Endochondral Ossification for Spinal Fusion: A Novel Perspective from Biological Mechanisms to Clinical Applications
Abstract
1. Introduction
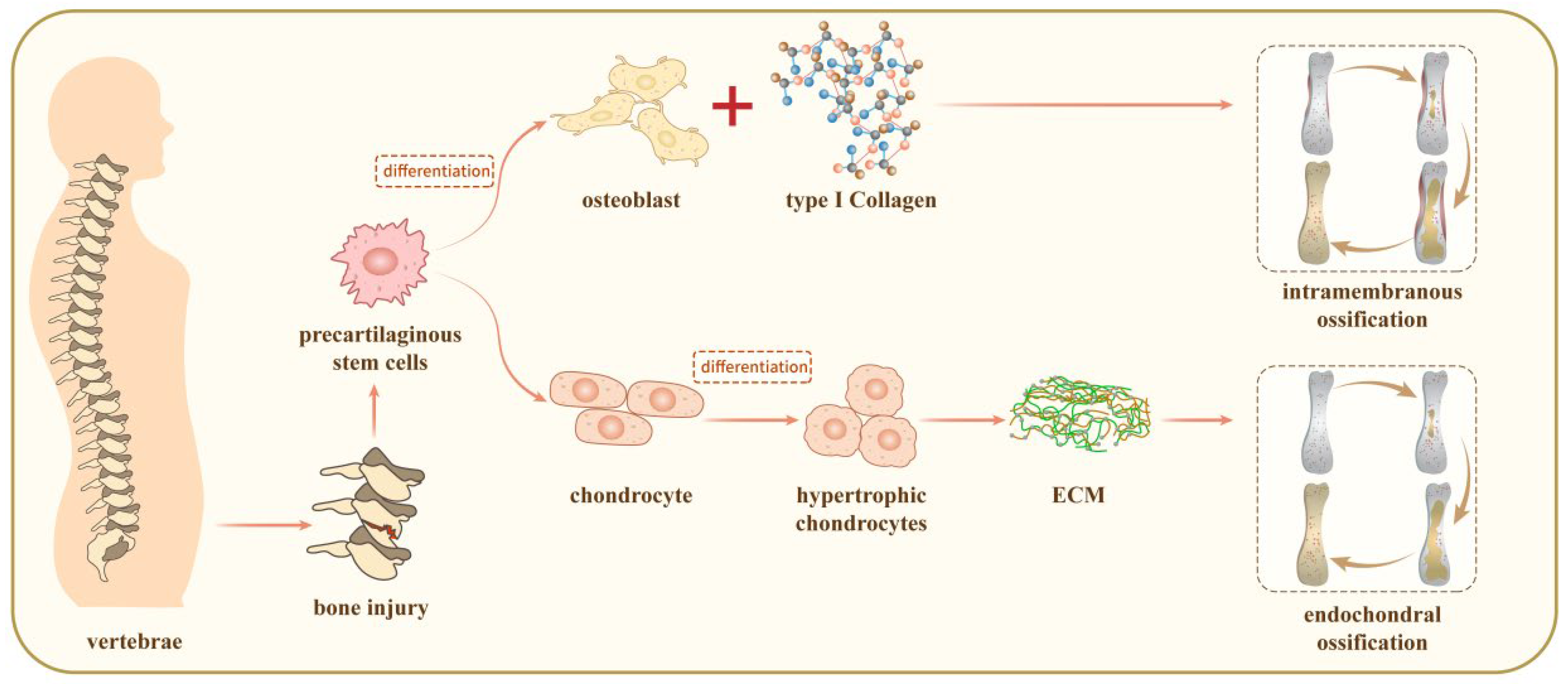
2. The Mechanisms for Vertebrae Growth
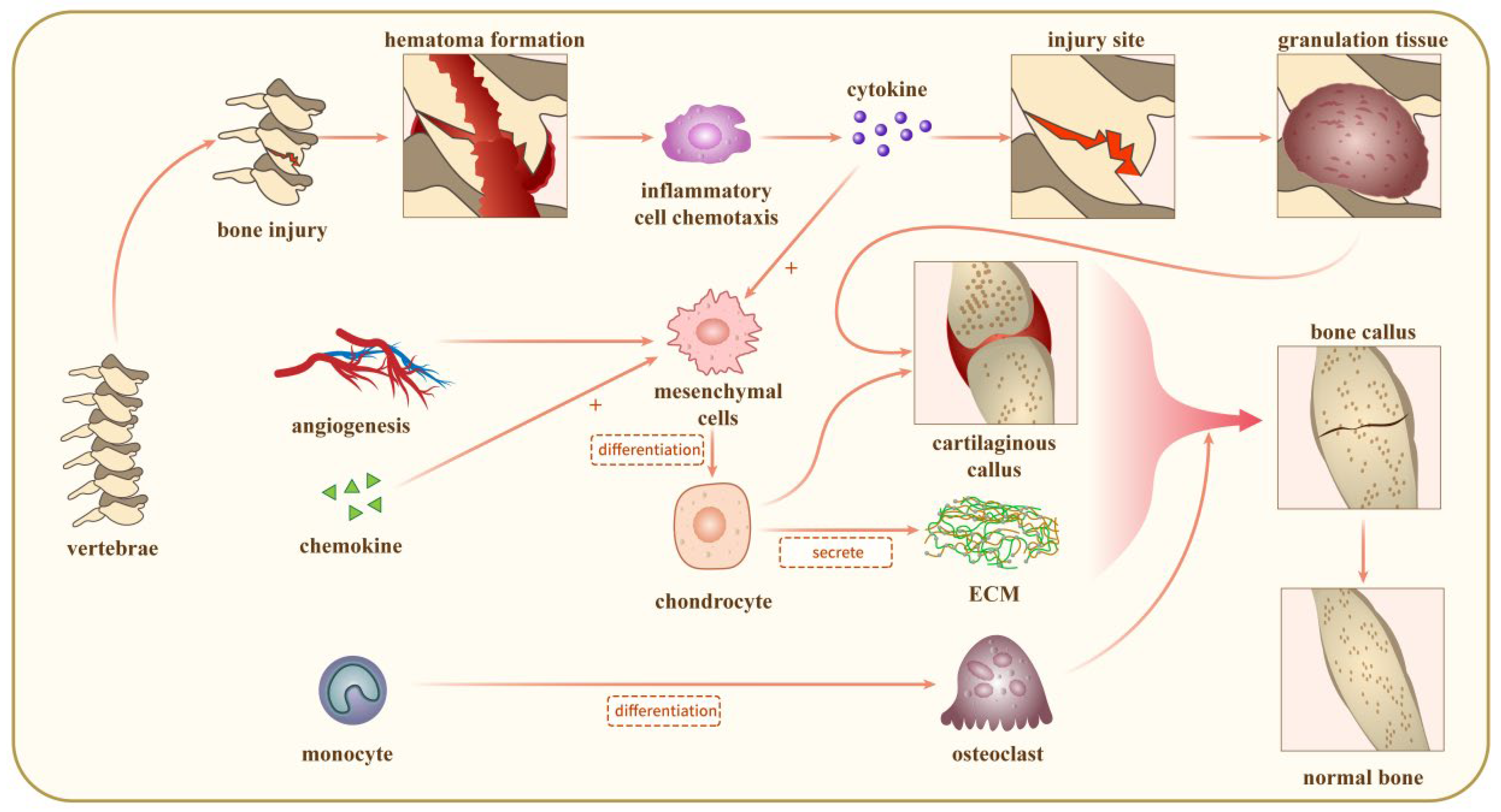
3. The Mechanisms for Bone Repair
3.1. Acute Inflammatory Response
3.2. Recruitment of MSCs
3.3. Cartilaginous Callus Generation
3.4. Revascularization
3.5. Mineralisation and Resorption of Cartilaginous Calluses
3.6. Bone Remodeling
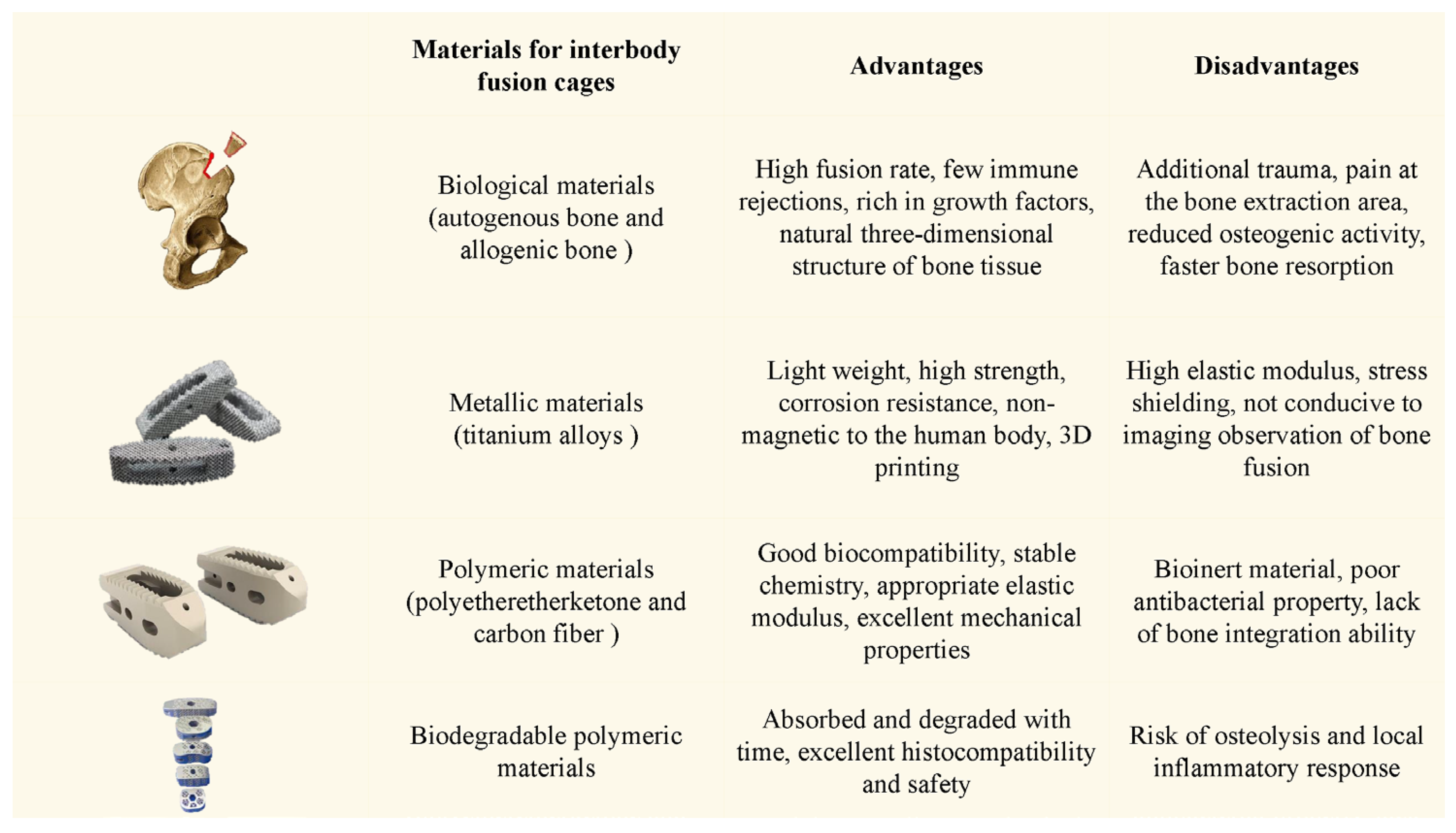
4. Endochondral Ossification and Bone Graft Substitutes for Lumbar Interbody Fusions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Petrosyan, E.; Fares, J.; Lesniak, M.S.; Koski, T.R.; El Tecle, N.E. Biological principles of adult degenerative scoliosis. Trends Mol. Med. 2023, 29, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, J.; Yang, Y.; Wei, X.; Chen, Z.; Li, M.; Zhai, X. Global research trends of adult degenerative scoliosis in this decade (2010–2019): A bibliometric study. Eur. Spine J. 2020, 29, 2970–2979. [Google Scholar] [CrossRef] [PubMed]
- de Kunder, S.L.; Rijkers, K.; Caelers, I.J.M.H.; de Bie, R.A.; Koehler, P.J.; van Santbrink, H. Lumbar Interbody Fusion: A Historical Overview and a Future Perspective. Spine 2018, 43, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhong, W.; Zhang, P.; Liu, X.; Huang, J.; Liu, F.; Li, J. Biomechanical evaluation of autologous bone-cage in posterior lumbar interbody fusion: A finite element analysis. BMC Musculoskelet. Disord. 2020, 21, 379. [Google Scholar] [CrossRef]
- Scott-Young, M.; Nielsen, D.; Riar, S. Fundamentals of Mechanobiology and Potential Applications in Spinal Fusion. Int. J. Spine Surg. 2023, 17, S61–S74. [Google Scholar] [CrossRef]
- Rao, P.J.; Pelletier, M.H.; Walsh, W.R.; Mobbs, R.J. Spine interbody implants: Material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef]
- Xie, M.; Gol’Din, P.; Herdina, A.N.; Estefa, J.; Medvedeva, E.V.; Li, L.; Newton, P.T.; Kotova, S.; Shavkuta, B.; Saxena, A.; et al. Secondary ossification center induces and protects growth plate structure. Elife 2020, 9, e55212. [Google Scholar] [CrossRef]
- Tong, W.; Tower, R.J.; Chen, C.; Wang, L.; Zhong, L.; Wei, Y.; Sun, H.; Cao, G.; Jia, H.; Pacifici, M.; et al. Periarticular Mesenchymal Progenitors Initiate and Contribute to Secondary Ossification Center Formation During Mouse Long Bone Development. Stem Cells 2019, 37, 677–689. [Google Scholar] [CrossRef]
- Michigami, T. Regulatory mechanisms for the development of growth plate cartilage. Cell. Mol. Life Sci. 2013, 70, 4213–4221. [Google Scholar] [CrossRef]
