Whole-Exome Sequencing Reveals Novel Candidate Driver Mutations and Potential Druggable Mutations in Patients with High-Risk Neuroblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. DNA Extraction and Whole-Exome Sequencing
2.3. Variant Calling and Annotation
2.4. Variant Refinement
2.5. Data Analysis and Visualization of Somatic Mutations
2.6. Tumor Mutational Burden
2.7. Mutational Signature Analysis
2.8. Survival Analysis
2.9. Cancer Driver Gene Identification
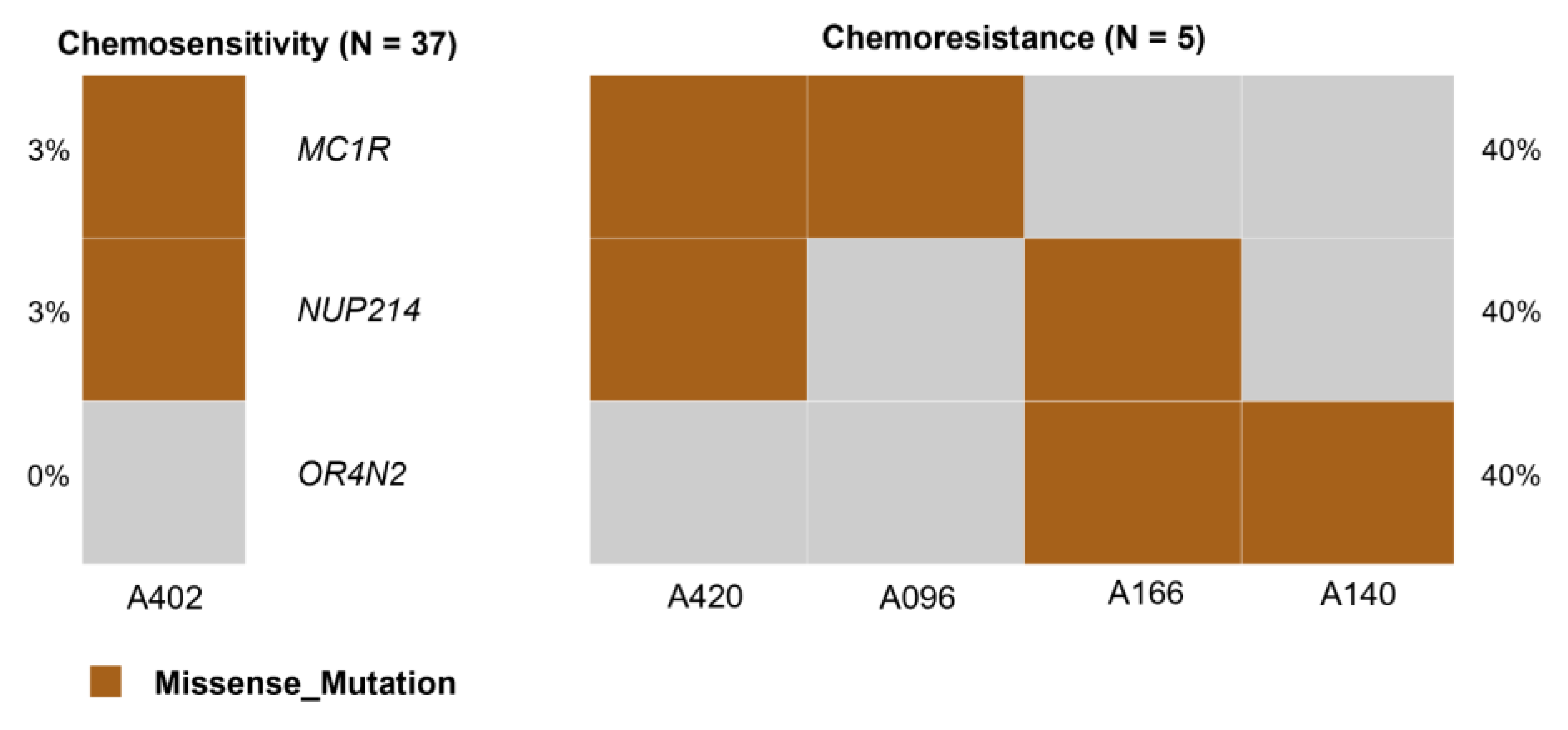
2.10. Identification of Chemoresistance Markers
2.11. GO and Pathway Enrichment Analysis
2.12. PPI Network and Identification of Hub Genes
2.13. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Whole-Exome Sequencing Statistics
3.3. Mutation Landscape of Patients with NB
3.4. Detection of Co-Occurring Mutated Genes
3.5. Distribution of Mutations in the Top Six Mutated Genes
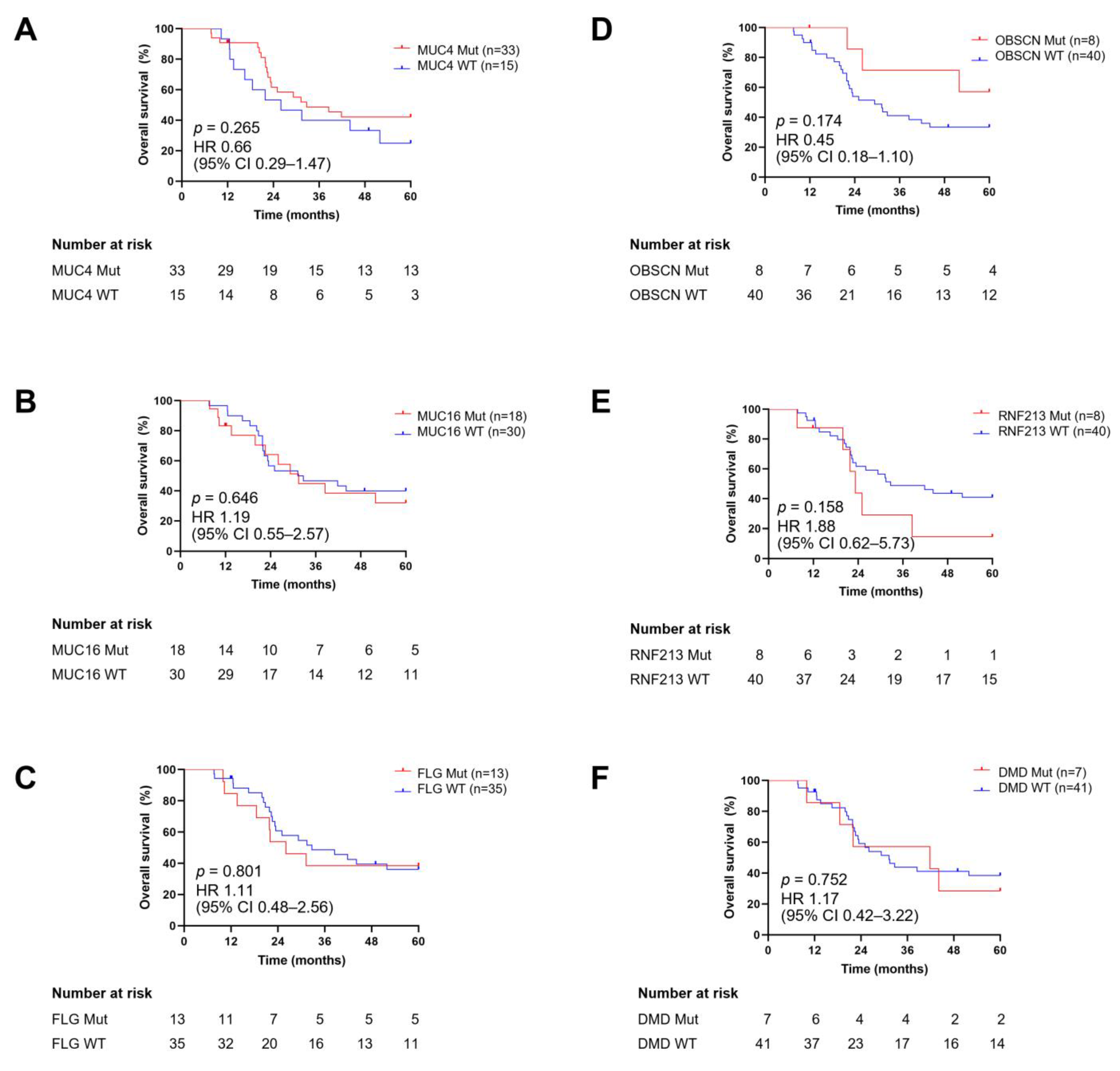
3.6. Correlation of Mutational Status of Frequently Mutated Genes with Clinicopathological Parameters and Their Prognostic Impact
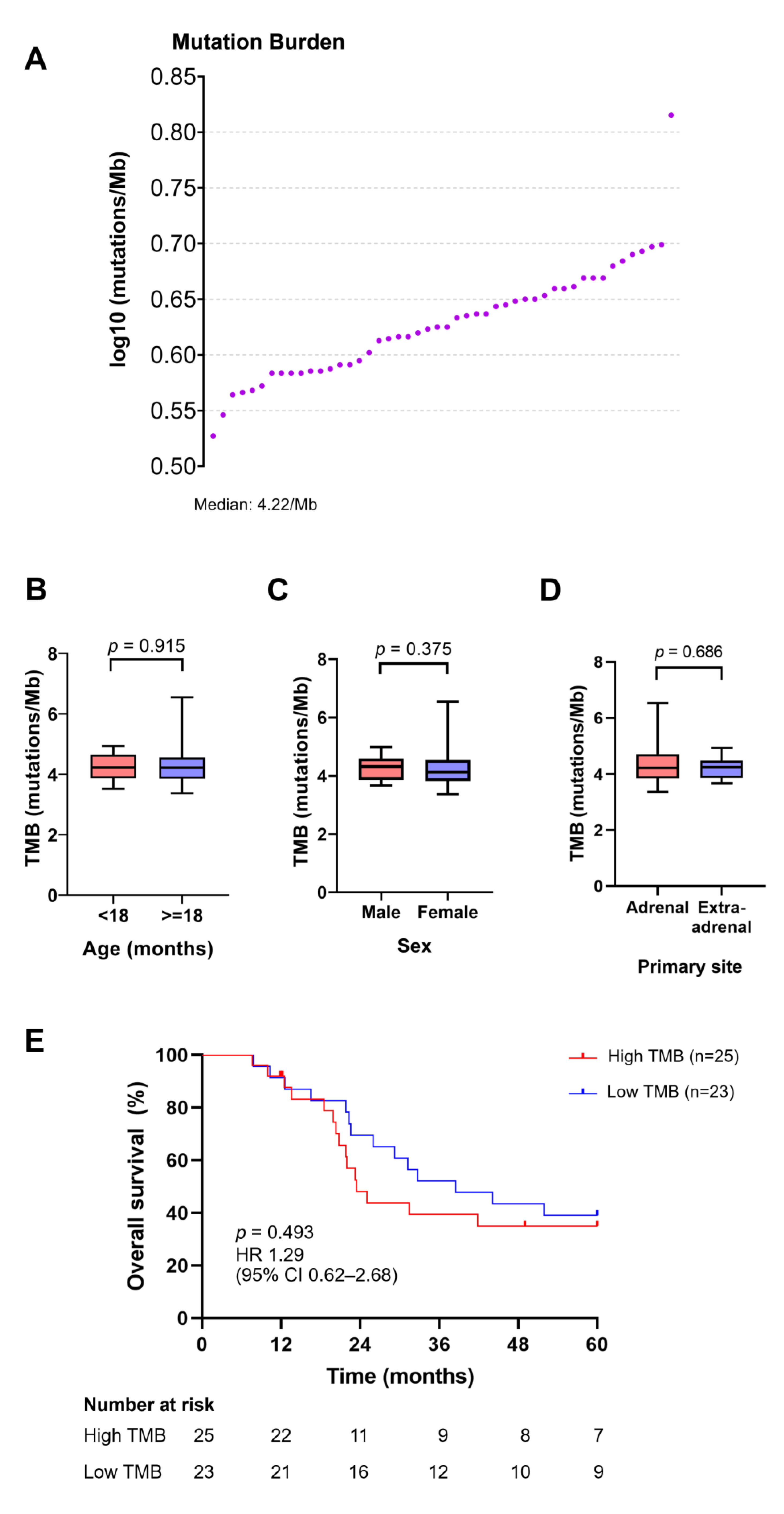
3.7. TMB Distribution and Its Association with Age, Sex, Primary Site of Tumors, and Survival Outcomes
3.8. Mutational Signature of NB
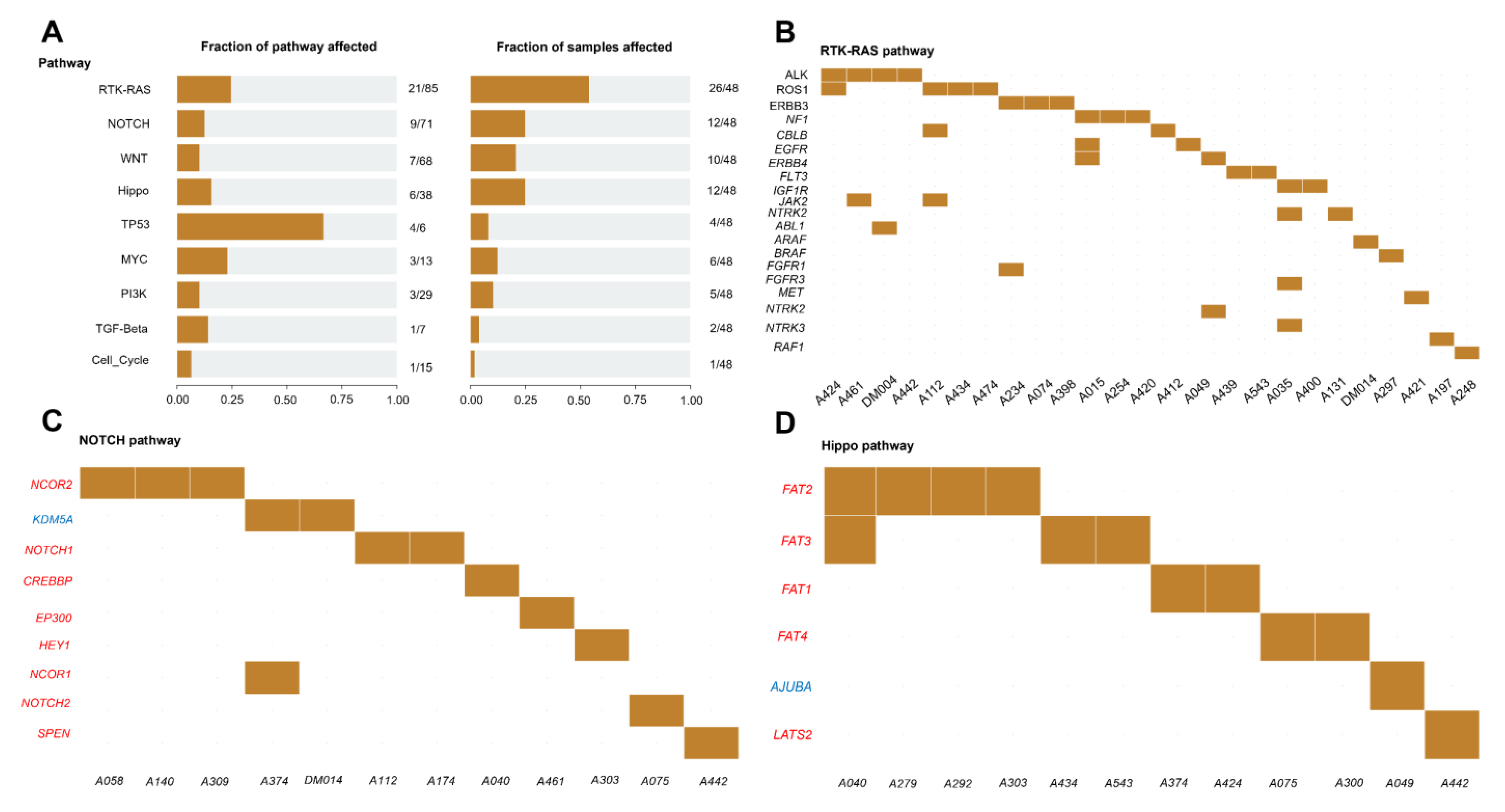
3.9. Enrichment of Mutated Genes in Oncogenic Signaling Pathways
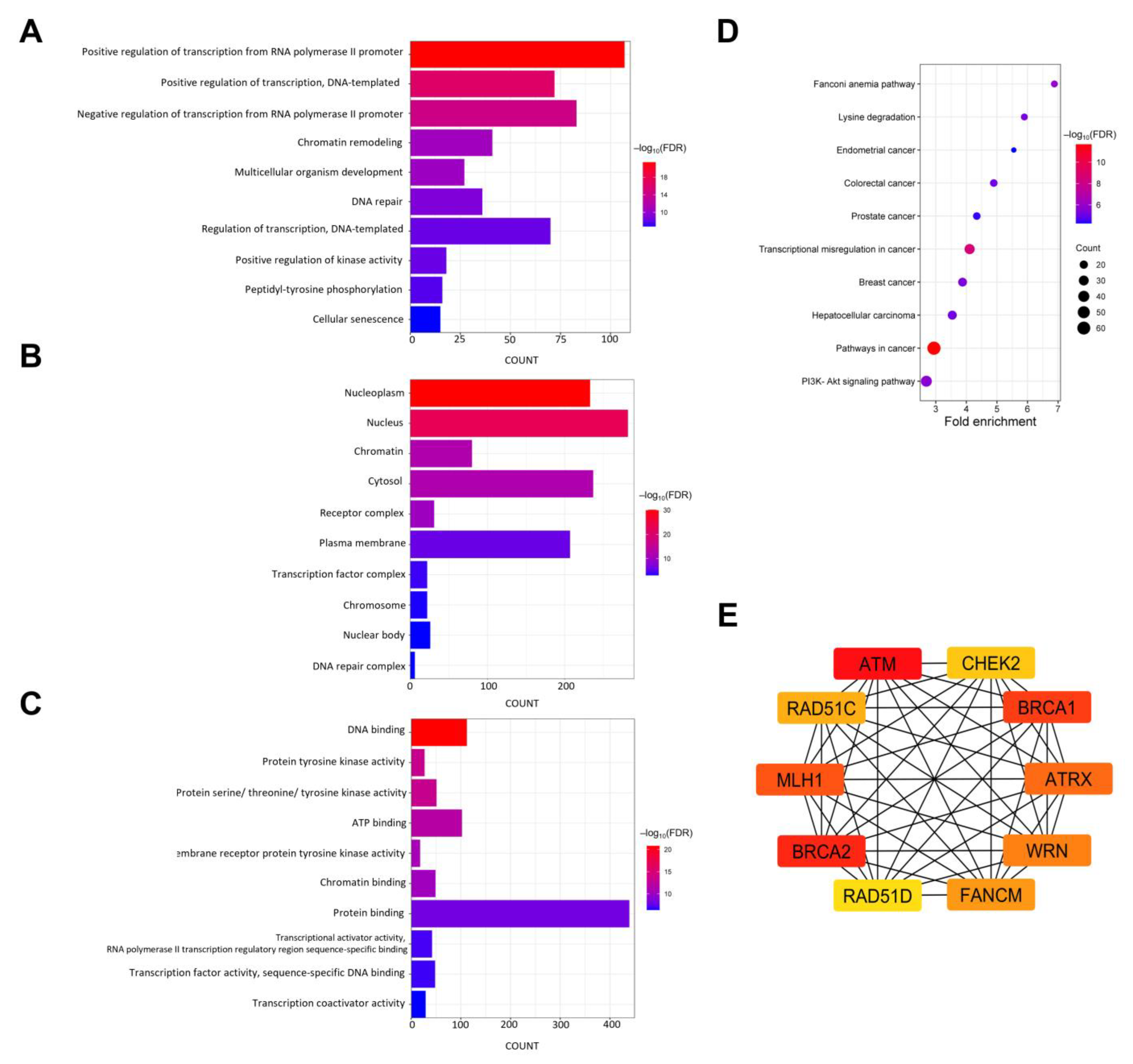
3.10. Functional Enrichment Analysis of Mutated Genes in NB
3.11. PPI Network and Hub Gene Identification
3.12. Identification of Cancer Driver Genes and Biomarkers for Therapeutic Target and Drug Response
3.13. Identification of Biomarkers Associated with Chemoresistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Stanke, J.; Lahti, J.M. The connections between neural crest development and neuroblastoma. Curr. Top. Dev. Biol. 2011, 94, 77–127. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, P. Epidemiology of childhood cancer. Cancer Treat. Rev. 2010, 36, 277–285. [Google Scholar] [CrossRef]
- Bidwell, S.S.; Peterson, C.C.; Demanelis, K.; Zarins, K.R.; Meza, R.; Sriplung, H.; Wiangnon, S.; Chotsampancharoen, T.; Chitapanarux, I.; Pongnikorn, D.; et al. Childhood cancer incidence and survival in Thailand: A comprehensive population-based registry analysis, 1990–2011. Pediatr. Blood Cancer 2019, 66, e27428. [Google Scholar] [CrossRef]
- Wiangnon, S.; Veerakul, G.; Nuchprayoon, I.; Seksarn, P.; Hongeng, S.; Krutvecho, T.; Sripaiboonkij, N. Childhood cancer incidence and survival 2003–2005, Thailand: Study from the Thai Pediatric Oncology Group. Asian Pac. J. Cancer Prev. 2011, 12, 2215–2220. [Google Scholar]
