Predicting and Avoiding Complications in Percutaneous Nephrolithotomy in the Era of Personalized Medicine: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Synthesis of Results
3. Results
3.1. Predictive A.I. Models
3.2. Three-Dimensional Presurgical Models
3.3. Intrasurgical Image and Guidance Systems
3.4. Biomarkers
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) Checklist
| SECTION | ITEM | PRISMA-ScR CHECKLIST ITEM | REPORTED ON PAGE |
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 2 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | 2 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 3 |
| Information sources * | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 3 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | 3 |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 3 |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 3 |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | 3 |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 3 |
| RESULTS | |||
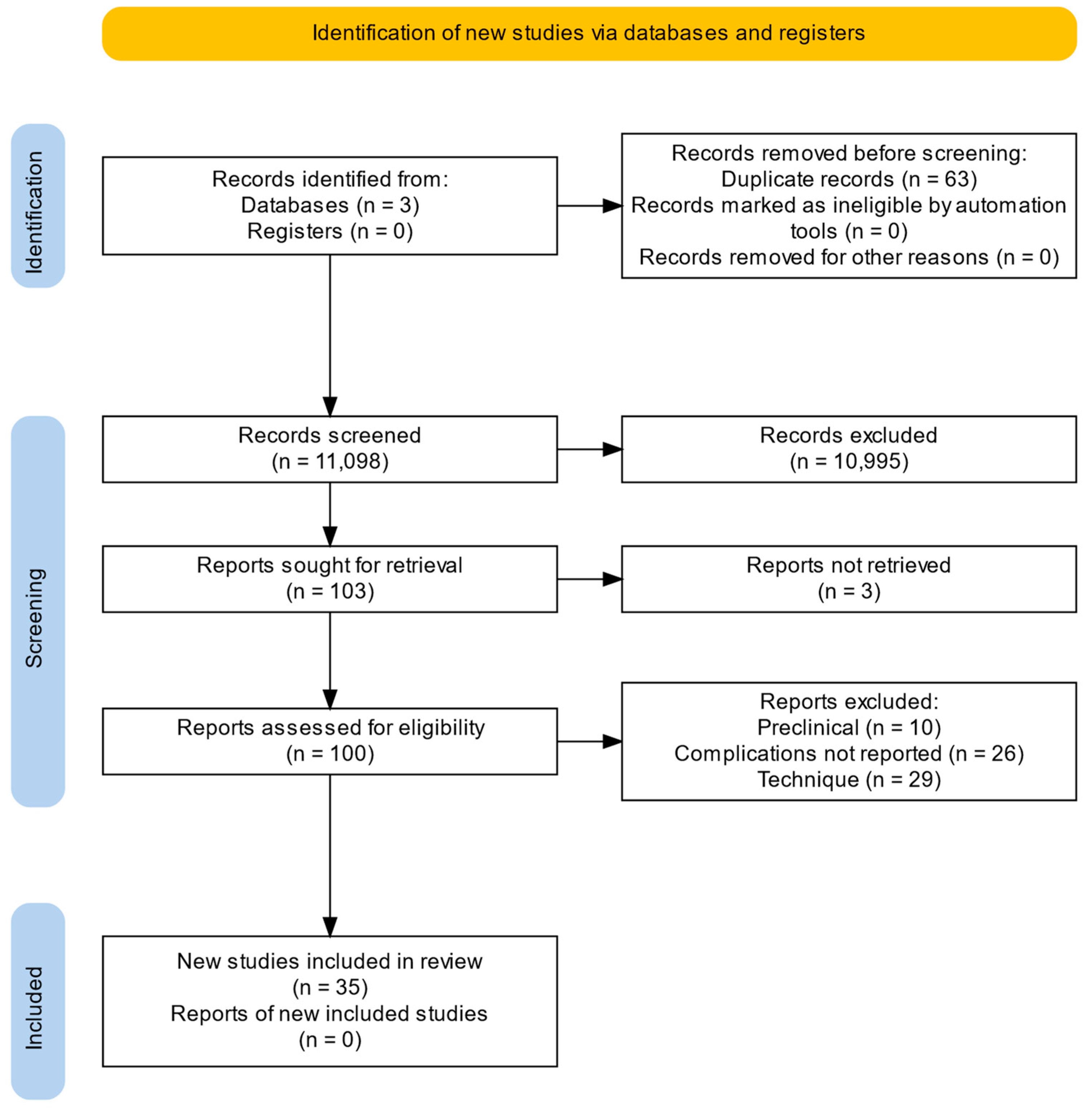
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 3–6 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 3–6 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | 3–6 |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 3–6 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 3–6 |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 7–8 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 8 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 8 |
| FUNDING | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 8 |
| From: Tricco et al. [68]. | |||
Appendix B. Search Strategy
- (Percutaneous nephrolithotomy and complications)
- Or
- (percutaneous nephrolitotomy and haemorhagic)
- Or
- (percutaneous nephrolitotomy and sepsis)
- Or
- (percutaneous nephrolitotomy and artificial intelligence)
- Or
- (percutaneous nephrolitotomy and neural networks)
- Or
- (percutaneous nephrolitotomy and access)
- Or
- (percutaneous nephrolitotomy and robotics)
- Or
- (percutaneous nephrolitotomy and infection)
- Or
- (percutaneous nephrolitotomy and biomarkers)
- Or
- (percutaneous nephrolitotomy and prediction)
- Or
- (percutaneous nephrolitotomy and genetics)
References
- Kyriazis, I.; Panagopoulos, V.; Kallidonis, P.; Ozsoy, M.; Vasilas, M.; Liatsikos, E. Complications in percutaneous nephrolithotomy. World J. Urol. 2015, 33, 1069–1077. [Google Scholar] [CrossRef]
- de la Rosette, J.; Assimos, D.; Desai, M.; Gutierrez, J.; Lingeman, J.; Scarpa, R.; Tefekli, A. The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: Indications, complications, and outcomes in 5803 patients. J. Endourol. 2011, 25, 11–17. [Google Scholar] [CrossRef]