- Burdan, F.; Szumiło, J.; Korobowicz, A.; Farooquee, R.; Patel, S.; Patel, A.; Dave, A.; Szumiło, M.; Solecki, M.; Klepacz, R.; et al. Morphology and physiology of the epiphyseal growth plate. Folia Histochem. Cytobiol. 2009, 47, 5–16. [Google Scholar] [CrossRef]
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013, 242, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Shu, C.; Whitelock, J.M.; Lord, M.S. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016, 52–54, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, U.E.; Reguzzoni, M.; Pagani, F.; Sibilia, V.; Congiu, T.; Salvi, A.G.; Benetti, A. Study of Endochondral Ossification in Human Fetalcartilage Anlagen of Metacarpals: Comparative Morphology of Mineral Deposition in Cartilage and in the Periosteal Bone Matrix. Anat. Rec. 2018, 301, 571–580. [Google Scholar] [CrossRef]
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, a008334. [Google Scholar] [CrossRef]
- Janicki, P.; Kasten, P.; Kleinschmidt, K.; Luginbuehl, R.; Richter, W. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: Enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater. 2010, 6, 3292–3301. [Google Scholar] [CrossRef]
- He, J.; Jiang, B.; Dai, Y.; Hao, J.; Zhou, Z.; Tian, Z.; Wu, F.; Gu, Z. Regulation of the osteoblastic and chondrocytic differentiation of stem cells by the extracellular matrix and subsequent bone formation modes. Biomaterials 2013, 34, 6580–6588. [Google Scholar] [CrossRef]
- He, J.; Meng, G.; Yao, R.; Jiang, B.; Wu, Y.; Wu, F. The essential role of inorganic substrate in the migration and osteoblastic differentiation of mesenchymal stem cells. J. Mech. Behav. Biomed. Mater. 2016, 59, 353–365. [Google Scholar] [CrossRef]
- Fan, L.; Chen, J.; Tao, Y.; Heng, B.C.; Yu, J.; Yang, Z.; Ge, Z. Enhancement of the chondrogenic differentiation of mesenchymal stem cells and cartilage repair by ghrelin. J. Orthop. Res. 2019, 37, 1387–1397. [Google Scholar] [CrossRef]
- Griffin, M.; Hindocha, S.; Khan, W.S. Chondrogenic differentiation of adult MSCs. Curr. Stem Cell Res. Ther. 2012, 7, 260–265. [Google Scholar] [CrossRef]
- Sandell, L.J.; Sugai, J.V.; Trippel, S.B. Expression of collagens I, II, X, and XI and aggrecan mRNAs by bovine growth plate chondrocytes in situ. J. Orthop. Res. 1994, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Billinghurst, C.; Wu, W.; Alini, M.; Webber, C.; Reiner, A.; Ionescu, M.; Poole, J.; Poole, A.R. Selective assembly and remodelling of collagens II and IX associated with expression of the chondrocyte hypertrophic phenotype. Dev. Dyn. 2000, 218, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.F.; Su, G.Y.; Hou, Y.; Chen, S.D.; Lin, D.K. Cartilage degradation in osteoarthritis: A process of osteochondral remodeling resembles the endochondral ossification in growth plate? Med. Hypotheses 2018, 121, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Haxaire, C.; Otero, M.; Lessard, S.; Weskamp, G.; McIlwain, D.R.; Mak, T.W.; Lichtenthaler, S.F.; Blobel, C.P. Role of iRhoms 1 and 2 in Endochondral Ossification. Int. J. Mol. Sci. 2020, 21, 8732. [Google Scholar] [CrossRef]