- Wongmeerit, P.; Suwanrungruang, K.; Jetsrisuparb, A.; Komvilaisak, P.; Wiangnon, S. Trends in Survival of Childhood Cancers in a University Hospital, Northeast Thailand, 19932012. Asian Pac. J. Cancer Prev. 2016, 17, 3515–3519. [Google Scholar]
- Suwannaying, K.; Monsereenusorn, C.; Rujkijyanont, P.; Techavichit, P.; Phuakpet, K.; Pongphitcha, P.; Chainansamit, S.O.; Chotsampancharoen, T.; Winaichatsak, A.; Traivaree, C.; et al. Treatment outcomes among high-risk neuroblastoma patients receiving non-immunotherapy regimen: Multicenter study on behalf of the Thai Pediatric Oncology Group. Pediatr. Blood Cancer 2022, 69, e29757. [Google Scholar] [CrossRef]
- Zhang, D.; Kaweme, N.M.; Duan, P.; Dong, Y.; Yuan, X. Upfront Treatment of Pediatric High-Risk Neuroblastoma With Chemotherapy, Surgery, and Radiotherapy Combination: The CCCG-NB-2014 Protocol. Front. Oncol. 2021, 11, 745794. [Google Scholar] [CrossRef]
- Wienke, J.; Dierselhuis, M.P.; Tytgat, G.A.M.; Künkele, A.; Nierkens, S.; Molenaar, J.J. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur. J. Cancer 2021, 144, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zeng, C.; Li, Z.; Wang, J.; Sun, F.; Huang, J.; Lu, S.; Zhu, J.; Zhang, Y.; Sun, X.; et al. Investigation of chemoresistance to first-line chemotherapy and its possible association with autophagy in high-risk neuroblastoma. Front. Oncol. 2022, 12, 1019106. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Berthold, F.; Borkhardt, A.; Kremens, B.; De Carolis, B.; Hero, B. Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: Results of German trials. Pediatr. Blood Cancer 2011, 56, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Cheng, Y.; Hu, A.; Li, D.; Wang, X.; Guo, Y.; Zhou, Y.; Chen, G.; Bao, B.; et al. Long-Term Survival of Neuroblastoma Patients Receiving Surgery, Chemotherapy, and Radiotherapy: A Propensity Score Matching Study. J. Clin. Med. 2023, 12, 754. [Google Scholar] [CrossRef]
- Liang, W.H.; Federico, S.M.; London, W.B.; Naranjo, A.; Irwin, M.S.; Volchenboum, S.L.; Cohn, S.L. Tailoring Therapy for Children With Neuroblastoma on the Basis of Risk Group Classification: Past, Present, and Future. JCO Clin. Cancer Inform. 2020, 4, 895–905. [Google Scholar] [CrossRef]
- Sokol, E.; Desai, A.V.; Applebaum, M.A.; Valteau-Couanet, D.; Park, J.R.; Pearson, A.D.J.; Schleiermacher, G.; Irwin, M.S.; Hogarty, M.; Naranjo, A.; et al. Age, Diagnostic Category, Tumor Grade, and Mitosis-Karyorrhexis Index Are Independently Prognostic in Neuroblastoma: An INRG Project. J. Clin. Oncol. 2020, 38, 1906–1918. [Google Scholar] [CrossRef]
