Addressing the Ethnicity Gap in Catechol O-Methyl Transferase Inhibitor Trials in Parkinson’s Disease: A Review of Available Global Data
Abstract
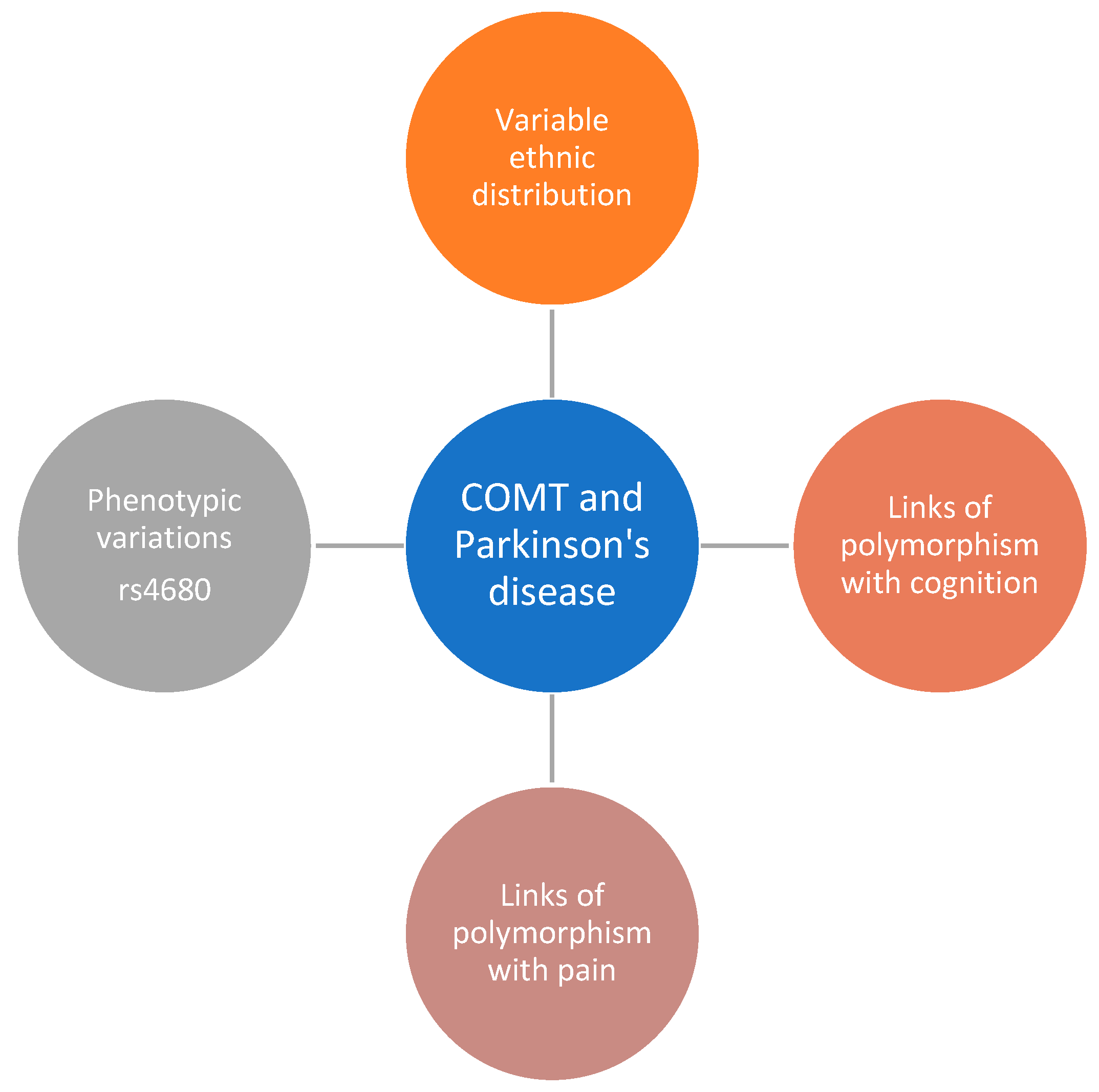
1. Introduction
2. Methodology
3. Results
3.1. Reporting of Ethnicity Data
3.2. Ethnicity Breakdown of All Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Rocha, J.-F.; Ferreira, J.J.; Rascol, O.; Soares-Da-Silva, P. Redefining the strategy for the use of COMT inhibitors in Parkinson’s disease: The role of opicapone. Expert. Rev. Neurother. 2021, 21, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, R.; Tsugawa, J.; Tsuboi, Y.; Fukae, J.; Mishima, T.; Fujioka, S. The impact of early morning off in Parkinson’s disease on patient quality of life and caregiver burden. J. Neurol. Sci. 2016, 364, 1–5. [Google Scholar] [CrossRef]
- Artusi, C.A.; Sarro, L.; Imbalzano, G.; Fabbri, M.; Lopiano, L. Safety and efficacy of tolcapone in Parkinsons disease: Systematic review. Eur. J. Clin. Pharmacol. 2021, 77, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Apud, J.A.; Mattay, V.; Chen, J.; Kolachana, B.S.; Callicott, J.H.; Rasetti, R.; Alce, G.; Iudicello, J.E.; Akbar, N.; Egan, M.F.; et al. Tolcapone Improves Cognition and Cortical Information Processing in Normal Human Subjects. Neuropsychopharmacology 2007, 32, 1011–1020. [Google Scholar] [CrossRef]
- Sheikh, H.I.; Kryski, K.R.; Smith, H.J.; Dougherty, L.R.; Klein, D.N.; Bufferd, S.J.; Singh, S.M.; Hayden, E.P. Catechol-O-methyltransferase gene val158met polymorphism and depressive symptoms during early childhood. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162, 245–252. [Google Scholar] [CrossRef]
- Corvol, J.; Bonnet, C.; Charbonnier-Beaupel, F.; Bonnet, A.; Fiévet, M.; Bellanger, A.; Roze, E.; Meliksetyan, G.; Djebara, M.B.; Hartmann, A.; et al. The COMT Val158Met polymorphism affects the response to entacapone in Parkinson’s disease: A randomized crossover clinical trial. Ann. Neurol. 2011, 69, 111–118. [Google Scholar] [CrossRef] [PubMed]
- McLeod, H.L.; Syvänen, A.-C.; Githang’a, J.; Indalo, A.; Ismail, D.; Dewar, K.; Ulmanen, I.; Sludden, J. Ethnic differences in catechol O-methyltransferase pharmacogenetics: Frequency of the codon 108/158 low activity allele is lower in Kenyan than Caucasian or South-west Asian individuals. Pharmacogenetics 1998, 8, 195–199. [Google Scholar] [CrossRef]
- Rivera-Calimlim, L.; Reilly, D.K. Difference in erythrocyte catechol-O-methyltransferase activity between Orientals and Caucasians: Difference in levodopa tolerance. Clin. Pharmacol. Ther. 1984, 35, 804–809. [Google Scholar] [CrossRef]
- Sampaio, T.F.; Dos Santos, E.U.D.; de Lima, G.D.C.; Dos Anjos, R.S.G.; da Silva, R.C.; Asano, A.G.C.; Asano, N.M.J.; Crovella, S.; de Souza, P.R.E. MAO-B and COMT Genetic Variations Associated with Levodopa Treatment Response in Patients With Parkinson’s Disease. J. Clin. Pharmacol. 2018, 58, 920–926. [Google Scholar] [CrossRef]
