Treatment Outcomes after Postoperative Radiotherapy in Triple-Negative Breast Cancer: Multi-Institutional Retrospective Study (KROG 17-05)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Characteristics
3.2. Treatments
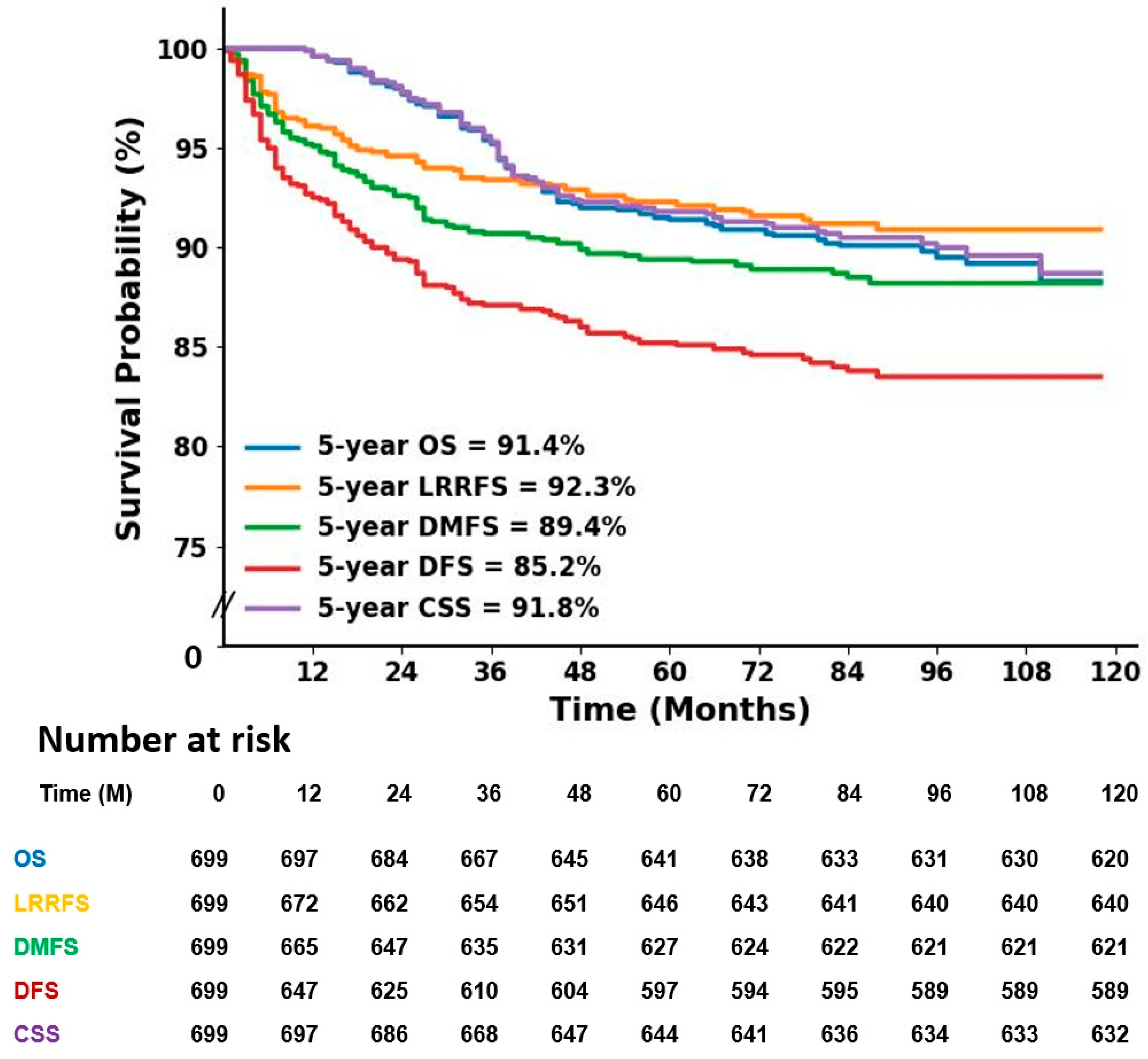
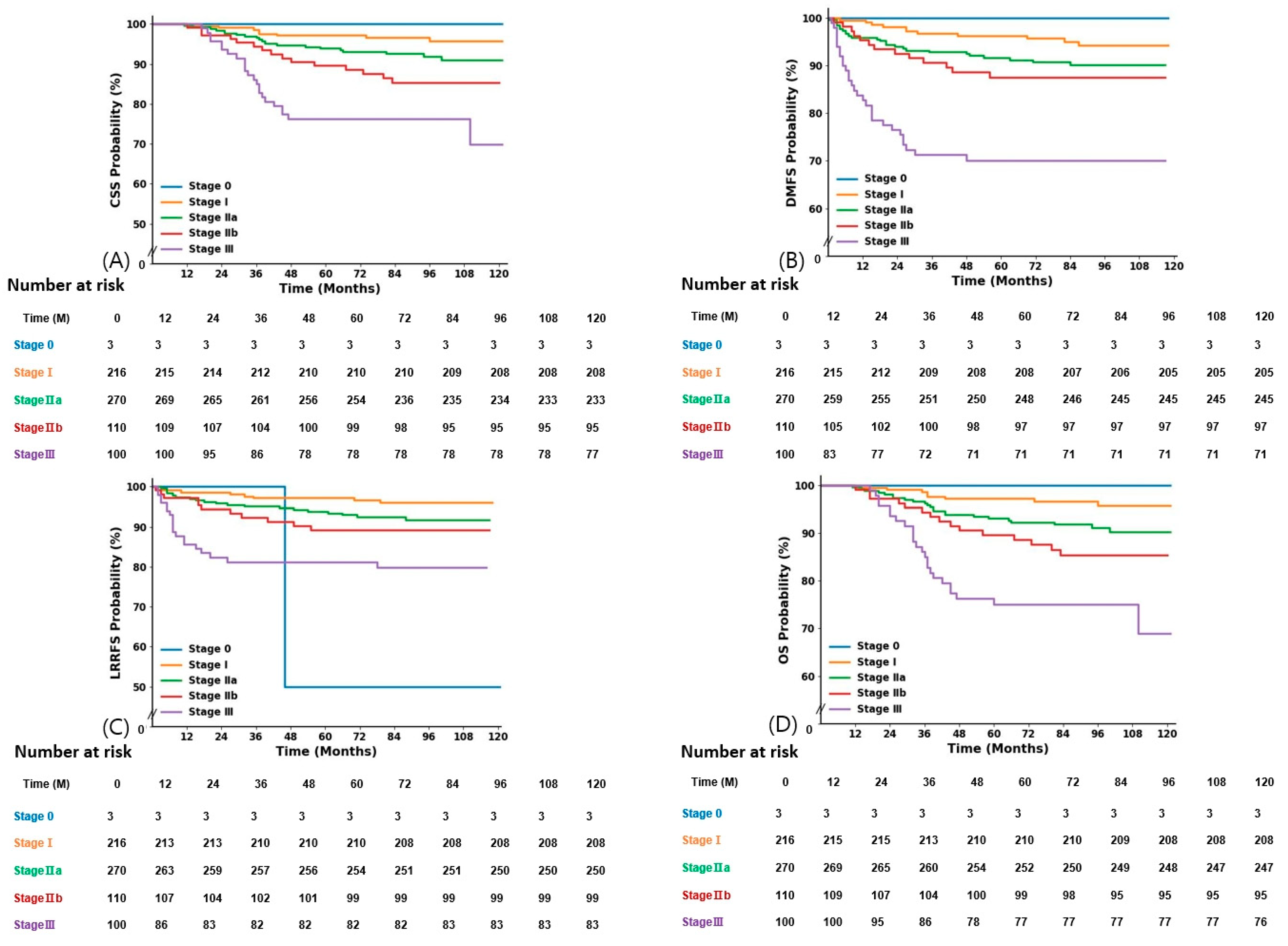
3.3. Outcomes and Prognostic Factors
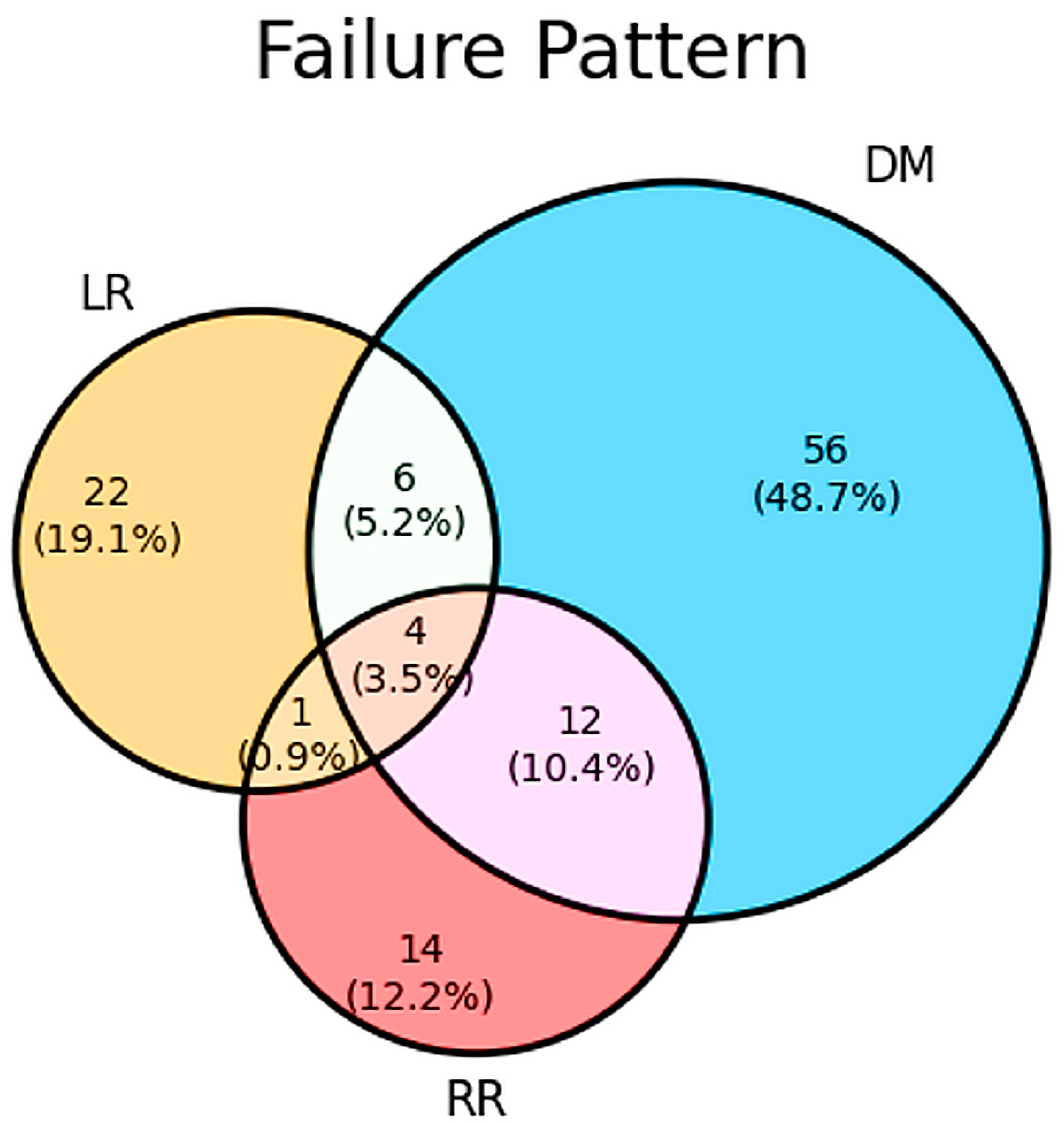
3.4. Patterns of Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Nanda, G.; Lal, P.; Mishra, A.; Agarwal, A.; Agrawal, V.; Krishnani, N. Outcomes of Triple-Negative Breast Cancers (TNBC) Compared with Non-TNBC: Does the Survival Vary for All Stages? World J. Surg. 2016, 40, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.T.; Gouveia, M.C.; Neto, F.L.; Testa, L.; Hoff, P.M.; de Azambuja, E.; Bonadio, R.C. Long-term outcomes of neoadjuvant immunotherapy plus chemotherapy in patients with early-stage triple-negative breast cancer: An extracted individual patient data and trial-level meta-analysis. Br. J. Cancer 2024, 130, 242–250. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Triple-negative breast cancer therapeutic resistance: Where is the Achilles’ heel? Cancer Lett. 2021, 497, 100–111. [Google Scholar] [CrossRef]
- Marra, A.; Curigliano, G. Adjuvant and Neoadjuvant Treatment of Triple-Negative Breast Cancer with Chemotherapy. Cancer J. 2021, 27, 41–49. [Google Scholar] [CrossRef]
- Sharma, P. Biology and Management of Patients With Triple-Negative Breast Cancer. Oncologist 2016, 21, 1050–1062. [Google Scholar]
- Wang, S.E.; Sun, Y.D.; Zhao, S.J.; Wei, F.; Yang, G. Breast conserving surgery (BCS) with adjuvant radiation therapy showed improved prognosis compared with mastectomy for early staged triple negative breast cancer patients Running title: BCS had better prognosis than mastectomy for early TNBC patients. Math. Biosci. Eng. 2019, 17, 92–104. [Google Scholar] [CrossRef]