- Tarantino, R.; Chiu, L.L.Y.; Weber, J.F.; Tse, M.Y.; Bardana, D.D.; Pang, S.C.; Waldman, S.D. Effect of nutrient metabolism on cartilaginous tissue formation. Biotechnol. Bioeng. 2021, 118, 4119–4128. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chung, U.-I.; Schipani, E.; Starbuck, M.; Karsenty, G.; Katagiri, T.; Goad, D.L.; Lanske, B.; Kronenberg, H.M. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 2002, 129, 2977–2986. [Google Scholar] [CrossRef]
- Liu, Z.; Mohan, S.; Yakar, S. Does the GH/IGF-1 axis contribute to skeletal sexual dimorphism? Evidence from mouse studies. Growth Horm. IGF Res. 2016, 27, 7–17. [Google Scholar] [CrossRef]
- Li, J.; Dong, S. The Signaling Pathways Involved in Chondrocyte Differentiation and Hypertrophic Differentiation. Stem Cells Int. 2016, 2016, 2470351. [Google Scholar] [CrossRef]
- Green, J.D.; Tollemar, V.; Dougherty, M.; Yan, Z.; Yin, L.; Ye, J.; Collier, Z.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. Multifaceted signaling regulators of chondrogenesis: Implications in cartilage regeneration and tissue engineering. Genes. Dis. 2015, 2, 307–327. [Google Scholar] [CrossRef]
- Cleary, M.A.; van Osch, G.J.V.M.; Brama, P.A.; Hellingman, C.A.; Narcisi, R. FGF, TGFβ and Wnt crosstalk: Embryonic to in vitro cartilage development from mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2015, 9, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef]
- Maes, C. Signaling pathways effecting crosstalk between cartilage and adjacent tissues: Seminars in cell and developmental biology: The biology and pathology of cartilage. Semin. Cell Dev. Biol. 2017, 62, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Racine, H.L.; Serrat, M.A. The Actions of IGF-1 in the Growth Plate and Its Role in Postnatal Bone Elongation. Curr. Osteoporos. Rep. 2020, 18, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Gentili, C.; Cancedda, R. Cartilage and bone extracellular matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar] [CrossRef]
- Prein, C.; Beier, F. ECM signaling in cartilage development and endochondral ossification. Curr. Top. Dev. Biol. 2019, 133, 25–47. [Google Scholar]
- Samsa, W.E.; Zhou, X.; Zhou, G. Signaling pathways regulating cartilage growth plate formation and activity. Semin. Cell Dev. Biol. 2017, 62, 3–15. [Google Scholar] [CrossRef]
