Automated Breast Ultrasound for Evaluating Response to Neoadjuvant Therapy: A Comparison with Magnetic Resonance Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Inclusion Criteria
2.2. MR Protocol
- -
- SURVEY localization sequences (axial, sagittal, and coronal) weighted in T1;
- -
- axial T2-weighted short tau inversion recovery (STIR) turbo spin-echo (TSE) sequences, repetition time/echo time/time interval (TR/TE/TI) = 3800/60/165 ms, field of view (FOV) = 250 × 450 mm (AP × RL), matrix 168 × 300, 50 slices with 3 mm slice thickness and no gaps, 3 averages, turbo factor 23, resulting in a voxel size of 1.5 × 1.5 × 3.0 mm3;
- -
- axial T2-weighted TSE sequences, TR/TE = 6300/130 ms, FOV = 250 × 450 mm (AP × RL), matrix 336 × 600, 50 slices with 3 mm slice thickness and no gaps, 3 averages, turbo factor 59, SENSE factor 1.7, resulting in a voxel size of 0.75 × 0.75 × 3.0 mm3;
- -
- T1 high-resolution isotropic volume examination (THRIVE), dynamic 3D acquisitions with contrast enhancement, gradient echo, TRE/TE = 4.4/2.0 ms, FOV = 250 × 450 × 150 mm (AP × RL × FH), matrix 168 × 300, 100 slices with 1.5 mm slice thickness, turbo factor 50, SENSE factor 1.6, 6 dynamic acquisitions, resulting in 1.5 mm3 isotropic voxels, a dynamic data acquisition time of 1 min 30 s, and a total sequence duration of 9 min;
- -
- diffusion-weighted imaging with background signal suppression (DWIB) sequences, TR/TE = 4600/66 ms, FOV = 300 × 338 × 145 mm (AP × RL × FH), matrix 168 × 300, b-value 2 (s/mm2) max b-value 700–1000 (s/mm2), 50 slices with 3 mm slice thickness, resulting in a voxel size of 1.34 × 1.33 × 3.0 mm3 (AP × RL × FH), with ADC mapping.