- Wang, J.; Xie, X.; Wang, X.; Tang, J.; Pan, Q.; Zhang, Y.; Di, M. Locoregional and distant recurrences after breast conserving therapy in patients with triple-negative breast cancer: A meta-analysis. Surg. Oncol. 2013, 22, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, H.J.; Shin, K.H.; Kim, J.H.; Choi, D.H.; Park, W.; Ahn, S.D.; Kim, S.S.; Kim, D.Y.; Kim, T.H.; et al. Breast Conservation Therapy versus Mastectomy in Patients with T1-2N1 Triple-Negative Breast Cancer: Pooled Analysis of KROG 14–18 and 14–23. Cancer Res. Treat. 2018, 50, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, M.A.; Murray, L.J.; Brand, J.S.; Bhoo-Pathy, N. The value of adjuvant radiotherapy on survival and recurrence in triple-negative breast cancer: A systematic review and meta-analysis of 5507 patients. Cancer Treat. Rev. 2016, 47, 12–21. [Google Scholar]
- Dixit, A.; Frampton, C.; Davey, V.; Robinson, B.; James, M. Radiation treatment in early stage triple-negative breast cancer in New Zealand: A national database study. J. Med. Imaging Radiat. Oncol. 2019, 63, 698–706. [Google Scholar] [PubMed]
- Moran, M.S. Radiation therapy in the locoregional treatment of triple-negative breast cancer. Lancet Oncol. 2015, 16, e113–e122. [Google Scholar] [CrossRef]
- Wang, J.; Shi, M.; Ling, R.; Xia, Y.; Luo, S.; Fu, X.; Xiao, F.; Li, J.; Long, X.; Wang, J.; et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: A prospective randomized controlled multi-center trial. Radiother. Oncol. 2011, 100, 200–204. [Google Scholar] [CrossRef]
- Ren, Y.X.; Hao, S.; Jin, X.; Ye, F.G.; Gong, Y.; Jiang, Y.Z.; Shao, Z.M. Effects of adjuvant chemotherapy in T1N0M0 triple-negative breast cancer. Breast 2019, 43, 97–104. [Google Scholar]
- Sharma, P. Update on the Treatment of Early-Stage Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2018, 19, 22. [Google Scholar]