- Tonolini, M.; Villa, F.; Ippolito, S.; Pagani, A.; Bianco, R. Cross-sectional imaging of iatrogenic complications after extracorporeal and endourological treatment of urolithiasis. Insights Into Imaging 2014, 5, 677–689. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Andonian, S.; Smith, A.D.; Siegel, D.N. Management of hemorrhagic complications associated with percutaneous nephrolithotomy. J. Endourol. 2009, 23, 1763–1767. [Google Scholar] [CrossRef]
- Lang, E.K. Percutaneous nephrostolithotomy and lithotripsy: A multi-institutional survey of complications. Radiology 1987, 162, 25–30. [Google Scholar] [CrossRef]
- Stoller, M.L.; Wolf, J.S., Jr.; St Lezin, M.A. Estimated blood loss and transfusion rates associated with percutaneous nephrolithotomy. J. Urol. 1994, 152, 1977–1981. [Google Scholar] [CrossRef]
- Kessaris, D.N.; Bellman, G.C.; Pardalidis, N.P.; Smith, A.G. Management of hemorrhage after percutaneous renal surgery. J. Urol. 1995, 153, 604–608. [Google Scholar] [CrossRef]
- Richstone, L.; Reggio, E.; Ost, M.C.; Seideman, C.; Fossett, L.K.; Okeke, Z.; Rastinehad, A.R.; Lobko, I.; Siegel, D.N.; Smith, A.D. First Prize (tie): Hemorrhage following percutaneous renal surgery: Characterization of angiographic findings. J. Endourol. 2008, 22, 1129–1135. [Google Scholar] [CrossRef]
- Sampaio, F.J. Renal collecting system anatomy: Its possible role in the effectiveness of renal stone treatment. Curr. Opin. Urol. 2001, 11, 359–366. [Google Scholar] [CrossRef]
- Sampaio, F.J. Renal anatomy. Endourologic considerations. Urol. Clin. 2000, 27, 585–607. [Google Scholar] [CrossRef]
- Michel, M.S.; Trojan, L.; Rassweiler, J.J. Complications in percutaneous nephrolithotomy. Eur. Urol. 2007, 51, 899–906; discussion 906. [Google Scholar] [CrossRef]
- O’Keeffe, N.K.; Mortimer, A.J.; Sambrook, P.A.; Rao, P.N. Severe sepsis following percutaneous or endoscopic procedures for urinary tract stones. Br. J. Urol. 1993, 72, 277–283. [Google Scholar] [CrossRef]
- Dogan, H.S.; Sahin, A.; Cetinkaya, Y.; Akdogan, B.; Ozden, E.; Kendi, S. Antibiotic prophylaxis in percutaneous nephrolithotomy: Prospective study in 81 patients. J. Endourol. 2002, 16, 649–653. [Google Scholar] [CrossRef]
- Lai, W.S.; Assimos, D. The Role of Antibiotic Prophylaxis in Percutaneous Nephrolithotomy. Rev. Urol. 2016, 18, 10–14. [Google Scholar]
- Charton, M.; Vallancien, G.; Veillon, B.; Brisset, J.M. Urinary tract infection in percutaneous surgery for renal calculi. J. Urol. 1986, 135, 15–17. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Wang, Y.; Hou, Y.; Zhang, H.; Chen, Q.; Xu, N.; Lu, Z.; Hu, J.; Lu, J.; et al. Post-percutaneous nephrolithotomy septic shock and severe hemorrhage: A study of risk factors. Urol. Int. 2012, 88, 307–310. [Google Scholar] [CrossRef]