2.3. ABUS Technique
2.4. Image Analysis
2.5. Statistical Analysis
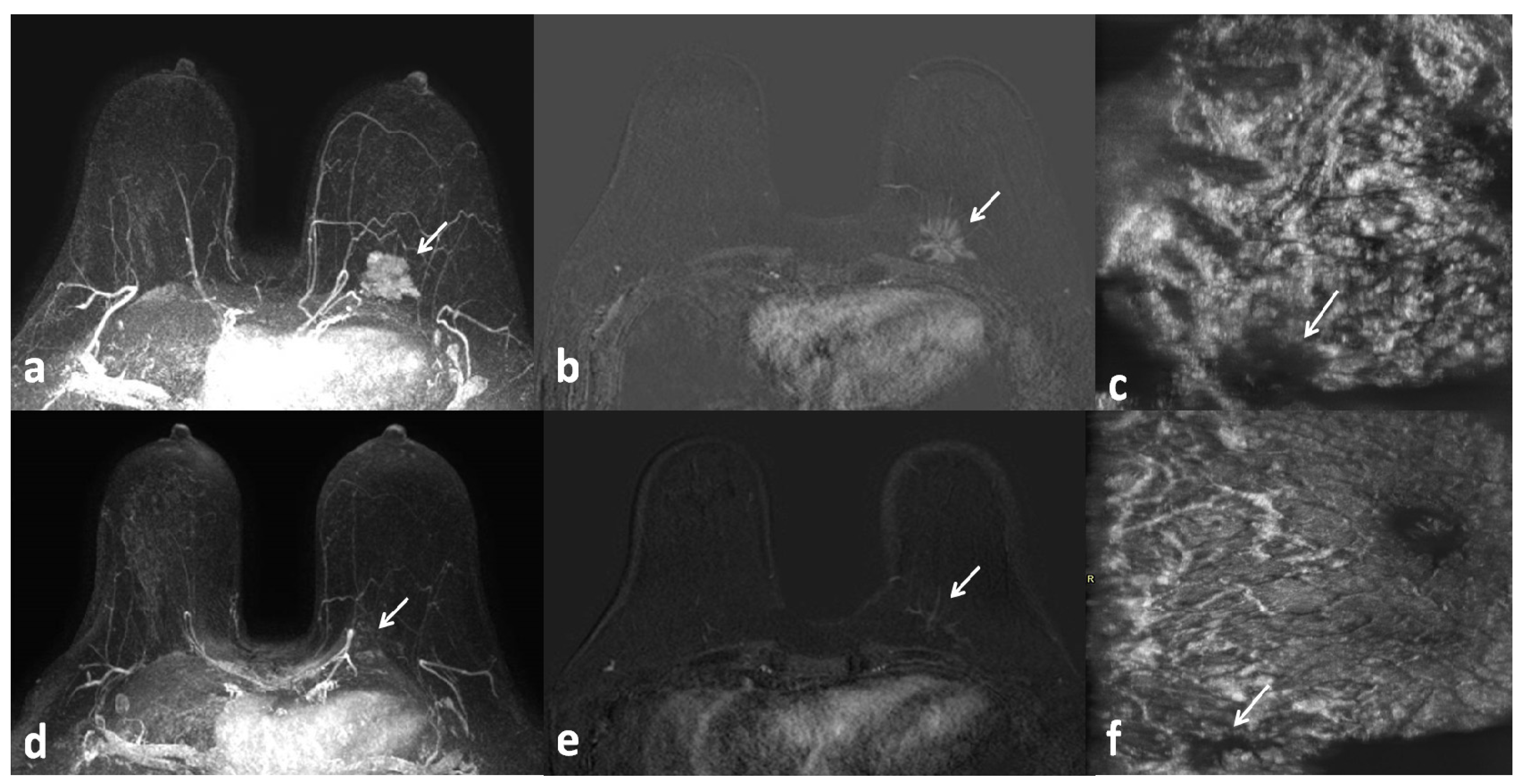
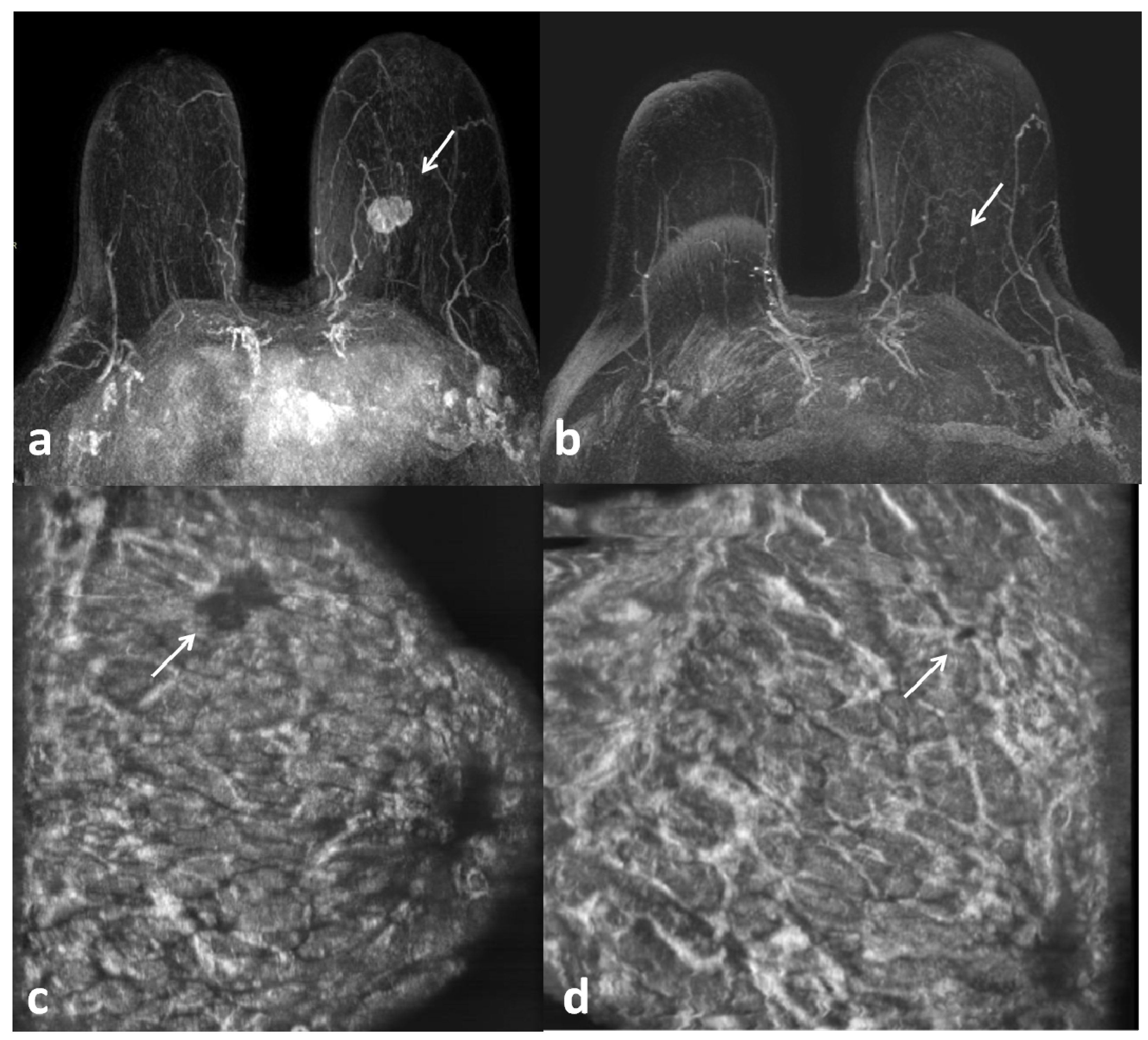
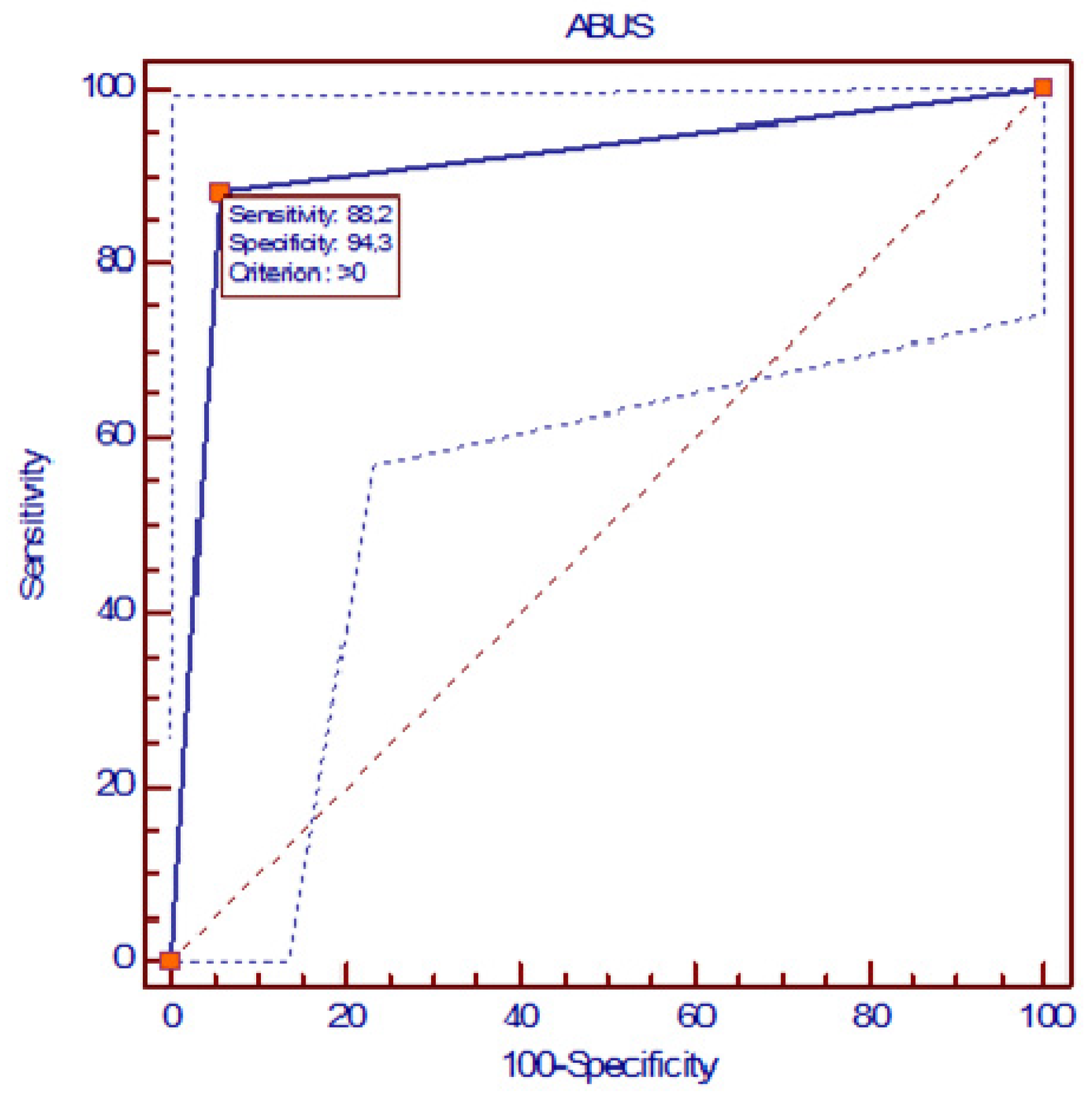
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Huo, L.; He, Y.; Fan, Z.; Wang, T.; Xie, Y.; Li, J.; Ouyang, T. Early prediction of pathological outcomes to neoadjuvant chemotherapy in breast cancer patients using automated breast ultrasound. Chin. J. Cancer Res. 2016, 28, 478–485. [Google Scholar] [CrossRef][Green Version]
- Colleoni, M.; Goldhirsch, A. Neoadjuvant chemotherapy for breast cancer: Any progress? Lancet Oncol. 2014, 15, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 2023, 41, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Moo, T.-A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of Imaging in Neoadjuvant Therapy for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Accardo, G.; Perillo, T.; Basso, L.; Garbino, N.; Nicolai, E.; Maurea, S.; Salvatore, M. Assessment and Prediction of Response to Neoadjuvant Chemotherapy in Breast Cancer: A Comparison of Imaging Modalities and Future Perspectives. Cancers 2021, 13, 3521. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Köbrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- Rezkallah, E.; Mekhaeil, K.; Tin, S.M.M.; Hanna, R.S. The Role of MRI in Assessing Residual Breast Cancer after Neoadjuvant Chemotherapy. Am. Surg. 2024, 90, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Reig, B.; Lewin, A.A.; Du, L.; Heacock, L.; Toth, H.K.; Hetller, S.L.; Gao, Y.; Moy, L. Breast MRI for Evaluation of Response to Neoadjuvant Therapy. RadioGraphics 2021, 41, 665–679. [Google Scholar] [CrossRef]