- Thompson, E.M.; Matsiko, A.; Kelly, D.J.; Gleeson, J.P.; O’Brien, F.J. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng. Part. A 2016, 22, 556–567. [Google Scholar] [CrossRef]
- Liu, Y.; Kuang, B.; Rothrauff, B.B.; Tuan, R.S.; Lin, H. Robust bone regeneration through endochondral ossification of human mesenchymal stem cells within their own extracellular matrix. Biomaterials 2019, 218, 119336. [Google Scholar] [CrossRef]
- Fahy, N.; Palomares Cabeza, V.; Lolli, A.; Witte-Bouma, J.; Merino, A.; Ridwan, Y.; Wolvius, E.B.; Hoogduijn, M.J.; Farrell, E.; Brama, P.A.J. Chondrogenically Primed Human Mesenchymal Stem Cells Persist and Undergo Early Stages of Endochondral Ossification in an Immunocompetent Xenogeneic Model. Front. Immunol. 2021, 12, 715267. [Google Scholar] [CrossRef]
- Herrmann, M.; Verrier, S.; Alini, M. Strategies to Stimulate Mobilization and Homing of Endogenous Stem and Progenitor Cells for Bone Tissue Repair. Front. Bioeng. Biotechnol. 2015, 3, 79. [Google Scholar] [CrossRef]
- Zigdon-Giladi, H.; Rudich, U.; Geller, G.M.; Evron, A. Recent advances in bone regeneration using adult stem cells. World J. Stem Cells 2015, 7, 630–640. [Google Scholar] [CrossRef]
- Dennis, S.C.; Berkland, C.J.; Bonewald, L.F.; Detamore, M.S. Endochondral ossification for enhancing bone regeneration: Converging native extracellular matrix biomaterials and developmental engineering in vivo. Tissue Eng. Part. B Rev. 2015, 21, 247–266. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-Conditioned Mesenchymal Stem Cells in Tissue Regeneration Application. Tissue Eng. Part. B Rev. 2022, 28, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Kruijt Spanjer, E.C.; Bittermann, G.K.P.; van Hooijdonk, I.E.M.; Rosenberg, A.J.W.P.; Gawlitta, D. Taking the endochondral route to craniomaxillofacial bone regeneration: A logical approach? J. Craniomaxillofac. Surg. 2017, 45, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Kushioka, J.; Chow, S.K.-H.; Toya, M.; Tsubosaka, M.; Shen, H.; Gao, Q.; Li, X.; Zhang, N.; Goodman, S.B. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflamm. Regen. 2023, 43, 29. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Schulz, N.; Hoff, P.; Perka, C.; Buttgereit, F.; Volk, H.-D.; Lienau, J.; Duda, G.N. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. 2012, 347, 567–573. [Google Scholar] [CrossRef]
- Wu, C.-L.; Harasymowicz, N.; Klimak, M.; Collins, K.; Guilak, F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr. Cartil. 2020, 28, 544–554. [Google Scholar] [CrossRef]