- van Roozendaal, L.M.; Smit, L.H.M.; Duijsens, G.; de Vries, B.; Siesling, S.; Lobbes, M.B.I.; de Boer, M.; de Wilt, J.H.W.; Smidt, M.L. Risk of regional recurrence in triple-negative breast cancer patients: A Dutch cohort study. Breast Cancer Res. Treat. 2016, 156, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.M.; Olopade, O.I. Epidemiology of Triple-Negative Breast Cancer: A Review. Cancer J. 2021, 27, 8–16. [Google Scholar] [CrossRef]
- Leon-Ferre, R.A.; Polley, M.Y.; Liu, H.; Gilbert, J.A.; Cafourek, V.; Hillman, D.W.; Elkhanany, A.; Akinhanmi, M.; Lilyquist, J.; Thomas, A.; et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 167, 89–99. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Liu, Y.; Liu, G.; Dong, G. Ki67 as a predictor of poor prognosis in patients with triple-negative breast cancer. Oncol. Lett. 2015, 9, 149–152. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Huang, B.; Wang, Y.; Ji, L.; Wu, J.; Di, G.; Liu, G.; Yu, K.; Shao, Z.; et al. The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Sci. Rep. 2020, 10, 225. [Google Scholar]
- Selz, J.; Stevens, D.; Jouanneau, L.; Labib, A.; Le Scodan, R. Prognostic value of molecular subtypes, ki67 expression and impact of postmastectomy radiation therapy in breast cancer patients with negative lymph nodes after mastectomy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1123–1132. [Google Scholar] [CrossRef]
- van den Ende, N.S.; Nguyen, A.H.; Jager, A.; Kok, M.; Debets, R.; van Deurzen, C.H.M. Triple-Negative Breast Cancer and Predictive Markers of Response to Neoadjuvant Chemotherapy: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2969. [Google Scholar] [CrossRef]
- Ahn, K.J.; Park, J.; Choi, Y. Lymphovascular invasion as a negative prognostic factor for triple-negative breast cancer after surgery. Radiat. Oncol. J. 2017, 35, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, S.; Demian, G.A.; El-Sherify, M.; Eissa, H.; Aziz, M.; Abuzallouf, S. Triple Negative Breast Cancer: 10-Year Survival Update of The Applied Treatment Strategy in Kuwait. Gulf J. Oncol. 