- Ackermann, S.; Cartolano, M.; Hero, B.; Welte, A.; Kahlert, Y.; Roderwieser, A.; Bartenhagen, C.; Walter, E.; Gecht, J.; Kerschke, L.; et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science 2018, 362, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Oberthuer, A.; Hero, B.; Berthold, F.; Juraeva, D.; Faldum, A.; Kahlert, Y.; Asgharzadeh, S.; Seeger, R.; Scaruffi, P.; Tonini, G.P.; et al. Prognostic impact of gene expression-based classification for neuroblastoma. J. Clin. Oncol. 2010, 28, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Nakagawara, A. Acceleration or Brakes: Which Is Rational for Cell Cycle-Targeting Neuroblastoma Therapy? Biomolecules 2021, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef]
- Brady, S.W.; Liu, Y.; Ma, X.; Gout, A.M.; Hagiwara, K.; Zhou, X.; Wang, J.; Macias, M.; Chen, X.; Easton, J.; et al. Pan-neuroblastoma analysis reveals age- and signature-associated driver alterations. Nat. Commun. 2020, 11, 5183. [Google Scholar] [CrossRef]
- Cheung, N.K.; Zhang, J.; Lu, C.; Parker, M.; Bahrami, A.; Tickoo, S.K.; Heguy, A.; Pappo, A.S.; Federico, S.; Dalton, J.; et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA 2012, 307, 1062–1071. [Google Scholar] [CrossRef]
- Peifer, M.; Hertwig, F.; Roels, F.; Dreidax, D.; Gartlgruber, M.; Menon, R.; Krämer, A.; Roncaioli, J.L.; Sand, F.; Heuckmann, J.M.; et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 2015, 526, 700–704. [Google Scholar] [CrossRef]
- Sausen, M.; Leary, R.J.; Jones, S.; Wu, J.; Reynolds, C.P.; Liu, X.; Blackford, A.; Parmigiani, G.; Diaz, L.A., Jr.; Papadopoulos, N.; et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat. Genet. 2013, 45, 12–17. [Google Scholar] [CrossRef]
- Valentijn, L.J.; Koster, J.; Zwijnenburg, D.A.; Hasselt, N.E.; van Sluis, P.; Volckmann, R.; van Noesel, M.M.; George, R.E.; Tytgat, G.A.; Molenaar, J.J.; et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat. Genet. 2015, 47, 1411–1414. [Google Scholar] [CrossRef]
- Tweddle, D.A.; Pearson, A.D.; Haber, M.; Norris, M.D.; Xue, C.; Flemming, C.; Lunec, J. The p53 pathway and its inactivation in neuroblastoma. Cancer Lett. 2003, 197, 93–98. [Google Scholar] [CrossRef]
- National Protocol for the Treatment of Childhood Cancers 2018. Available online: https://www.nhso.go.th/storage/files/shares/PDF/Protocol_UC03.pdf (accessed on 1 June 2024).
