 (2.4) | 19 (16.5) | - | - | <0.001 | - | <5.00 |
| Atherogenic index in plasma (AIP) | 59 (48.0) | 81 (70.4) | - | - | <0.001 | - | <0.21 |
| Liver function tests | |||||||
| Aspartate aminotransferase (AST), U/L | 7 (5.7) | 8 (7.0) | - | - | 0.79 | - | W ≤ 32.00 M ≤ 40.00 |
| Alanine aminotransferase (ALT), U/L | 12 (9.8) | 17 (14.8) | - | - | 0.32 | - | W ≤ 33.00 M ≤ 41.00 |
| Gamma-glutamyl transferase (GGT), U/L | 6 (4.9) | 7 (6.1) | - | - | 0.77 | - | W ≤ 40.00 M ≤ 60.00 |
| Alkaline phosphatase (ALP), U/L | 8 (6.5) | 12 (10.4) | 1 (0.8) | 0 (0.0) | 0.35 | 1.00 | W: 35.00–104.00 M: 40.00–129.00 |
| Lactate dehydrogenase (LDH), U/L | 34 (27.6) | 38 (33.0) | 8 (6.5) | 4 (3.5) | 0.39 | 0.37 | W: 135.00–214.00 M: 135.00–225.00 |
| Blood chemistry | |||||||
| Glucose, g/dL | 3 (2.4) | 4 (3.5) | 8 (6.5) | 2 (1.7) | 0.71 | 0.10 | 74.00–106.00 |
| Urea, mg/dL | 1 (0.8) | 2 (1.7) | 3 (2.4) | 1 (0.9) | 0.61 | 0.62 | 16.60–48.50 |
| Creatinine, mg/dL | 8 (6.5) | 2 (1.7) | 1 (0.8) | 4 (3.5) | 0.10 | 0.20 | W: 0.50–0.90 M: 0.70–1.20 |
| Uric acid, mg/dL | 8 (6.5) | 19 (16.5) | 3 (2.4) | 0 (0.0) | 0.02 | 0.25 | W: 2.40–5.70 M: 3.40–7.00 |
| Serum electrolytes | |||||||
| Calcium, mg/dL | 14 (11.4) | 37 (32.2) | 0 (0.0) | 0 (0.0) | <0.001 | 1.00 | 8.60–10.00 |
| Sodium, mg/dL | 4 (3.3) | 1 (0.9) | 5 (4.1) | 2 (1.7) | 0.37 | 0.44 | 136.00–145.00 |
| Potassium, meq/L | 8 (6.5) | 6 (5.2) | 0 (0.0) | 0 (0.0) | 0.78 | 1.00 | 3.50–5.10 |
| Magnesium, mg/dL | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.00 | 1.00 | 1.60–2.60 |
| Iron, μg/dL | 0 (0.0) | 5 (4.3) | 7 (5.7) | 0 (0.0) | 0.025 | 0.014 | 33.00–193.00 |
| Phosphorus, mg/dL | 1 (0.8) | 2 (1.7) | 4 (3.3) | 0 (0.0) | 0.611 | 0.122 | 2.50–4.50 |
| Chloride, meq/L | 7 (5.7) | 2 (1.7) | 0 (0.0) | 2 (1.7) | 0.174 | 0.232 | 98.00–107.00 |
| Pancreatic enzymes | |||||||
| Amylase, U/L | 13 (10.6) | 9 (7.8) | 3 (2.4) | 0 (0.0) | 0.509 | 0.248 | 28.00–100.00 |
| Lipase, U/L | 5 (4.1) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0.214 | 1.00 | 13.00–60.00 |
| C-reactive protein | 28 (22.8) | 14 (12.2) | - | - | 0.04 | - | 0.10–3.00 |
| General urine test | |||||||
| Leucocyte esterase (> 0) | 56 (45.5) | 7 (6.1) | - | - | <0.0001 | - | 0 |
| Erythrocytes (1 per camp | 73 (59.3) | 39 (33.9) | - | - | <0.0001 | - | 0 |
| Leucocytes (>1 per camp) | 106 (86.2) | 36 (31.3) | - | - | <0.0001 | - | 0 |
| Nitrites (1 per camp) | 5 (4.1) | 0 (0.0) | - | - | 0.06 | - | 0 |
| Variable | Women, n (%) Women = 123 | Men, n (%) Men = 115 | p Value |
|---|---|---|---|
| Body mass index (BMI) > 25.0 | 49 (39.8) | 53 (46.1) | 0.36 |
| Waist-to-hip ratio (WHR), M > 0.90, W > 0.86 | 9 (7.3) | 32 (27.8) | <0.0001 |
| Systolic blood pressure (SBP) | <0.001 | ||
| Normal ≤ 120 mmHg | 113 (91.9) | 57 (49.6) | |
| Pre-hypertension (121–139 mmHg) | 7 (5.7) | 48 (41.7) | |
| High ≥ 140 mmHg | 3 (2.4) | 10 (8.7) | |
| Diastolic blood pressure (DBP) | 0.009 | ||