- Fan, S.; Sun, X.; Su, C.; Xue, Y.; Song, X.; Deng, R. Macrophages-bone marrow mesenchymal stem cells crosstalk in bone healing. Front. Cell Dev. Biol. 2023, 11, 1193765. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Stefanowski, J.; Lang, A.; Rauch, A.; Aulich, L.; Köhler, M.; Fiedler, A.F.; Buttgereit, F.; Schmidt-Bleek, K.; Duda, G.N.; Gaber, T.; et al. Spatial Distribution of Macrophages During Callus Formation and Maturation Reveals Close Crosstalk between Macrophages and Newly Forming Vessels. Front. Immunol. 2019, 10, 2588. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014, 8, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, B.; Yan, F.; Yang, W. The Influence of Inflammatory Cytokines on the Proliferation and Osteoblastic Differentiation of MSCs. Curr. Stem Cell Res. Ther. 2017, 12, 401–408. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, C.; Yang, P. The Promotional Effect of Mesenchymal Stem Cell Homing on Bone Tissue Regeneration. Curr. Stem Cell Res. Ther. 2017, 12, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Greenfield, S.; Pan, H.; Vasanji, A.; Kumagai, K.; Midura, R.J.; Kiedrowski, M.; Penn, M.S.; Muschler, G.F. Stromal cell-derived factor-1 and monocyte chemotactic protein-3 improve recruitment of osteogenic cells into sites of musculoskeletal repair. J. Orthop. Res. 2011, 29, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, R.; Li, Z.; Wang, Y.; Hu, R. Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1α signalling. J. Cell. Mol. Med. 2019, 23, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Yellowley, C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2013, 2, 300. [Google Scholar] [CrossRef]
- Yu, X.; Wan, Q.; Cheng, G.; Cheng, X.; Zhang, J.; Pathak, J.L.; Li, Z. CoCl(2), a mimic of hypoxia, enhances bone marrow mesenchymal stem cells migration and osteogenic differentiation via STAT3 signaling pathway. Cell Biol. Int. 2018, 42, 1321–1329. [Google Scholar] [CrossRef]
- Wong, S.A.; Rivera, K.O.; Miclau, T.; Alsberg, E.; Marcucio, R.S.; Bahney, C.S. Microenvironmental Regulation of Chondrocyte Plasticity in Endochondral Repair—A New Frontier for Developmental Engineering. Front. Bioeng. Biotechnol. 2018, 6, 58. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 19. [Google Scholar] [CrossRef]
- Gandica, Y.; Schwarz, T.; Oliveira, O.; Travasso, R.D.M. Hypoxia in vascular networks: A complex system approach to unravel the diabetic paradox. PLoS ONE 2014, 9, e113165. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, H.; Shen, H.; Wang, B.; Lei, G.; Tuan, R.S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018, 69, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Block, T.; Singh-Varma, A.; Sheldrake, A.; Leeth, R.; Griffey, S.; Kohn, J. Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation. Acta Biomater. 2019, 85, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, K.; Kwarciak, A.; Freeman, C.; Brook, I.; Hatton, P.; Crawford, A. Repair of bone defects in vivo using tissue engineered hypertrophic cartilage grafts produced from nasal chondrocytes. Biomaterials 2017, 112, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, J.; Ferguson, J.; Rieder, B.; Heimel, P.; Nau, T.; Tangl, S.; Redl, H.; Vunjak-Novakovic, G. Tissue-engineered hypertrophic chondrocyte grafts enhanced long bone repair. Biomaterials 2017, 139, 202–212. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn. 2017, 246, 227–234. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Park, J.; Lee, D.-J.; Mizuno, S.; Shinohara, M.; Hong, C.P.; Jeong, Y.; Yun, R.; Park, H.; Park, S.; et al. Mast4 determines the cell fate of MSCs for bone and cartilage development. Nat. Commun. 2022, 13, 3960. [Google Scholar] [CrossRef]
- Zhou, X.; von der Mark, K.; Henry, S.; Norton, W.; Adams, H.; de Crombrugghe, B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014, 10, e1004820. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Nagano, K.; Moriishi, T.; Miyazaki, T.; Komori, H.; Ito, K.; von der Mark, K.; Sakane, C.; Kaneko, H.; et al. Runx2 is essential for the transdifferentiation of chondrocytes into osteoblasts. PLoS Genet. 2020, 16, e1009169. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Komori, H.; Sakane, C.; Fukuyama, R.; Matsuo, Y.; Ito, K.; Miyazaki, T.; Komori, T. Runt-related transcription factor-2 (Runx2) is required for bone matrix protein gene expression in committed osteoblasts in mice. J. Bone Miner. Res. 2021, 36, 2081–2095. [Google Scholar] [CrossRef]
- Wu, C.W.; Tchetina, E.V.; Mwale, F.; Hasty, K.; Pidoux, I.; Reiner, A.; Chen, J.; van Wart, H.E.; Poole, A.R.; Poole, D.A.R. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J. Bone Miner. Res. 2002, 17, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Zhang, W.; Ling, C.; Li, X.; Sheng, R.; Liu, H.; Zhang, A.; Jiang, Y.; Chen, J.; Yao, Q. Cell-Free Biomimetic Scaffold with Cartilage Extracellular Matrix-Like Architectures for In Situ Inductive Regeneration of Osteochondral Defects. ACS Biomater. Sci. Eng. 2020, 6, 6917–6925. [Google Scholar] [CrossRef]