- Watanabe, M.; Harada, S.; Nakamura, T.; Ohkoshi, N.; Yoshizawa, K.; Hayashi, A.; Shoji, S. Association between Catechol-O-Methyltransferase Gene Polymorphisms and Wearing-Off and Dyskinesia in Parkinson’s Disease. Neuropsychobiology 2003, 48, 190–193. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Zhang, B.; Yue, Y.; Zhang, J. Genetic variations in catechol-O-methyltransferase gene are associated with levodopa response variability in Chinese patients with Parkinson’s disease. Sci. Rep. 2020, 10, 9521. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Nanko, S.; Ueki, A.; Otsuka, E.; Hattori, M.; Hoda, F.; Vallada, H.P.; Arranz, M.J.; Collier, D.A. High and low activity alleles of catechol-O-methyltransferase gene: Ethnic difference and possible association with Parkinson’s disease. Neurosci. Lett. 1997, 221, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A. COMT gene and risk for Parkinson’s disease: A systematic review and meta-analysis. Pharmacogenet Genom. 2014, 24, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Lechun, L.; Yu, S.; Pengling, H.; Changqi, H. The COMT Val158Met polymorphism as an associated risk factor for Parkinson’s disease in Asian rather than Caucasian populations. Neurol. India 2013, 61, 12–16. [Google Scholar] [CrossRef]
- Chuan, L.; Gao, J.; Lei, Y.; Wang, R.; Lu, L.; Zhang, X. Val158Met polymorphism of COMT gene and Parkinson’s disease risk in Asians. Neurol. Sci. 2015, 36, 109–115. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Zou, Y.-B.; Xiao, J.; Pan, C.-D.; Jiang, S.-D.; Zheng, Z.-J.; Yan, Z.-R.; Tang, K.-Y.; Tan, L.-M.; Tang, M.-S. COMT Val158Met polymorphism and Parkinson’s disease risk: A pooled analysis in different populations. Neurol. Res. 2019, 41, 319–325. [Google Scholar] [CrossRef]
- Lau, Y.H.; Podlewska, A.; Ocloo, J.; Gupta, A.; Gonde, C.; Bloem, B.R.; Chaudhuri, K.R. Does Ethnicity Influence Recruitment into Clinical Trials of Parkinsons Disease? J. Park. Dis. 2022, 12, 975–981. [Google Scholar] [CrossRef]
- Reichmann, H.; Lees, A.; Rocha, J.-F.; Magalhães, D.; Soares-Da-Silva, P. Effectiveness and safety of opicapone in Parkinson’s disease patients with motor fluctuations: The OPTIPARK open-label study. Transl. Neurodegener. 2020, 9, 9. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Rocha, J.F.; Falcão, A.; Santos, A.; Pinto, R.; Nunes, T.; Soares-Da-Silva, P. Effect of opicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity and motor fluctuations in patients with Parkinson’s disease. Eur. J. Neurol. 2015, 22, 815-e56. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Ratziu, V.; Tolosa, E.; Oertel, W.H. Safety and tolerability of adjunctive tolcapone treatment in patients with early Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 944–948. [Google Scholar] [CrossRef]
- The Entacapone to Tolcapone Switch Study Investigators. Entacapone to tolcapone switch: Multicenter double-blind, randomized, active-controlled trial in advanced Parkinson’s disease. Mov. Disord. 2007, 22, 14–19. [Google Scholar] [CrossRef]
- Rocha, J.-F.; Falcão, A.; Santos, A.; Pinto, R.; Lopes, N.; Nunes, T.; Wright, L.C.; Vaz-Da-Silva, M.; Soares-Da-Silva, P. Effect of opicapone and entacapone upon levodopa pharmacokinetics during three daily levodopa administrations. Eur. J. Clin. Pharmacol. 2014, 70, 1059–1071. [Google Scholar] [CrossRef]