2019, 1, 53–59. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Tricarico, C.; Gabani, P.; Weiner, A.A.; Altman, M.B.; Ochoa, L.L.; Thomas, M.A.; Margenthaler, J.A.; Sanati, S.; Peterson, L.L.; et al. Predictors of Distant Metastases in Triple-Negative Breast Cancer without Pathologic Complete Response after Neoadjuvant Chemotherapy. J. Natl. Compr. Cancer Netw. 2020, 18, 288–296. [Google Scholar]
- Radosa, J.C.; Eaton, A.; Stempel, M.; Khander, A.; Liedtke, C.; Solomayer, E.F.; Karsten, M.; Pilewskie, M.; Morrow, M.; King, T.A. Evaluation of Local and Distant Recurrence Patterns in Patients with Triple-Negative Breast Cancer According to Age. Ann. Surg. Oncol. 2017, 24, 698–704. [Google Scholar] [PubMed]
- Chow, R.; Hasan, S.; Choi, J.I.; Fox, J.; Chhabra, A.M.; Marshall, D.C.; Bakst, R.L.; Simone, C.B., 2nd. Effect of treatment interruptions on overall survival in patients with triple-negative breast cancer. J. Natl. Cancer Inst. 2023, 115, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Polley, M.C.; Leon-Ferre, R.A.; Leung, S.; Cheng, A.; Gao, D.; Sinnwell, J.; Liu, H.; Hillman, D.W.; Eyman-Casey, A.; Gilbert, J.A.; et al. A clinical calculator to predict disease outcomes in women with triple-negative breast cancer. Breast Cancer Res. Treat. 2021, 185, 557–566. [Google Scholar] [PubMed]
- Chen, J.; Jiang, P.; Wang, H.J.; Zhang, J.Y.; Xu, Y.; Guo, M.H.; Zhang, B.; Tang, C.Y.; Cao, H.Y.; Wang, S. The efficacy of molecular subtyping in predicting postoperative recurrence in breast-conserving therapy: A 15-study meta-analysis. World J. Surg. Oncol. 2014, 12, 212. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Michaels, E.; Chen, N.; Nanda, R. The Role of Immunotherapy in Triple-Negative Breast Cancer (TNBC). Clin. Breast Cancer 2024, 24, 263–270. [Google Scholar]
| Characteristics | Number | (%) | |
|---|---|---|---|
| Age (year, median, range) | 49 | (range: 24–80) | |