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 26 August 2024).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.11–11.10.33. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Broad Institute. Data Pre-Processing for Variant Discovery. Available online: https://gatk.broadinstitute.org/hc/en-us/articles/360035535912-Data-pre-processing-for-variant-discovery (accessed on 4 March 2024).
- Mose, L.E.; Perou, C.M.; Parker, J.S. Improved indel detection in DNA and RNA via realignment with ABRA2. Bioinformatics 2019, 35, 2966–2973. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Conesa, A.; García-Alcalde, F. Qualimap 2: Advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 2016, 32, 292–294. [Google Scholar] [CrossRef]
- Cibulskis, K.; Lawrence, M.S.; Carter, S.L.; Sivachenko, A.; Jaffe, D.; Sougnez, C.; Gabriel, S.; Meyerson, M.; Lander, E.S.; Getz, G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 2013, 31, 213–219. [Google Scholar] [CrossRef]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- de Andrade, K.C.; Frone, M.N.; Wegman-Ostrosky, T.; Khincha, P.P.; Kim, J.; Amadou, A.; Santiago, K.M.; Fortes, F.P.; Lemonnier, N.; Mirabello, L.; et al. Variable population prevalence estimates of germline TP53 variants: A gnomAD-based analysis. Hum. Mutat. 2019, 40, 97–105. [Google Scholar] [CrossRef]
- Broad Institute. Somatic Short Variant Discovery (SNVs + Indels). Available online: https://gatk.broadinstitute.org/hc/en-us/articles/360035894731-Somatic-short-variant-discovery-SNVs-Indels (accessed on 4 March 2024).
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Sukhai, M.A.; Misyura, M.; Thomas, M.; Garg, S.; Zhang, T.; Stickle, N.; Virtanen, C.; Bedard, P.L.; Siu, L.L.; Smets, T.; et al. Somatic Tumor Variant Filtration Strategies to Optimize Tumor-Only Molecular Profiling Using Targeted Next-Generation Sequencing Panels. J. Mol. Diagn. 2019, 21, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Byrjalsen, A.; Diets, I.J.; Bakhuizen, J.; Hansen, T.V.O.; Schmiegelow, K.; Gerdes, A.M.; Stoltze, U.; Kuiper, R.P.; Merks, J.H.M.; Wadt, K.; et al. Selection criteria for assembling a pediatric cancer predisposition syndrome gene panel. Fam. Cancer 2021, 20, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, M.N.; Patel, A.N.; Hedges, D.J.; Wang, Z.; Rampersaud, E.; Kesserwan, C.A.; Zhou, X.; Liu, Y.; Newman, S.; Rusch, M.C.; et al. Pediatric Cancer Variant Pathogenicity Information Exchange (PeCanPIE): A cloud-based platform for curating and classifying germline variants. Genome Res. 2019, 29, 1555–1565. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Priestley, P.; Baber, J.; Lolkema, M.P.; Steeghs, N.; de Bruijn, E.; Shale, C.; Duyvesteyn, K.; Haidari, S.; van Hoeck, A.; Onstenk, W.; et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 2019, 575, 210–216. [Google Scholar] [CrossRef]
- Sondka, Z.; Dhir, N.B.; Carvalho-Silva, D.; Jupe, S.; Madhumita; McLaren, K.; Starkey, M.; Ward, S.; Wilding, J.; Ahmed, M.; et al. COSMIC: A curated database of somatic variants and clinical data for cancer. Nucleic Acids Res. 2024, 52, D1210–D1217. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Mayoh, C.; Lau, L.M.S.; Khuong-Quang, D.A.; Pinese, M.; Kumar, A.; Barahona, P.; Wilkie, E.E.; Sullivan, P.; Bowen-James, R.; et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat. Med. 2020, 26, 1742–1753. [Google Scholar] [CrossRef]
- Mayakonda, A.; Lin, D.C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef]
- Lee, J.; Lee, A.J.; Lee, J.K.; Park, J.; Kwon, Y.; Park, S.; Chun, H.; Ju, Y.S.; Hong, D. Mutalisk: A web-based somatic MUTation AnaLyIS toolKit for genomic, transcriptional and epigenomic signatures. Nucleic Acids Res. 2018, 46, W102–W108. [Google Scholar] [CrossRef]
- Tamborero, D.; Rubio-Perez, C.; Deu-Pons, J.; Schroeder, M.P.; Vivancos, A.; Rovira, A.; Tusquets, I.; Albanell, J.; Rodon, J.; Tabernero, J.; et al. Cancer Genome Interpreter annotates the biological and clinical relevance of tumor alterations. Genome Med. 2018, 10, 25. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef]
- Boscolo Bielo, L.; Trapani, D.; Repetto, M.; Crimini, E.; Valenza, C.; Belli, C.; Criscitiello, C.; Marra, A.; Subbiah, V.; Curigliano, G. Variant allele frequency: A decision-making tool in precision oncology? Trends Cancer 2023, 9, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Rujkijyanont, P.; Photia, A.; Traivaree, C.; Monsereenusorn, C.; Anurathapan, U.; Seksarn, P.; Sosothikul, D.; Techavichit, P.; Sanpakit, K.; Phuakpet, K.; et al. Clinical outcomes and prognostic factors to predict treatment response in high risk neuroblastoma patients receiving topotecan and cyclophosphamide containing induction regimen: A prospective multicenter study. BMC Cancer 2019, 19, 961. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Dessypris, N.; Baka, M.; Moschovi, M.; Papadakis, V.; Polychronopoulou, S.; Kourti, M.; Hatzipantelis, E.; Stiakaki, E.; Dana, H.; et al. Neuroblastoma among children in Southern and Eastern European cancer registries: Variations in incidence and temporal trends compared to US. Int. J. Cancer 2018, 142, 1977–1985. [Google Scholar] [CrossRef]
- Khan, S.; AlSayyad, K.; Siddiqui, K.; AlAnazi, A.; AlSeraihy, A.; AlAhmari, A.; ElSolh, H.; Ghemlas, I.; AlSaedi, H.; AlJefri, A.; et al. Pediatric high risk neuroblastoma with autologous stem cell transplant—20 years of experience. Int. J. Pediatr. Adolesc. Med. 2021, 8, 253–257. [Google Scholar] [CrossRef]
- Hishiki, T.; Horie, H.; Higashimoto, Y.; Yotsumoto, K.; Komatsu, S.; Okimoto, Y.; Kakuda, H.; Taneyama, Y.; Saito, T.; Terui, K.; et al. Histological features of primary tumors after induction or high-dose chemotherapy in high-risk neuroblastoma. Pediatr. Surg. Int. 2014, 30, 919–926. [Google Scholar] [CrossRef]
- Liu, S.; Yin, W.; Lin, Y.; Huang, S.; Xue, S.; Sun, G.; Wang, C. Metastasis pattern and prognosis in children with neuroblastoma. World J. Surg. Oncol. 2023, 21, 130. [Google Scholar] [CrossRef]
- Chen, W.M.; Fang, Y.Y.; Lin, P.; Bai, J.X.; Fang, Y.F.; Zhang, B. A novel nomogram for predicting post-recurrence survival in recurrent neuroblastoma patients. Am. J. Cancer Res. 2023, 13, 2254–2268. [Google Scholar] [PubMed]
- Gallia, G.L.; Zhang, M.; Ning, Y.; Haffner, M.C.; Batista, D.; Binder, Z.A.; Bishop, J.A.; Hann, C.L.; Hruban, R.H.; Ishii, M.; et al. Genomic analysis identifies frequent deletions of Dystrophin in olfactory neuroblastoma. Nat. Commun. 2018, 9, 5410. [Google Scholar] [CrossRef]