- Gamblin, A.L.; Renaud, A.; Charrier, C.; Hulin, P.; Louarn, G.; Heymann, D.; Trichet, V.; Layrolle, P. Osteoblastic and osteoclastic differentiation of human mesenchymal stem cells and monocytes in a miniaturized three-dimensional culture with mineral granules. Acta Biomater. 2014, 10, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Kameo, Y.; Adachi, T.; Hojo, M. Effects of loading frequency on the functional adaptation of trabeculae predicted by bone remodeling simulation. J. Mech. Behav. Biomed. Mater. 2011, 4, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.A.; Cardenas, E.R.; Xu, H.; Jiang, J.X. The Role of Connexin Channels in the Response of Mechanical Loading and Unloading of Bone. Int. J. Mol. Sci. 2020, 21, 1146. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care 2017, 26, e12740. [Google Scholar] [CrossRef]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Suda, T.; Udagawa, N.; Nakamura, I.; Miyaura, C.; Takahashi, N. Modulation of osteoclast differentiation by local factors. Bone 1995, 17, S87–S91. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.; Jing, Y.; Jing, J.; Feng, J. Roles of Chondrocytes in Endochondral Bone Formation and Fracture Repair. J. Dent. Res. 2017, 96, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Laperre, K.; Eelen, G.; Rinaldi, G.; Fraisl, P.; Torrekens, S.; van Looveren, R.; Loopmans, S.; Bultynck, G.; Vinckier, S.; et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature 2019, 565, 511–515. [Google Scholar] [CrossRef]
- Sielatycki, J.A.; Saito, M.; Yuasa, M.; Moore-Lotridge, S.N.; Uppuganti, S.; Colazo, J.M.; Hysong, A.A.; Robinette, J.P.; Okawa, A.; Yoshii, T.; et al. Autologous chondrocyte grafting promotes bone formation in the posterolateral spine. JOR Spine 2018, 1, e1001. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.G.; James, A.W.; Asatrian, G.; Chang, L.; Nguyen, A.; Le, K.; Bayani, G.; Lee, R.; Stoker, D.; Pang, S.; et al. Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl. Med. 2014, 3, 1231–1241. [Google Scholar]
- de Kunder, S.L.; van Kuijk, S.M.J.; Rijkers, K.; Caelers, I.J.M.H.; van Hemert, W.L.W.; de Bie, R.A.; van Santbrink, H. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: A systematic review and meta-analysis. Spine J. 2017, 17, 1712–1721. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- McGilvray, K.C.; Easley, J.; Seim, H.B.; Regan, D.; Berven, S.H.; Hsu, W.K.; Mroz, T.E.; Puttlitz, C.M. Bony ingrowth potential of 3D-printed porous titanium alloy: A direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J. 2018, 18, 1250–1260. [Google Scholar] [CrossRef]
- Vlad, M.D.; Aguado, E.F.; González, S.G.; Ivanov, I.C.; Şindilar, E.V.; Poeată, I.; Iencean, A.Ş.; Butnaru, M.; Avădănei, E.R.; López, J.L. Novel titaniumapatite hybrid scaffolds with spongy bone-like micro architecture intended for spinal application: In vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110658. [Google Scholar] [CrossRef]