- Farrell, S.M.; Tunbridge, E.M.; Braeutigam, S.; Harrison, P.J. COMT Val158Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol. Psychiatry 2012, 71, 538–544. [Google Scholar] [CrossRef]
- Dingemanse, J.; Jorga, K.; Zürcher, G.; Fotteler, B.; Sedek, G.; Nielsen, T.; Van Brummelen, P. Multiple-dose clinical pharmacology of the catechol-O-methyl-transferase inhibitor tolcapone in elderly subjects. Eur. J. Clin. Pharmacol. 1996, 50, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Ferreira, J.; Rascol, O.; Poewe, W.; Rocha, J.-F.; McCrory, M.; Soares-da-Silva, P. Opicapone as Adjunct to Levodopa Therapy in Patients with Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial. JAMA Neurol. 2017, 74, 197–206. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Hu, M.T.M.; Brooks, D.J. Atypical parkinsonism in Afro-Caribbean and Indian origin immigrants to the UK. Mov. Disord. 2000, 15, 18–23. [Google Scholar] [CrossRef]
- Geller, S.; Wilhelm, O.; Wacker, J.; Hamm, A.; Hildebrandt, A. Associations of the COMT Val158Met polymorphism with working memory and intelligence—A review and meta-analysis. Intelligence 2017, 65, 75–92. [Google Scholar] [CrossRef]
- Correa, D.D.; Satagopan, J.; Cheung, K.; Arora, A.K.; Kryza-Lacombe, M.; Xu, Y.; Karimi, S.; Lyo, J.; DeAngelis, L.M.; Orlow, I. COMT, BDNF, and DTNBP1 polymorphisms and cognitive functions in patients with brain tumors. Neuro-Oncol. 2016, 18, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Kjærstad, H.L.; Støttrup, M.M.; Svendsen, A.M.; Demant, K.M.; Hoeffding, L.K.; Werge, T.M.; Burdick, K.E.; Domschke, K.; Carvalho, A.F.; et al. The catechol-O-methyltransferase (COMT) Val158Met genotype modulates working memory-related dorsolateral prefrontal response and performance in bipolar disorder. Bipolar Disord. 2017, 19, 214–224. [Google Scholar] [CrossRef]
- Cha, E.; Ahn, H.J.; Kang, W.; Jung, K.I.; Ohn, S.H.; Bashir, S.; Yoo, W.K. Correlations between COMT polymorphism and brain structure and cognition in elderly subjects: An observational study. Medicine 2022, 101, e29214. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Cedillo, T.; González-Figueroa, E.; Martínez-Rodríguez, N.; Fragosos, J.M.; Garrido-Acosta, O.; Vargas-Alarcón, G. Influence of COMT polymorphism in cognitive performance on dementia in community-dwelling elderly Mexican (SADEM study). Metab. Brain Dis. 2021, 36, 1223–1229. [Google Scholar] [CrossRef]
- Fiocco, A.J.; Lindquist, K.; Ferrell, R.; Li, R.; Simonsick, E.M.; Nalls, M.; Harris, T.B.; Yaffe, K. COMT genotype and cognitive function: An 8-year longitudinal study in white and black elders. Neurology 2010, 74, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Karacetin, G.; Topal, M.; Yuksel, M.E.; Eseroglu, T.; Akdeniz, G.B.; Demir, T.; Erkiran, M.; Dirican, A.; Bayoglu, B. COMT rs4680 and DRD2 rs6275 variants and their association with YMRS scores in children with early-onset bipolar disorder. Eur. J. Psychiatry 2023, 37, 8–14. [Google Scholar] [CrossRef]
- Devrimci-Ozguven, H.; Alıcı, Y.H.; Oz, M.D.; Suzen, H.; Kale, H.; Baskak, B. The role of COMT polymorphism in modulation of prefrontal activity during verbal fluency in bipolar disorder. Neurosci. Lett. 2020, 738, 135310. [Google Scholar] [CrossRef]