- Niba, E.T.E.; Yamanaka, R.; Rani, A.Q.M.; Awano, H.; Matsumoto, M.; Nishio, H.; Matsuo, M. DMD transcripts in CRL-2061 rhabdomyosarcoma cells show high levels of intron retention by intron-specific PCR amplification. Cancer Cell Int. 2017, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, M.; Wu, M.; Fang, H.; Xiao, B.; Xie, L.; Zhu, X. RNF213 suppresses carcinogenesis in glioblastoma by affecting MAPK/JNK signaling pathway. Clin. Transl. Oncol. 2020, 22, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Dong, J.J.; Xie, T.; Guan, X. Integrative Analysis of MUC4 to Prognosis and Immune Infiltration in Pan-Cancer: Friend or Foe? Front. Cell Dev. Biol. 2021, 9, 695544. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Gu, H.; Xiang, L.; Xiong, Y.; Wang, R.; Zhou, H.; Wang, J. Mucin 4 mutation is associated with tumor mutation burden and promotes antitumor immunity in colon cancer patients. Aging 2021, 13, 9043–9055. [Google Scholar] [CrossRef]
- Abreu, M.T.; Baptista, R.; Girão, H. Immune cell subsets as a marker of development of heart failure: The application of bioinformatics tools. Rev. Port. Cardiol. (Engl. Ed.) 2021, 40, 849–851. [Google Scholar] [CrossRef]
- Gu, C.; Gu, X.; Wang, Y.; Yao, Z.; Zhou, C. Construction and Validation of a Novel Immunosignature for Overall Survival in Uveal Melanoma. Front. Cell Dev. Biol. 2021, 9, 710558. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, L. MUC16 mutation predicts a favorable clinical outcome and correlates decreased Warburg effect in gastric cancer. Biochem. Biophys. Res. Commun. 2018, 506, 780–786. [Google Scholar] [CrossRef]
- Yicheng, F.; Xin, L.; Tian, Y.; Huilin, L. Association of FLG mutation with tumor mutation load and clinical outcomes in patients with gastric cancer. Front. Genet. 2022, 13, 808542. [Google Scholar] [CrossRef]
- Chen, L.; Huang, X.; Xiong, L.; Chen, W.; An, L.; Wang, H.; Hong, Y.; Wang, H. Analysis of prognostic oncogene filaggrin (FLG) wild-type subtype and its implications for immune checkpoint blockade therapy in bladder urothelial carcinoma. Transl. Androl. Urol. 2022, 11, 1419–1432. [Google Scholar] [CrossRef]
- Yi, H.; Liao, Z.W.; Chen, J.J.; Shi, X.Y.; Chen, G.L.; Wu, G.T.; Zhou, D.Y.; Zhou, G.Q.; Huang, J.Y.; Lian, L.; et al. Genome variation in colorectal cancer patient with liver metastasis measured by whole-exome sequencing. J. Gastrointest. Oncol. 2021, 12, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, B.K.; Deng, C.X. A comprehensive genomic meta-analysis identifies confirmatory role of OBSCN gene in breast tumorigenesis. Oncotarget 2017, 8, 102263–102276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, L.; Guo, C.; Liu, L.; Jiao, D.; Sun, Z.; Wu, K.; Zhao, Y.; Han, X. TTN/OBSCN ‘Double-Hit’ predicts favourable prognosis, ‘immune-hot’ subtype and potentially better immunotherapeutic efficacy in colorectal cancer. J. Cell Mol. Med. 2021, 25, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Luce, L.N.; Abbate, M.; Cotignola, J.; Giliberto, F. Non-myogenic tumors display altered expression of dystrophin (DMD) and a high frequency of genetic alterations. Oncotarget 2017, 8, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, F.; Lasorsa, V.A.; Vetrella, S.; Iolascon, A.; Capasso, M. A Targeted Gene Panel for Circulating Tumor DNA Sequencing in Neuroblastoma. Front. Oncol. 2020, 10, 596191. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.W.; Lee, B.; Park, K.; Shim, J.; Yoo, K.H.; Koo, H.H.; Sung, K.W.; Park, W.Y. Genomic profile of MYCN non-amplified neuroblastoma and potential for immunotherapeutic strategies in neuroblastoma. BMC Med. Genomics 2020, 13, 171. [Google Scholar] [CrossRef]
- Miller, A.L.; Garcia, P.L.; Pressey, J.G.; Beierle, E.A.; Kelly, D.R.; Crossman, D.K.; Council, L.N.; Daniel, R.; Watts, R.G.; Cramer, S.L.; et al. Whole exome sequencing identified sixty-five coding mutations in four neuroblastoma tumors. Sci. Rep. 2017, 7, 17787. [Google Scholar] [CrossRef]
- Jahan, R.; Macha, M.A.; Rachagani, S.; Das, S.; Smith, L.M.; Kaur, S.; Batra, S.K. Axed MUC4 (MUC4/X) aggravates pancreatic malignant phenotype by activating integrin-β1/FAK/ERK pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2538–2549. [Google Scholar] [CrossRef]
- Kumar, S.; Cruz, E.; Joshi, S.; Patel, A.; Jahan, R.; Batra, S.K.; Jain, M. Genetic variants of mucins: Unexplored conundrum. Carcinogenesis 2017, 38, 671–679. [Google Scholar] [CrossRef][Green Version]
- Wu, W.; Xu, W.J.; Liu, J.B.; Sun, J.; Huang, Y.M.; Lv, Z.B. Exome sequencing identifies predisposing and fusion gene in ganglioneuroma, ganglioneuroblastoma and neuroblastoma. Math. Biosci. Eng. 2019, 16, 7217–7229. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, W.X.; Zhang, Q.Y.; Li, M.; Wang, H.Y.; Li, D.; Liu, J.; Zhuo, Z.; He, J.; Miao, L.; et al. MUC15 is an independent prognostic factor that promotes metastases of MYCN non-amplified neuroblastoma. J. Cancer 2023, 14, 3496–3507. [Google Scholar] [CrossRef]
- Fransson, S.; Martinez-Monleon, A.; Johansson, M.; Sjöberg, R.M.; Björklund, C.; Ljungman, G.; Ek, T.; Kogner, P.; Martinsson, T. Whole-genome sequencing of recurrent neuroblastoma reveals somatic mutations that affect key players in cancer progression and telomere maintenance. Sci. Rep. 2020, 10, 22432. [Google Scholar] [CrossRef] [PubMed]
- Bouanene, H.; Hadj Kacem, H.; Ben Fatma, L.; Ben Limem, H.; Ben Ahmed, S.; Yakoub, S.; Miled, A. Polymorphisms in the MUC16 gene: Potential implication in epithelial ovarian cancer. Pathol. Oncol. Res. 2011, 17, 295–299. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, P.; Xia, Y.; Dong, L.; Li, Y.; Liu, L.; Liu, Y.; Wang, Y. A signature based on five immune-related genes to predict the survival and immune characteristics of neuroblastoma. BMC Med. Genomics 2022, 15, 242. [Google Scholar] [CrossRef]
- Rajendran, B.K.; Deng, C.X. Characterization of potential driver mutations involved in human breast cancer by computational approaches. Oncotarget 2017, 8, 50252–50272. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Sekiguchi, M.; Watanabe, K.; Hiwatarai, M.; Seki, M.; Yoshida, K.; Isobe, T.; Shiozawa, Y.; Suzuki, H.; Hoshino, N.; et al. Association of high-risk neuroblastoma classification based on expression profiles with differentiation and metabolism. PLoS ONE 2021, 16, e0245526. [Google Scholar] [CrossRef]