- Zhong, W.; Zeng, G.; Wu, K.; Li, X.; Chen, W.; Yang, H. Does a smaller tract in percutaneous nephrolithotomy contribute to high renal pelvic pressure and postoperative fever? J. Endourol. 2008, 22, 2147–2151. [Google Scholar] [CrossRef]
- Geraghty, R.M.; Thakur, A.; Howles, S.; Finch, W.; Fowler, S.; Rogers, A.; Sriprasad, S.; Smith, D.; Dickinson, A.; Gall, Z.; et al. Use of Temporally Validated Machine Learning Models To Predict Outcomes of Percutaneous Nephrolithotomy Using Data from the British Association of Urological Surgeons Percutaneous Nephrolithotomy Audit. Eur. Urol. Focus 2024. [Google Scholar] [CrossRef]
- Alexander Izrailevich, N.; Boris Alexandrovich, N.; Artem Vladimirovich, E.; Leonid Grigoryevich, S.; Dmitry Olegovich, K.; Dmitry Georgievich, T.; Leonid Moiseevich, R. The use of intelligent analysis (IA) in determining the tactics of treating patients with nephrolithiasis. Urologia 2023, 90, 663–669. [Google Scholar] [CrossRef]
- Meng, R.; Wang, W.; Zhai, Z.; Zuo, C. Machine learning algorithm to predict postoperative bleeding complications after lateral decubitus percutaneous nephrolithotomy. Medicine 2024, 103, e37050. [Google Scholar] [CrossRef]
- Cui, D.; Yan, F.; Yi, J.; He, D.; Zhang, Y.; Zhang, Z.; Chen, Y.; Jiao, Y.; Zhang, B. Efficacy and safety of 3D printing-assisted percutaneous nephrolithotomy in complex renal calculi. Sci. Rep. 2022, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, A.; Melnyk, R.; Farooq, S.; Bell, A.; Holler, T.; Saba, P.; Joseph, J. Validity of a patient-specific percutaneous nephrolithotomy (PCNL) simulated surgical rehearsal platform: Impact on patient and surgical outcomes. World J. Urol. 2022, 40, 627–637. [Google Scholar] [CrossRef]
- Liu, Y.; Song, H.; Xiao, B.; Hu, W.; Zhang, G.; Fu, M.; Li, J. PCNL Combined with 3D Printing Technology for the Treatment of Complex Staghorn Kidney Stones. J. Healthc. Eng. 2022, 2022, 7554673. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Xie, Y.; Zhang, X.; Wang, W.; Yuan, H.; Lin, C. Assessment of Three-Dimensional Reconstruction in Percutaneous Nephrolithotomy for Complex Renal Calculi Treatment. Front. Surg. 2021, 8, 701207. [Google Scholar] [CrossRef]
- Huang, Y.S.; Zhu, X.S.; Wan, G.Y.; Zhu, Z.W.; Huang, H.P. Application of simulated puncture in percutaneous nephrolithotomy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 190–197. [Google Scholar] [CrossRef]
- Zhu, X.S.; Yin, X.Y.; Fu, D.H.; Huang, H.P.; Wu, M.; Wang, C.H.; Huang, Y.S. Application of image overlapping in percutaneous nephrolithotomy. Int. Urol. Nephrol. 2023, 55, 3057–3063. [Google Scholar] [CrossRef]
- Hosseini, S.R.; Tehranipour, E.; Khadem, A.; Alwedaie, S.M.J. Three-Dimensional Virtual Reconstruction Method versus Standard Fluoroscopy as a Guiding Tool for an Optimal Puncture Rout in Patients Undergoing Percutaneous Nephrolithotomy: A Randomized Trial Study. Urol. J. 2024, 21, 29–34. [Google Scholar] [CrossRef]