- Rella, R.; Belli, P.; Giuliani, M.; Bufi, E.; Carlino, G.; Rinaldi, P.; Manfredi, R. Automated Breast Ultrasonography (ABUS) in the Screening and Diagnostic Setting. Acad. Radiol. 2018, 25, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Ferrari, F.; Bozzini, A.C.; Latronico, A.; Trentin, C.; Meneghetti, L.; Pesapane, F.; Pizzamiglio, M.; Balesetreri, N.; Cassano, E. Automatic breast ultrasound: State of the art and future perspectives. ecancermedicalscience 2020, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis after Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Man, V.C.; Cheung, P.S. Neoadjuvant chemotherapy increases rates of breast-conserving surgery in early operable breast cancer. Hong Kong Med. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pasquero, G.; Surace, A.; Ponti, A.; Bortolini, M.; Tota, D.; Mano, M.P.; Arisio, R.; Benedetto, C.; Balù, M.G. Role of Magnetic Resonance Imaging in the Evaluation of Breast Cancer Response to Neoadjuvant Chemotherapy. Vivo 2020, 34, 909–915. [Google Scholar] [CrossRef]
- Schrading, S.; Kuhl, C.K. Breast Cancer: Influence of Taxanes on Response Assessment with Dynamic Contrast-enhanced MR Imaging. Radiology 2015, 277, 687–696. [Google Scholar] [CrossRef] [PubMed]
- van Egdom, L.S.E.; Lagendijk, M.; Heijkoop, E.H.M.; Koning, A.; van Deurzen, C.; Jager, A.; van Lankeren, W.; Koppert, L. Three-dimensional ultrasonography of the breast; An adequate replacement for MRI in neoadjuvant chemotherapy tumour response evaluation?—RESPONDER trial. Eur. J. Radiol. 2018, 104, 94–100. [Google Scholar] [CrossRef]