- Qin, X.; Chen, B. Comprehensive analysis and validation reveal potential MYCN regulatory biomarkers associated with neuroblastoma prognosis. J. Biomol. Struct. Dyn. 2023, 41, 8902–8917. [Google Scholar] [CrossRef]
- Wei, Y.; Ke, X.; Yu, J.; Jing, Q.; Bu, H.; Zeng, X.; Wei, B. Clinical and genomic analyses of neuroendocrine neoplasms of the breast. Mod. Pathol. 2022, 35, 495–505. [Google Scholar] [CrossRef]
- Wang, J.; Zhuo, Z.; Chen, M.; Zhu, J.; Zhao, J.; Zhang, J.; Chen, S.; He, J.; Zhou, H. RAN/RANBP2 polymorphisms and neuroblastoma risk in Chinese children: A three-center case-control study. Aging 2018, 10, 808–818. [Google Scholar] [CrossRef]
- Schnepp, R.W.; Diskin, S.J. LIN28B: An orchestrator of oncogenic signaling in neuroblastoma. Cell Cycle 2016, 15, 772–774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, S.K.; Jin, U.H.; Kim, K.W.; Lee, Y.C.; Park, Y.G.; Kim, C.H. Disialoganglioside GD3 increases in the secretion of apoB-containing lipoproteins. Biochem. Biophys. Res. Commun. 2007, 356, 418–423. [Google Scholar] [CrossRef]
- Evangelisti, C.; Bianco, F.; Pradella, L.M.; Puliti, A.; Goldoni, A.; Sbrana, I.; Rossi, M.; Vargiolu, M.; Seri, M.; Romeo, G.; et al. Apolipoprotein B is a new target of the GDNF/RET and ET-3/EDNRB signalling pathways. Neurogastroenterol. Motil. 2012, 24, e497–e508. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lin, J.; Yu, S.; Sun, S. Upregulation of PREX2 promotes the proliferation and migration of hepatocellular carcinoma cells via PTEN-AKT signaling. Oncol. Lett. 2016, 11, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Xiao, F.; Ding, Q.; Liu, J.; Liu, J.; Li, J.; Zhang, J.; Tian, D.A. The effect of CXCL9 on the invasion ability of hepatocellular carcinoma through up-regulation of PREX2. J. Mol. Histol. 2014, 45, 689–696. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Xu, J.; Zhang, B.; Wei, M.; Li, J.; Xu, H.; Yu, X.; Wang, W.; Shi, S. Intra-tumoral infiltration of adipocyte facilitates the activation of antitumor immune response in pancreatic ductal adenocarcinoma. Transl. Oncol. 2023, 27, 101561. [Google Scholar] [CrossRef]
- Roosan, M.R.; Mambetsariev, I.; Pharaon, R.; Fricke, J.; Baroz, A.R.; Chao, J.; Chen, C.; Nasser, M.W.; Chirravuri-Venkata, R.; Jain, M.; et al. Evaluation of Somatic Mutations in Solid Metastatic Pan-Cancer Patients. Cancers 2021, 13, 2776. [Google Scholar] [CrossRef]
- Peng, C.; Jian, X.; Xie, Y.; Li, L.; Ouyang, J.; Tang, L.; Zhang, X.; Su, J.; Zhao, S.; Liu, H.; et al. Genomic alterations of dermatofibrosarcoma protuberans revealed by whole-genome sequencing. Br. J. Dermatol. 2022, 186, 997–1009. [Google Scholar] [CrossRef]
- Lin, P.H.; Huang, C.Y.; Yu, K.J.; Kan, H.C.; Liu, C.Y.; Chuang, C.K.; Lu, Y.C.; Chang, Y.H.; Shao, I.H.; Pang, S.T. Genomic characterization of clear cell renal cell carcinoma using targeted gene sequencing. Oncol. Lett. 2021, 21, 169. [Google Scholar] [CrossRef]
- Ramarao-Milne, P.; Kondrashova, O.; Patch, A.M.; Nones, K.; Koufariotis, L.T.; Newell, F.; Addala, V.; Lakis, V.; Holmes, O.; Leonard, C.; et al. Comparison of actionable events detected in cancer genomes by whole-genome sequencing, in silico whole-exome and mutation panels. ESMO Open 2022, 7, 100540. [Google Scholar] [CrossRef]
- Parikh, K.; Huether, R.; White, K.; Hoskinson, D.; Beaubier, N.; Dong, H.; Adjei, A.A.; Mansfield, A.S. Tumor Mutational Burden From Tumor-Only Sequencing Compared With Germline Subtraction From Paired Tumor and Normal Specimens. JAMA Netw. Open 2020, 3, e200202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wan, Z.; Zhang, E.; Piao, Y. Genomic profiling and immune landscape of olfactory neuroblastoma in China. Front. Oncol. 2023, 13, 1226494. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiao, Y.; Qiao, Y.; Zeng, N.; Yu, R. A novel approach combined transfer learning and deep learning to predict TMB from histology image. Pattern Recognit. Lett. 2020, 135, 244–248. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef]
- Liu, L.; Bai, X.; Wang, J.; Tang, X.R.; Wu, D.H.; Du, S.S.; Du, X.J.; Zhang, Y.W.; Zhu, H.B.; Fang, Y.; et al. Combination of TMB and CNA Stratifies Prognostic and Predictive Responses to Immunotherapy Across Metastatic Cancer. Clin. Cancer Res. 2019, 25, 7413–7423. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Chen, J.; Zang, M.; Hu, X.; Li, R.; Hou, J.; Zhou, J. Characterization of somatic mutations and pathway alterations during hepatocellular carcinoma vascular invasion using next-generation sequencing. J. Gastrointest. Oncol. 2022, 13, 1864–1874. [Google Scholar] [CrossRef]
- Hwang, W.L.; Wolfson, R.L.; Niemierko, A.; Marcus, K.J.; DuBois, S.G.; Haas-Kogan, D. Clinical Impact of Tumor Mutational Burden in Neuroblastoma. J. Natl. Cancer Inst. 2019, 111, 695–699. [Google Scholar] [CrossRef]
- Ma, R.; Jing, C.; Zhang, Y.; Cao, H.; Liu, S.; Wang, Z.; Chen, D.; Zhang, J.; Wu, Y.; Wu, J.; et al. The somatic mutation landscape of Chinese Colorectal Cancer. J. Cancer 2020, 11, 1038–1046. [Google Scholar] [CrossRef]
- Banelli, B.; Di Vinci, A.; Gelvi, I.; Casciano, I.; Allemanni, G.; Bonassi, S.; Romani, M. DNA methylation in neuroblastic tumors. Cancer Lett. 2005, 228, 37–41. [Google Scholar] [CrossRef]
- Byron, S.A.; Hendricks, W.P.D.; Nagulapally, A.B.; Kraveka, J.M.; Ferguson, W.S.; Brown, V.I.; Eslin, D.E.; Mitchell, D.; Cornelius, A.; Roberts, W.; et al. Genomic and Transcriptomic Analysis of Relapsed and Refractory Childhood Solid Tumors Reveals a Diverse Molecular Landscape and Mechanisms of Immune Evasion. Cancer Res. 2021, 81, 5818–5832. [Google Scholar] [CrossRef] [PubMed]
- Waanders, E.; Gu, Z.; Dobson, S.M.; Antić, Ž.; Crawford, J.C.; Ma, X.; Edmonson, M.N.; Payne-Turner, D.; van de Vorst, M.; Jongmans, M.C.J.; et al. Mutational landscape and patterns of clonal evolution in relapsed pediatric acute lymphoblastic leukemia. Blood Cancer Discov. 2020, 1, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fos, E.; Planas-Fèlix, M.; Burkert, M.; Puiggròs, M.; Toedling, J.; Thiessen, N.; Blanc, E.; Szymansky, A.; Hertwig, F.; Ishaque, N.; et al. Mutational topography reflects clinical neuroblastoma heterogeneity. Cell Genom. 2023, 3, 100402. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Tapon, N.; Kanda, H.; Cigizoglu, S.; Edelmann, L.; Pellock, B.; White, K.; Hariharan, I.K. Capicua regulates cell proliferation downstream of the receptor tyrosine kinase/ras signaling pathway. Curr. Biol. 2007, 17, 728–733. [Google Scholar] [CrossRef]