- Fan, L.; Chen, S.; Yang, M.; Liu, Y.; Liu, J. Metallic Materials for Bone Repair. Adv. Healthcare Mater. 2024, 13, e2302132. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhou, C.; Zhang, H.; Bai, J.; Jiang, B.; Jiang, C.; Ming, W.; Zhang, H.; Long, H.; Huang, X.; et al. Recent advances in 3D printing of biodegradable metals for orthopaedic applications. J. Biol. Eng. 2023, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Kashii, M.; Kitaguchi, K.; Makino, T.; Kaito, T. Comparison in the same intervertebral space between titanium-coated and uncoated PEEK cages in lumbar interbody fusion surgery. J. Orthop. Sci. 2020, 25, 565–570. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Y.; Yang, L.; Shi, X.; Yang, W.; Chen, Z.-G. Enhanced antibacterial property and osteodifferentiation activity on plasma treated porous polyetheretherketone with hierarchical micro/nano-topography. J. Biomater. Sci. Polym. Ed. 2018, 29, 520–542. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, Z.; Sun, A.; Huang, J.; Wu, W.; Chen, M.; Hao, X.; Huang, Z.; Lin, X.; Weng, S. Rapid construction of polyetheretherketone (PEEK) biological implants incorporated with brushite (CaHPO(4)·2H(2)O) and antibiotics for anti-infection and enhanced osseointegration. Mater. Sci. Eng. C 2020, 111, 110782. [Google Scholar] [CrossRef]
- Hojo, Y.; Kotani, Y.; Ito, M.; Abumi, K.; Kadosawa, T.; Shikinami, Y.; Minami, A. A biomechanical and histological evaluation of a bioresorbable lumbar interbody fusion cage. Biomaterials 2005, 26, 2643–2651. [Google Scholar] [CrossRef]
- Lowe, T.G.; Coe, J.D. Resorbable polymer implants in unilateral transforaminal lumbar interbody fusion. J. Neurosurg. Spine 2002, 97, 464–467. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, R.; Liu, C.; Zhao, Y.; Wang, K.; Wang, X. Endochondral Ossification for Spinal Fusion: A Novel Perspective from Biological Mechanisms to Clinical Applications. J. Pers. Med. 2024, 14, 957. https://doi.org/10.3390/jpm14090957
Ge R, Liu C, Zhao Y, Wang K, Wang X. Endochondral Ossification for Spinal Fusion: A Novel Perspective from Biological Mechanisms to Clinical Applications. Journal of Personalized Medicine. 2024; 14(9):957. https://doi.org/10.3390/jpm14090957
Chicago/Turabian StyleGe, Rile, Chenjun Liu, Yuhong Zhao, Kaifeng Wang, and Xiluan Wang. 2024. "Endochondral Ossification for Spinal Fusion: A Novel Perspective from Biological Mechanisms to Clinical Applications" Journal of Personalized Medicine 14, no. 9: 957. https://doi.org/10.3390/jpm14090957
APA StyleGe, R., Liu, C., Zhao, Y., Wang, K., & Wang, X. (2024). Endochondral Ossification for Spinal Fusion: A Novel Perspective from Biological Mechanisms to Clinical Applications. Journal of Personalized Medicine, 14(9), 957. https://doi.org/10.3390/jpm14090957