| Pathology | |||
| IDC | 622 | (88.9) | |
| Metaplastic | 23 | (3.3) | |
| medullary | 13 | (1.9) | |
| Apocrine | 11 | (1.6) | |
| DCIS | 10 | (1.4) | |
| microinvasive | 7 | (1.0) | |
| Others | 13 | (1.9) | |
| Histologic grade | |||
| 1 | 10 | (1.4) | |
| 2 | 103 | (14.7) | |
| 3 | 538 | (77.0) | |
| unknown | 48 | (6.9) | |
| Lympho-vascular invasion | |||
| positive | 240 | (34.3) | |
| negative | 435 | (62.3) | |
| unknown | 24 | (3.4) | |
| Extracapsular extension | |||
| positive | 49 | (7.0) | |
| negative | 557 | (79.7) | |
| unknown | 93 | (13.3) | |
| Ki-67 | |||
| negative | 3 | (0.4) | |
| ≤5% | 131 | (18.7) | |
| 6–25% | 211 | (30.2) | |
| >25% | 198 | (28.3) | |
| unknown | 156 | (22.3) | |
| T stage | |||
| in situ | 3 | (0.4) | |
| 1 | 268 | (38.3) | |
| 2 | 355 | (50.8) | |
| 3 | 61 | (8.7) | |
| unknown | 12 | (17.1) | |
| N stage | |||
| positive | 254 | (36.3) | |
| negative | 443 | (63.4) | |
| unknown | 2 | (0.3) | |
| Stage | |||
| 0 | 3 | (0.4) | |
| I | 216 | (30.9) | |
| IIA | 270 | (38.6) | |
| IIB | 110 | (15.7) | |
| III | 100 | (14.3) | |
| Characteristics | Number | (%) | |
|---|---|---|---|
| Neoadjuvant chemotherapy | |||
| Yes | 130 | (18.6) | |
| No | 569 | (82.4) | |
| Neoadjuvant chemotherapy regimen | |||
| AC | 92 | (70.8) | |
| AC-T | 26 | (20.0) | |
| other | 12 | (9.2) | |
| Surgery | |||
| BCS only | 1 | (0.1) | |
| BCS and SLNB | 329 | (47.1) | |
| BCS and ALND | 304 | (43.5) | |
| Mastectomy and SLNB | 1 | (0.1) | |
| Mastectomy and ALNB | 64 | (9.2) | |
| Radiotherapy dose (mainly) | |||
| Breast/chest wall | 45–50.4 Gy in 25–28 fractions | ||
| Tumor bed boost | 10–16 Gy in 5–8 fractions | ||
| SCLN/IMN | 45–50 Gy in 25 fractions | ||
| Adjuvant chemotherapy | |||
| Yes | 596 | (85.3) | |
| No | 103 | (14.7) | |
| Adjuvant regimen | |||
| FAC | 259 | (43.5) | |
| AC | 118 | (19.8) | |
| AC-T | 68 | (11.4) | |
| AC-D | 46 | (7.7) | |