- Rosswog, C.; Fassunke, J.; Ernst, A.; Schömig-Markiefka, B.; Merkelbach-Bruse, S.; Bartenhagen, C.; Cartolano, M.; Ackermann, S.; Theissen, J.; Blattner-Johnson, M.; et al. Genomic ALK alterations in primary and relapsed neuroblastoma. Br. J. Cancer 2023, 128, 1559–1571. [Google Scholar] [CrossRef]
- Zage, P.E.; Nolo, R.; Fang, W.; Stewart, J.; Garcia-Manero, G.; Zweidler-McKay, P.A. Notch pathway activation induces neuroblastoma tumor cell growth arrest. Pediatr. Blood Cancer 2012, 58, 682–689. [Google Scholar] [CrossRef]
- Altun, Z.; Yuan, H.; Baran, B.; Aktaş, S.; Sönmez, E.E.; Küçük, C.; Olgun, N. Whole-exome sequencing reveals genetic variants in low-risk and high-risk neuroblastoma. Gene 2023, 860, 147233. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Mohamed, A.D.; Gener, M.; Li, W.; Taboada, E. YAP and the Hippo pathway in pediatric cancer. Mol. Cell Oncol. 2017, 4, e1295127. [Google Scholar] [CrossRef]
- Caglar, H.O. Bioinformatics analysis of recurrent deletion regions in neuroblastoma. Med. Oncol. 2022, 39, 31. [Google Scholar] [CrossRef]
- Otte, J.; Dyberg, C.; Pepich, A.; Johnsen, J.I. MYCN Function in Neuroblastoma Development. Front. Oncol. 2020, 10, 624079. [Google Scholar] [CrossRef]
- Quan, J.; Adelmant, G.; Marto, J.A.; Look, A.T.; Yusufzai, T. The chromatin remodeling factor CHD5 is a transcriptional repressor of WEE1. PLoS ONE 2014, 9, e108066. [Google Scholar] [CrossRef]
- Robbins, H.L.; Hague, A. The PI3K/Akt Pathway in Tumors of Endocrine Tissues. Front. Endocrinol. 2015, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, S.J.; Valentijn, L.J.; Lesne, L.; Betts, D.R.; Marino, D.; Boudal-Khoshbeen, M.; London, W.B.; Rougemont, A.L.; Attiyeh, E.F.; Maris, J.M.; et al. Ataxia-telangiectasia mutated (ATM) silencing promotes neuroblastoma progression through a MYCN independent mechanism. Oncotarget 2015, 6, 18558–18576. [Google Scholar] [CrossRef] [PubMed]
- Varol, U.; Kucukzeybek, Y.; Alacacioglu, A.; Somali, I.; Altun, Z.; Aktas, S.; Oktay Tarhan, M. BRCA genes: BRCA 1 and BRCA 2. J. BUON 2018, 23, 862–866. [Google Scholar] [PubMed]
- Martínez-Jiménez, F.; Muiños, F.; Sentís, I.; Deu-Pons, J.; Reyes-Salazar, I.; Arnedo-Pac, C.; Mularoni, L.; Pich, O.; Bonet, J.; Kranas, H.; et al. A compendium of mutational cancer driver genes. Nat. Rev. Cancer 2020, 20, 555–572. [Google Scholar] [CrossRef]
- Kumar, S.; Warrell, J.; Li, S.; McGillivray, P.D.; Meyerson, W.; Salichos, L.; Harmanci, A.; Martinez-Fundichely, A.; Chan, C.W.Y.; Nielsen, M.M.; et al. Passenger Mutations in More Than 2,500 Cancer Genomes: Overall Molecular Functional Impact and Consequences. Cell 2020, 180, 915–927.e916. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Pamarthy, S.; Shah, A.N.; Sagar, V.; Unno, K.; Han, H.; Yang, X.J.; Costa, R.B.; Nagy, R.J.; Lanman, R.B.; et al. Anaplastic Lymphoma Kinase Mutation (ALK F1174C) in Small Cell Carcinoma of the Prostate and Molecular Response to Alectinib. Clin. Cancer Res. 2018, 24, 2732–2739. [Google Scholar] [CrossRef]
- Ou, S.H.; Milliken, J.C.; Azada, M.C.; Miller, V.A.; Ali, S.M.; Klempner, S.J. ALK F1174V mutation confers sensitivity while ALK I1171 mutation confers resistance to alectinib. The importance of serial biopsy post progression. Lung Cancer 2016, 91, 70–72. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.M.; Yu, Y.S.; Kim, H.; Nam, H.J.; Moon, H.G.; Noh, D.Y.; Kim, K.I.; Fang, S.; Baek, S.H. RORα2 requires LSD1 to enhance tumor progression in breast cancer. Sci. Rep. 2017, 7, 11994. [Google Scholar] [CrossRef]
- Mann, K.M.; Ward, J.M.; Yew, C.C.; Kovochich, A.; Dawson, D.W.; Black, M.A.; Brett, B.T.; Sheetz, T.E.; Dupuy, A.J.; Chang, D.K.; et al. Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2012, 109, 5934–5941. [Google Scholar] [CrossRef]
- Sebestyén, E.; Zawisza, M.; Eyras, E. Detection of recurrent alternative splicing switches in tumor samples reveals novel signatures of cancer. Nucleic Acids Res. 2015, 43, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.; Li, F.; Wu, M.; Luo, T.; Hammarström, K.; Lundin, E.; Ljuslinder, I.; Mezheyeuski, A.; Edqvist, P.-H.; Löfgren-Burström, A.; et al. Prognostic whole-genome and transcriptome signatures in colorectal cancers. medRxiv 2023. [Google Scholar] [CrossRef]
- Ono, S.; Saito, T.; Terui, K.; Yoshida, H.; Enomoto, H. Generation of conditional ALK F1174L mutant mouse models for the study of neuroblastoma pathogenesis. Genesis 2019, 57, e23323. [Google Scholar] [CrossRef]
- Pacenta, H.L.; Macy, M.E. Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma. Drug Des. Dev. Ther. 2018, 12, 3549–3561. [Google Scholar] [CrossRef]
- Umapathy, G.; Mendoza-Garcia, P.; Hallberg, B.; Palmer, R.H. Targeting anaplastic lymphoma kinase in neuroblastoma. APMIS 2019, 127, 288–302. [Google Scholar] [CrossRef]
- Moore, N.F.; Azarova, A.M.; Bhatnagar, N.; Ross, K.N.; Drake, L.E.; Frumm, S.; Liu, Q.S.; Christie, A.L.; Sanda, T.; Chesler, L.; et al. Molecular rationale for the use of PI3K/AKT/mTOR pathway inhibitors in combination with crizotinib in ALK-mutated neuroblastoma. Oncotarget 2014, 5, 8737–8749. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.K.; Gunnes, M.W. Therapeutic Targeting of the Anaplastic Lymphoma Kinase (ALK) in Neuroblastoma-A Comprehensive Update. Pharmaceutics 2021, 13, 1427. [Google Scholar] [CrossRef]
- Yau, N.K.; Fong, A.Y.; Leung, H.F.; Verhoeft, K.R.; Lim, Q.Y.; Lam, W.Y.; Wong, I.C.; Lui, V.W. A Pan-Cancer Review of ALK Mutations: Implications for Carcinogenesis and Therapy. Curr. Cancer Drug Targets 2015, 15, 327–336. [Google Scholar] [CrossRef]
- Ben Abdelali, R.; Roggy, A.; Leguay, T.; Cieslak, A.; Renneville, A.; Touzart, A.; Banos, A.; Randriamalala, E.; Caillot, D.; Lioure, B.; et al. SET-NUP214 is a recurrent γδ lineage-specific fusion transcript associated with corticosteroid/chemotherapy resistance in adult T-ALL. Blood 2014, 123, 1860–1863. [Google Scholar] [CrossRef]
- Nizialek, E.; Antonarakis, E.S. PARP Inhibitors in Metastatic Prostate Cancer: Evidence to Date. Cancer Manag. Res. 2020, 12, 8105–8114. [Google Scholar] [CrossRef]
- Pich, O.; Muiños, F.; Lolkema, M.P.; Steeghs, N.; Gonzalez-Perez, A.; Lopez-Bigas, N. The mutational footprints of cancer therapies. Nat. Genet. 2019, 51, 1732–1740. [Google Scholar] [CrossRef]