| Normal ≤ 80 mmHg | 95 (77.2) | 68 (59.1) | |
| Pre-hypertension (81–89 mmHg) | 21 (17.1) | 31 (27.0) | |
| High ≥ 90 mmHg | 7 (5.7) | 16 (13.9) |
| Variable | Age | BMI | WHR | SBP | DBP | Uric Acid | CRI-I | AIP |
|---|---|---|---|---|---|---|---|---|
| CRP | 0.297 * | 0.526 ** | 0.304 ** | 0.248 ** | 0.363 ** | 0.294 ** | 0.412 ** | 0.469 ** |
| Age | - | 0.384 ** | 0.443 ** | 0.294 ** | 0.268 * | 0.116 | 0.396 ** | 0.375 ** |
| BMI | - | - | 0.485 ** | 0.324 ** | 0.302 ** | 0.145 | 0.587 ** | 0.515 ** |
| WHR | - | - | - | 0.215 * | 0.193 * | 0.214 * | 0.427 ** | 0.378 ** |
| SBP | - | - | - | - | 0.722 ** | 0.236 ** | 0.141 | 0.082 |
| DBP | - | - | - | - | - | 0.252 ** | 0.113 | 0.114 |
| Uric acid | - | - | - | - | - | - | 0.202 * | 0.239 ** |
| CRI-I | - | - | - | - | - | - | - | 0.810 ** |
| Variable | Age | BMI | WHR | SBP | DBP | Uric Acid | CRI-I | AIP |
|---|---|---|---|---|---|---|---|---|
| CRP | 0.184 | 0.393 ** | 0.402 ** | 0.116 | 0.190 * | 0.218 * | 0.277 ** | 0.287 ** |
| Age | - | 0.352 ** | 0.680 ** | 0.095 | 0.349 ** | 0.069 | 0.455 ** | 0.512 ** |
| BMI | - | - | 0.595 ** | 0.324 ** | 0.274 ** | 0.275 * | 0.376 ** | 0.368 ** |
| WHR | - | - | - | 0.181 | 0.336 ** | 0.353 ** | 0.573 ** | 0.599 ** |
| SBP | - | - | - | - | 0.656 ** | 0.19 | 0.174 | 0.123 |
| DBP | - | - | - | - | - | 0.260 ** | 0.193 * | 0.301 ** |
| Uric acid | - | - | - | - | - | - | 0.099 | 0.234 ** |
| CRI-I | - | - | - | - | - | - | - | 0.815 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brambila-Tapia, A.J.L.; González-Gómez, A.S.; Carrillo-Delgadillo, L.A.; Saldaña-Cruz, A.M.; Dávalos-Rodríguez, I.P. Sex Differences in Biochemical Analyses, Cardiometabolic Risk Factors and Their Correlation with CRP in Healthy Mexican Individuals. J. Pers. Med. 2024, 14, 904. https://doi.org/10.3390/jpm14090904
Brambila-Tapia AJL, González-Gómez AS, Carrillo-Delgadillo LA, Saldaña-Cruz AM, Dávalos-Rodríguez IP. Sex Differences in Biochemical Analyses, Cardiometabolic Risk Factors and Their Correlation with CRP in Healthy Mexican Individuals. Journal of Personalized Medicine. 2024; 14(9):904. https://doi.org/10.3390/jpm14090904
Chicago/Turabian StyleBrambila-Tapia, Aniel Jessica Leticia, Alejandra Soledad González-Gómez, Laura Arely Carrillo-Delgadillo, Ana Míriam Saldaña-Cruz, and Ingrid Patricia Dávalos-Rodríguez. 2024. "Sex Differences in Biochemical Analyses, Cardiometabolic Risk Factors and Their Correlation with CRP in Healthy Mexican Individuals" Journal of Personalized Medicine 14, no. 9: 904. https://doi.org/10.3390/jpm14090904
APA StyleBrambila-Tapia, A. J. L., González-Gómez, A. S., Carrillo-Delgadillo, L. A., Saldaña-Cruz, A. M., & Dávalos-Rodríguez, I. P. (2024). Sex Differences in Biochemical Analyses, Cardiometabolic Risk Factors and Their Correlation with CRP in Healthy Mexican Individuals. Journal of Personalized Medicine, 14(9), 904. https://doi.org/10.3390/jpm14090904