| CMF | 44 | (7.4) | |
| DA | 35 | (5.9) | |
| other | 26 | (4.3) | |
| Characteristics | No. of Patient | OS | LRRFS | DMFS | DFS | CSS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 yr (%) | p | 5 yr (%) | p | 5 yr (%) | p | 5 yr (%) | p | 5 yr (%) | p | ||
| Age | 0.16 | NS | 0.01 | 0.048 | NS | ||||||
| 50 or younger | 404 | 90.5 | 91.3 | 87.1 | 82.6 | 90.8 | |||||
| Older than 50 | 295 | 92.9 | 93.6 | 92.7 | 88.8 | 93.3 | |||||
| T stage | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| T1 | 268 | 96.6 | 96.6 | 95.0 | 93.2 | 96.6 | |||||
| T2 | 355 | 90.7 | 91.8 | 88.1 | 83.6 | 90.9 | |||||
| T3 | 61 | 75.1 | 77.1 | 71.0 | 59.3 | 75.1 | |||||
| N stage | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Negative | 443 | 94.9 | 94.9 | 93.3 | 90.1 | 95.1 | |||||
| Positive | 254 | 85.5 | 87.6 | 82.5 | 76.5 | 85.8 | |||||
| Grade | 0.01 | NS | 0.051 | 0.091 | 0.014 | ||||||
| Low | 113 | 96.3 | 96.3 | 93.6 | 90.0 | 96.3 | |||||
| High | 538 | 92.7 | 91.9 | 88.2 | 84.3 | 90.4 | |||||
| LVI | 0.04 | 0.01 | <0.001 | <0.001 | 0.046 | ||||||
| Negative | 435 | 93.2 | 94.5 | 92.7 | 89.4 | 93.6 | |||||
| Positive | 240 | 87.6 | 88.8 | 82.9 | 77.6 | 88.1 | |||||
| ECE | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Negative | 557 | 92.4 | 93.3 | 91.9 | 88.1 | 92.6 | |||||
| Positive | 49 | 74.4 | 77.1 | 60.3 | 50.1 | 74.4 | |||||
| Ki-67 | 0.036 | 0.016 | 0.041 | 0.002 | 0.027 | ||||||
| ≤5% | 134 | 94.7 | 97.7 | 93.8 | 93.1 | 95.4 | |||||
| >5% | 410 | 88.2 | 89.1 | 85.4 | 80.3 | 88.7 | |||||
| Characteristics | No. of Patient | OS | LRRFS | DMFS | DFS | CSS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| T stage | 0.003 | 0.001 | 0.014 | 0.000 | 0.006 | ||||||
| T1 | 268 | 1 | 1 | 1 | 1 | 1 | |||||
| T2 | 355 | 1.856 (1.213–2.84) | 2.203 (1.378–3.523) | 1.649 (1.106–2.457) | 1.991 (1.403–2.825) | 1.839 (1.194–2.832) | |||||
| T3 | 61 | ||||||||||
| N stage | 0.004 | 0.026 | 0.002 | 0.001 | 0.001 | ||||||