- Sagud, M.; Tudor, L.; Erjavec, G.N.; Perkovic, M.N.; Uzun, S.; Mimica, N.; Madzarac, Z.; Zivkovic, M.; Kozumplik, O.; Konjevod, M.; et al. Genotypic and Haplotypic Association of Catechol-O-Methyltransferase rs4680 and rs4818 Gene Polymorphisms with Particular Clinical Symptoms in Schizophrenia. Genes 2023, 14, 1358. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Niu, L.; Ma, Y.; Qiu, Y.; Li, S.; Guobule, N.; Cao, H.; Li, J. Polymorphisms of COMT and CREB1 are associated with treatment-resistant depression in a Chinese Han population. J. Neural Transm. 2022, 129, 85–93. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Yin, B.; Zhang, L. Pain in Parkinson’s disease associated with COMT gene polymorphisms. Behav. Neurol. 2014, 2014, 304203. [Google Scholar] [CrossRef][Green Version]
- Lin, C.-H.; Chaudhuri, K.R.; Fan, J.-Y.; Ko, C.-I.; Rizos, A.; Chang, C.-W.; Lin, H.-I.; Wu, Y.-R. Depression and Catechol-O-methyltransferase (COMT) genetic variants are associated with pain in Parkinson’s disease. Sci. Rep. 2017, 7, 6306. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Podlewska, A.; Lau, Y.H.; Gonde, C.; McIntosh, A.; Qamar, M.A.; O’Donoghue, S.; Larcombe, K.; Adeeko, M.; Gupta, A.; et al. Addressing the gap for racially diverse research involvement: The King’s Model for minority ethnic research participant recruitment. Public Health Pract. 2023, 6, 100426. [Google Scholar] [CrossRef]
- Popławska-Domaszewicz, K.; Falup-Pec, C. Urariu, and K. Chaudhuri, An Overview of a Stepped-care Approach to Modern Holistic and Subtype-driven Care for Parkinson’s Disease in the Clinic. Touchrev. Neurol. 2024, 20, 27. [Google Scholar]
- Allison, K.; Patel, D.; Kaur, R. Assessing Multiple Factors Affecting Minority Participation in Clinical Trials: Development of the Clinical Trials Participation Barriers Survey. Cureus 2022, 14, e24424. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Jutlla, K.; Raghavan, R.; Wilson, A.; Uddin, M.S.; Akroyd, C.; Patel, N.; Campbell-Morris, P.P.; Farooqi, A.T. Developing a toolkit for increasing the participation of black, Asian and minority ethnic communities in health and social care research. BMC Med. Res. Methodol. 2022, 22, 17. [Google Scholar] [CrossRef]
- Clark, L.T.; Watkins, L.; Piña, I.L.; Elmer, M.; Akinboboye, O.; Gorham, M.; Jamerson, B.; McCullough, C.; Pierre, C.; Polis, A.B.; et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr. Probl. Cardiol. 2019, 44, 148–172. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, D.G.; Macklin, E.A.; Hodgeman, K.; Lopez, G.; Pothier, L.; Callahan, K.F.; Lowell, J.; Chan, J.; Videnovic, A.; Lungu, C.; et al. Enrollment of Participants From Marginalized Racial and Ethnic Groups: A Comparative Assessment of the STEADY-PD III and SURE-PD3 Trials. Neurol. Clin. Pract. 2023, 13, e200113. [Google Scholar] [CrossRef] [PubMed]
- Poplawska-Domaszewicz, K.; Limbachiya, N.; Lau, Y.H.; Chaudhuri, K.R. Parkinson’s Kinetigraph for Wearable Sensor Detection of Clinically Unrecognized Early-Morning Akinesia in Parkinson’s Disease: A Case Report-Based Observation. Sensors 2024, 24, 3045. [Google Scholar] [CrossRef]