- Park, J.; Chae, E.Y.; Cha, J.H.; Shin, H.J.; Choi, W.J.; Choi, Y.-W.; Kim, H.H. Comparison of mammography, digital breast tomosynthesis, automated breast ultrasound, magnetic resonance imaging in evaluation of residual tumor after neoadjuvant chemotherapy. Eur. J. Radiol. 2018, 108, 261–268. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B. Operator Dependence of Physician-performed Whole-Breast US: Lesion Detection and Characterization. Radiology 2006, 241, 355–365. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xw, J.H.; Li, J.L.; Huang, Y.; Tang, J. Comparison of automated breast volume scanning to hand-held ultrasound and mammography. La Radiol. Medica 2012, 117, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Hatzipanagiotou, M.E.; Huber, D.; Gerthofer, V.; Hetterich, M.; Ripoll, B.R.; Ortmann, O.; Seitz, S. Feasibility of ABUS as an Alternative to Handheld Ultrasound for Response Control in Neoadjuvant Breast Cancer Treatment. Clin. Breast Cancer 2021, 22, e142–e146. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, Y.; Wang, Q.; Li, B.; Shang, H.; Jing, H. Early Prediction of Response to Neoadjuvant Chemotherapy Using Quantitative Parameters on Automated Breast Ultrasound Combined with Contrast-Enhanced Ultrasound in Breast Cancer. Ultrasound Med. Biol. 2023, 49, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]

| IMAGING TOOL | ABUS | n = 52 | |
|---|---|---|---|