| Negative | 443 | 1 2.551 (1.460–4.459) | 1 1.966 (1.085–3.564) | 1 2.287 (1.347–3.884) | 1 2.132 (1.360–3.341) | 1 2.606 (1.474–4.605) | |||||
| Positive | 254 | ||||||||||
| LVI | 0.044 | 0.05 | |||||||||
| Negative | 435 | 1 | 0.600 (0.360–1.000) | ||||||||
| Positive | 240 | 1.227 (1.006–1.496) | 1 | ||||||||
| Ki-67 | 0.015 | 0.019 | |||||||||
| ≤5% | 134 | 1 | 1 | ||||||||
| >5% | 410 | 3.148 (1.246–7.955) | 2.188 (1.140–4.197) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Byun, S.J.; Kim, M.; Shin, K.H.; Kim, D.Y.; Lee, H.B.; Kim, T.H.; Kim, Y.J.; Kim, Y.B.; Chang, J.S.; et al. Treatment Outcomes after Postoperative Radiotherapy in Triple-Negative Breast Cancer: Multi-Institutional Retrospective Study (KROG 17-05). J. Pers. Med. 2024, 14, 941. https://doi.org/10.3390/jpm14090941
Kim JH, Byun SJ, Kim M, Shin KH, Kim DY, Lee HB, Kim TH, Kim YJ, Kim YB, Chang JS, et al. Treatment Outcomes after Postoperative Radiotherapy in Triple-Negative Breast Cancer: Multi-Institutional Retrospective Study (KROG 17-05). Journal of Personalized Medicine. 2024; 14(9):941. https://doi.org/10.3390/jpm14090941
Chicago/Turabian StyleKim, Jin Hee, Sang Jun Byun, Myeongsoo Kim, Kyung Hwan Shin, Dong Yun Kim, Han Byoel Lee, Tae Hyun Kim, Yeon Joo Kim, Yong Bae Kim, Jee Suk Chang, and et al. 2024. "Treatment Outcomes after Postoperative Radiotherapy in Triple-Negative Breast Cancer: Multi-Institutional Retrospective Study (KROG 17-05)" Journal of Personalized Medicine 14, no. 9: 941. https://doi.org/10.3390/jpm14090941
APA StyleKim, J. H., Byun, S. J., Kim, M., Shin, K. H., Kim, D. Y., Lee, H. B., Kim, T. H., Kim, Y. J., Kim, Y. B., Chang, J. S., Kim, K., & Lee, S. Y. (2024). Treatment Outcomes after Postoperative Radiotherapy in Triple-Negative Breast Cancer: Multi-Institutional Retrospective Study (KROG 17-05). Journal of Personalized Medicine, 14(9), 941. https://doi.org/10.3390/jpm14090941