| COMT Inhibitor | Location Performed in | Trial Registration | Study Design | Sample Size | Ethnicity | Citation | |
|---|---|---|---|---|---|---|---|
| Caucasian | Non-Caucasian | ||||||
| Opicapone | Germany and UK | NCT02847442 | Open-label, single-arm, multicentre trial | 495 | 495 | 0 | [20] |
| Opicapone | Romania (three centres) and Ukraine (four centres). | EudraCT No. 2009-012897-12 | Randomized, multicentre, double-blind, placebo-controlled study in four parallel groups | 40 | 40 | 0 | [21] |
| Tolcapone | 92 sites in Europe, the United States, and Canada | NS | Multicentre, randomised, placebo-controlled, double-blind, parallel groups | 677 | 661 | 3 (Black), 5 (Asian), 6 (Hispanic), 2 (Other) | [22] |
| Tolcapone | 32 centres in Finland, France, Germany, Spain, Sweden, Switzerland, and the United States | NS | Randomized, double-blind, active-controlled study | 150 | 146 | 4 (Asian) | [23] |
| Opicapone and Entacapone | Paris, France | EudraCT No. 2011-000173-31 | Randomized, double-blind, gender-balanced, placebo-controlled study | 80 | 42 | 38 (No further specification) | [24] |
| Tolcapone | NS | NS | Randomized, double-blind | 67 | 59 | 5 (Indian), 2 (Chinese), 1 (African) | [25] |
| Tolcapone | NS | NS | Double-blind, randomised, placebo-controlled, | 48 | 47 | 1 (Oriental) | [26] |
| Opicapone | Belgium, United Kingdom, Israel, Estonia, Czech Republic, Russia; South Africa, Australia, South Korea, India; Argentina, and Chile | NCT01227655 | Randomised, double-blind, placebo-controlled and active-controlled trial | 427 | 294 | 102 (Asian), 10 (Other) | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poplawska-Domaszewicz, K.; Limbachiya, N.; Qamar, M.; Batzu, L.; Jones, S.; Sauerbier, A.; Rota, S.; Lau, Y.H.; Chaudhuri, K.R. Addressing the Ethnicity Gap in Catechol O-Methyl Transferase Inhibitor Trials in Parkinson’s Disease: A Review of Available Global Data. J. Pers. Med. 2024, 14, 939. https://doi.org/10.3390/jpm14090939
Poplawska-Domaszewicz K, Limbachiya N, Qamar M, Batzu L, Jones S, Sauerbier A, Rota S, Lau YH, Chaudhuri KR. Addressing the Ethnicity Gap in Catechol O-Methyl Transferase Inhibitor Trials in Parkinson’s Disease: A Review of Available Global Data. Journal of Personalized Medicine. 2024; 14(9):939. https://doi.org/10.3390/jpm14090939
Chicago/Turabian StylePoplawska-Domaszewicz, Karolina, Naomi Limbachiya, Mubasher Qamar, Lucia Batzu, Shelley Jones, Anna Sauerbier, Silvia Rota, Yue Hui Lau, and K. Ray Chaudhuri. 2024. "Addressing the Ethnicity Gap in Catechol O-Methyl Transferase Inhibitor Trials in Parkinson’s Disease: A Review of Available Global Data" Journal of Personalized Medicine 14, no. 9: 939. https://doi.org/10.3390/jpm14090939
APA StylePoplawska-Domaszewicz, K., Limbachiya, N., Qamar, M., Batzu, L., Jones, S., Sauerbier, A., Rota, S., Lau, Y. H., & Chaudhuri, K. R. (2024). Addressing the Ethnicity Gap in Catechol O-Methyl Transferase Inhibitor Trials in Parkinson’s Disease: A Review of Available Global Data. Journal of Personalized Medicine, 14(9), 939. https://doi.org/10.3390/jpm14090939





_Chaudhuri_also_Ray-Chaudhuri.png)