- Gomez, R.L.; Ibragimova, S.; Ramachandran, R.; Philpott, A.; Ali, F.R. Tumoral heterogeneity in neuroblastoma. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188805. [Google Scholar] [CrossRef]




| Characteristics | No. of Patient (n = 48) (%) |
|---|---|
| Sex | |
| Male | 32 (66.7) |
| Female | 16 (33.3) |
| Age at diagnosis (years) | |
| Mean ± SD | 3.63 ± 3.41 |
| Median (range) | 2.7 (0.3–16.8) |
| <18 months | 12 (25) |
| ≥18 months | 36 (75) |
| INRG stage | |
| L2 | 2 (4.2) |
| M | 43 (89.6) |
| MS | 3 (6.2) |
| INSS stage | |
| 1 | 1 (2.1) |
| 2A | 1 (2.1) |
| 3 | 1 (2.1) |
| 4 | 43 (89.6) |
| 4S | 2 (4.1) |
| Pre-treatment risk group | |
| High risk | 44 (91.6) |
| Standard risk | 2 (4.2) |
| Low risk | 2 (4.2) |
| MYCN amplification | |
| Yes | 5 (10.4) |
| No | 4 (8.3) |
| Unknown | 39 (81.3) |
| Tumor size (cm), n = 43 | |
| Mean ± SD | 6.1 ± 3.2 |
| Median (range) | 5.0 (2.5–15.0) |
| Tumor histology | |
| Ganglioneuroblastoma | 9 (18.8) |
| Ganglioneuroma | 6 (12.5) |
| Neuroblastoma | 30 (62.5) |
| Unknown | 3 (6.2) |
| Primary site | |
| Adrenal | 26 (54.2) |
| Extra-adrenal | 22 (45.8) |
| Metastasis | |
| Yes | 46 (95.8) |
| No | 2 (4.2) |
| Metastatic site, n = 45 | |
| BM | 26 (57.8) |
| Bone | 38 (84.4) |
| CNS | 1 (2.2) |
| Liver | 8 (17.8) |
| LN | 6 (13.3) |
| Lung | 7 (15.6) |
| Orbit | 2 (4.4) |
| Skin | 1 (2.2) |
| Spine | 3 (6.7) |
| Recurrence | |
| Yes | 5 (10.4) |
| No | 43 (89.6) |
| Stage collection | |
| Pre-CM | 2 (4.2) |
| Post-CM | 43 (89.6) |
| Unknown | 3 (6.2) |
| Response to treatment | |
| Complete response | 27 (56.3) |
| Partial response | 11 (22.9) |
| Stable disease | 5 (10.4) |
| Unknown | 5 (10.4) |
| Radiation | |
| Yes | 6 (12.5) |
| No | 39 (81.3) |
| Unknown | 3 (6.2) |
| Vital status at last follow-up Alive | 40 (83.3) |
| Dead | 8 (16.7) |
| Vital status at 5 years | |
| Alive | 17 (35.4) |
| Dead | 31 (64.6) |
| Gene | Chr | Start | End | Ref | Alt | Amino Acid Change | COSMIC ID |
|---|---|---|---|---|---|---|---|
| MUC4 | 3 | 195,786,542 | 195,786,542 | - | GGTGGCGTGACCTGTGGATACTGA GGAATTGTCGGTGACAGGAAGAG GGGTGGCGTGACCGGTGGATGCTG AGGAAGTGCTGGTGACAGGAAGA GA | p.T1679_P1680insSL PVTSTSSASTGHAT PLPVTDNSSVSTG HAT | NA |
| MUC4 | 3 | 195,783,453 | 195,783,596 | GTCGGTGACAGGAAGAGAGGTGG TGTCACCTGTGGATGCTGAGGAAG TGTCGGTGACAGGAAGAGAGGTG GCATGACCGGTGGATGCTGAGGAA GGGCTAGTGACAGGAAGAGGCGT GGTGTCACCTGTGGATACTGAGGA AAG | - | p.L2662_D2709del | NA |
| MUC16 | 19 | 8,851,677 | 8,851,677 | C | G | - | NA |
| ALK | 2 | 29,220,829 | 29,220,829 | G | T | p.F1174L | COSV66555460 |
| CTNND1 | 11 | 57,802,091 | 57,802,091 | C | T | p.R439C | COSV62385889 |
| Gene | Total Number of Mutations | Total Number of Samples (n = 48) | Frequency of Mutated Samples (%) |
|---|---|---|---|
| ALK | 3 | 3 | 6.3 |
| BUB1B | 2 | 2 | 4.2 |
| CD209 | 2 | 2 | 4.2 |
| CSMD3 | 2 | 2 | 4.2 |
| CTNND1 | 2 | 2 | 4.2 |
| ERBB4 | 2 | 2 | 4.2 |
| ITGAV | 2 | 2 | 4.2 |
| KAT6A | 2 | 2 | 4.2 |
| KMT2C | 5 | 4 | 8.3 |
| MUC16 | 2 | 2 | 4.2 |
| MUC4 | 14 | 12 | 25.0 |
| MYH11 | 2 | 2 | 4.2 |
| NF1 | 3 | 2 | 4.2 |
| POLQ | 2 | 2 | 4.2 |
| PREX2 | 4 | 4 | 8.3 |
| SETD2 | 2 | 2 | 4.2 |
| Gene | Alteration | Drug | Diseases | Response | Evidence |
|---|---|---|---|---|---|
| NF1 | Q1798* and Q2616* | Retinoic acids | Neuroblastoma | Resistant | D |
| NF1 | Q1798* and Q2616* | Cobimetinib + trametinib | Any cancer type | Responsive | D |
| NF1 | S636X | Retinoic Acids | Neuroblastoma | Resistant | D |
| NF1 | S636X | Cobimetinib + Trametinib | Any cancer type | Responsive | D |
| ALK | F1174L | Crizotinib | Neuroblastoma | Resistant | D |
| ALK | F1174L | Crizotinib | Neuroblastoma | Responsive | D |
| ALK | F1174L | Crizotinib | Neuroblastoma | Responsive | C |
| ALK | F1174L | Alectinib | Neuroblastoma | Responsive | D |
| ALK | F1174L | TAE684 | Neuroblastoma | Responsive | D |
| ALK | F1174L | AZD3463 | Neuroblastoma | Responsive | D |
| ALK | F1174L | Lorlatinib | Neuroblastoma | Responsive | D |
| ALK | R1275Q | TAE684 | Neuroblastoma | Resistant | D |
| ALK | R1275Q | TAE684 | Neuroblastoma | Responsive | D |
| ALK | R1275Q | Crizotinib | Neuroblastoma | Responsive | D |
| ALK | R1275Q | Crizotinib | Neuroblastoma | Responsive | C |
| ALK | R1275Q | Lorlatinib | Neuroblastoma | Responsive | D |
| SETD2 | P10L | WEE1 inhibitors | Any cancer type | Responsive | D |
| SETD2 | Q1829E | WEE1 inhibitors | Any cancer type | Responsive | D |
| BRCA1 | R612S | WEE1 inhibitors | Any cancer type | Responsive | C |
| NOTCH1 | D1670V | Gamma secretase inhibitors (Ro4929097,Pf-03084014,Mk-0752,etc) | Any cancer type | Responsive | C |
| ATR | S1372L | Olaparib (PARP inhibitor) | Ovary, Any cancer type | Responsive | D |
| FGFR1 | N577K | Azd4547 + Nvp-Bgj398 + Erdafitinib + 1265229-25-1 | Any cancer type | Responsive | D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokchan, N.; Suthapot, P.; Choochuen, P.; Khongcharoen, N.; Hongeng, S.; Anurathapan, U.; Surachat, K.; Sangkhathat, S.; Thai Pediatric Cancer Atlas (TPCA) Consortium. Whole-Exome Sequencing Reveals Novel Candidate Driver Mutations and Potential Druggable Mutations in Patients with High-Risk Neuroblastoma. J. Pers. Med. 2024, 14, 950. https://doi.org/10.3390/jpm14090950
Nokchan N, Suthapot P, Choochuen P, Khongcharoen N, Hongeng S, Anurathapan U, Surachat K, Sangkhathat S, Thai Pediatric Cancer Atlas (TPCA) Consortium. Whole-Exome Sequencing Reveals Novel Candidate Driver Mutations and Potential Druggable Mutations in Patients with High-Risk Neuroblastoma. Journal of Personalized Medicine. 2024; 14(9):950. https://doi.org/10.3390/jpm14090950
Chicago/Turabian StyleNokchan, Natakorn, Praewa Suthapot, Pongsakorn Choochuen, Natthapon Khongcharoen, Suradej Hongeng, Usanarat Anurathapan, Komwit Surachat, Surasak Sangkhathat, and Thai Pediatric Cancer Atlas (TPCA) Consortium. 2024. "Whole-Exome Sequencing Reveals Novel Candidate Driver Mutations and Potential Druggable Mutations in Patients with High-Risk Neuroblastoma" Journal of Personalized Medicine 14, no. 9: 950. https://doi.org/10.3390/jpm14090950
APA StyleNokchan, N., Suthapot, P., Choochuen, P., Khongcharoen, N., Hongeng, S., Anurathapan, U., Surachat, K., Sangkhathat, S., & Thai Pediatric Cancer Atlas (TPCA) Consortium. (2024). Whole-Exome Sequencing Reveals Novel Candidate Driver Mutations and Potential Druggable Mutations in Patients with High-Risk Neuroblastoma. Journal of Personalized Medicine, 14(9), 950. https://doi.org/10.3390/jpm14090950