| MRI | Complete Response | Persistent Disease | |
| Complete Response to NAC | 31 | 0 | 31 (60%) |
| Persistent Disease | 4 | 17 | 21 (40%) |
| n = 52 | 35 (67%) | 17 (33%) | p = 0.1250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telegrafo, M.; Stucci, S.L.; Gurrado, A.; Catacchio, C.; Cofone, F.; Maruccia, M.; Stabile Ianora, A.A.; Moschetta, M. Automated Breast Ultrasound for Evaluating Response to Neoadjuvant Therapy: A Comparison with Magnetic Resonance Imaging. J. Pers. Med. 2024, 14, 930. https://doi.org/10.3390/jpm14090930
Telegrafo M, Stucci SL, Gurrado A, Catacchio C, Cofone F, Maruccia M, Stabile Ianora AA, Moschetta M. Automated Breast Ultrasound for Evaluating Response to Neoadjuvant Therapy: A Comparison with Magnetic Resonance Imaging. Journal of Personalized Medicine. 2024; 14(9):930. https://doi.org/10.3390/jpm14090930
Chicago/Turabian StyleTelegrafo, Michele, Stefania Luigia Stucci, Angela Gurrado, Claudia Catacchio, Federico Cofone, Michele Maruccia, Amato Antonio Stabile Ianora, and Marco Moschetta. 2024. "Automated Breast Ultrasound for Evaluating Response to Neoadjuvant Therapy: A Comparison with Magnetic Resonance Imaging" Journal of Personalized Medicine 14, no. 9: 930. https://doi.org/10.3390/jpm14090930
APA StyleTelegrafo, M., Stucci, S. L., Gurrado, A., Catacchio, C., Cofone, F., Maruccia, M., Stabile Ianora, A. A., & Moschetta, M. (2024). Automated Breast Ultrasound for Evaluating Response to Neoadjuvant Therapy: A Comparison with Magnetic Resonance Imaging. Journal of Personalized Medicine, 14(9), 930. https://doi.org/10.3390/jpm14090930