- Qin, F.; Sun, Y.F.; Wang, X.N.; Li, B.; Zhang, Z.L.; Zhang, M.X.; Xie, F.; Liu, S.H.; Wang, Z.J.; Cao, Y.C.; et al. Application of a novel computer-assisted surgery system in percutaneous nephrolithotomy: A controlled study. World J. Clin. Cases 2022, 10, 6039–6049. [Google Scholar] [CrossRef]
- Keyu, G.; Shuaishuai, L.; Raj, A.; Shuofeng, L.; Shuai, L.; Yuan, Z.; Haitao, Z.; Junqi, W. A 3D printing personalized percutaneous puncture guide access plate for percutaneous nephrolithotomy: A pilot study. BMC Urol. 2021, 21, 184. [Google Scholar] [CrossRef]
- Özbir, S.; Atalay, H.A.; Canat, H.L.; Çulha, M.G. Do 3D-calculated volume distribution of a stone in pelvicalyceal system affect complications of percutaneous nephrolithotomy? Urolithiasis 2019, 47, 557–565. [Google Scholar] [CrossRef]
- Rassweiler-Seyfried, M.C.; Rassweiler, J.J.; Weiss, C.; Müller, M.; Meinzer, H.P.; Maier-Hein, L.; Klein, J.T. iPad-assisted percutaneous nephrolithotomy (PCNL): A matched pair analysis compared to standard PCNL. World J. Urol. 2020, 38, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Checcucci, E.; Amparore, D.; Peretti, D.; Piramide, F.; De Cillis, S.; Piana, A.; Niculescu, G.; Verri, P.; Manfredi, M.; et al. Percutaneous Kidney Puncture with Three-dimensional Mixed-reality Hologram Guidance: From Preoperative Planning to Intraoperative Navigation. Eur. Urol. 2022, 81, 588–597. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Wang, G.; Zhou, J.; Zhu, H.; Guo, H.; Huang, H.; Yu, M.; Zhu, G.; Li, N.; et al. Application of a three-dimensional visualization model in intraoperative guidance of percutaneous nephrolithotomy. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2022, 29, 838–844. [Google Scholar] [CrossRef]
- Xia, D.; Peng, E.; Yu, Y.; Yang, X.; Liu, H.; Tong, Y.; Wang, X.; Xu, H.; Ye, Z.; Tang, K.; et al. Comparison of contrast-enhanced ultrasound versus conventional ultrasound-guided percutaneous nephrolithotomy in patients with nondilated collecting system: A randomized controlled trial. Eur. Radiol. 2021, 31, 6736–6746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Xie, J.; Zhao, C.B.; Liu, Y.F.; Li, Z.S.; Guo, J.N.; Jiang, H.T.; Xiao, K.F. Feasibility of contrast-enhanced ultrasound and flank position during percutaneous nephrolithotomy in patients with no apparent hydronephrosis: A randomized controlled trial. World J. Urol. 2022, 40, 1043–1048. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Liu, Z.; Fu, H.; Gao, X.; Yang, H.; Gao, P.; Li, X.; Ai, W.; He, Z.; et al. Assessment of the Contrast-Enhanced Ultrasound in Percutaneous Nephrolithotomy for the Treatment of Patients with Nondilated Collecting System. J. Endourol. 2021, 35, 436–443. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Liu, P.; Zhang, L.; Cao, J. Evaluation of the safety and efficiency of color Doppler ultrasound-guided percutaneous nephrolithotomy in clinical practice: Results from a retrospective study. Ren. Fail. 2023, 45, 2275714. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Mathis, B.J.; Li, W. Can Color Doppler Ultrasound Challenge the Paradigm in Percutaneous Nephrolithotomy? J. Endourol. 2022, 36, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Zhang, Z.; Sun, Z.; Wang, Y.; Han, X. Percutaneous nephrolithotripsy: C-arm CT with 3D virtual navigation in non-dilated renal collecting systems. Diagn. Interv. Radiol. 2018, 24, 17–22. [Google Scholar] [CrossRef]
- Taguchi, K.; Hamamoto, S.; Okada, A.; Sugino, T.; Unno, R.; Kato, T.; Fukuta, H.; Ando, R.; Kawai, N.; Tan, Y.K.; et al. A Randomized, Single-Blind Clinical Trial Comparing Robotic-Assisted Fluoroscopic-Guided with Ultrasound-Guided Renal Access for Percutaneous Nephrolithotomy. J. Urol. 2022, 208, 684–694. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Abol-Enein, H.; Awadalla, A.; El Degla, H.; El-Shehaby, O.A. Investigation of Virulence Genes of the Predominant Bacteria Associated with Renal Stones and their Correlation with Postoperative Septic Complications. Infect. Drug Resist. 2022, 15, 3643–3655. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, S.; Shi, C.; Zhang, W.; Liu, Z.; Jiang, J.; Zhang, Y.; Chen, Z.; Zheng, B.; Zhu, H. Risk factors and predictors of urogenous sepsis after percutaneous nephrolithotomy for idiopathic calcium oxalate nephrolithiasis. Transl. Androl. Urol. 2023, 12, 1002–1015. [Google Scholar] [CrossRef]
- Hou, H.F.; Liu, Y.; Zhang, X.; Han, Z.; Chen, T. The value of postoperative HLA-DR expression and high mobility group box 1 level in predictive diagnosis of sepsis in percutaneous nephrolithotomy surgery. Ren. Fail. 2022, 44, 1338–1344. [Google Scholar] [CrossRef]
- He, Y.; Xia, D.; Tong, Y.; Shang, H.; Liu, X.; Peng, E.; Huang, Q.; Tang, K.; Chen, Z. Predictive value of CD3(+) cells and interleukin 2 receptor in systemic inflammatory response syndrome after percutaneous nephrolithotomy. Front. Immunol. 2022, 13, 1017219. [Google Scholar] [CrossRef]
- Li, D.; Sha, M.; Chen, L.; Xiao, Y.; Lu, J.; Shao, Y. A preliminary study: The role of preoperative procalcitonin in predicting postoperative fever after mini-percutaneous nephrolithotomy in patients with a negative baseline urine culture. Urolithiasis 2019, 47, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, Z.; Cui, Y.; Zeng, H.; Li, Y.; Huang, F.; Cui, Z.; Zeng, F.; Chen, Z.; Li, Y.; et al. The value of procalcitonin for predicting urosepsis after mini-percutaneous nephrolithotomy or flexible ureteroscopy based on different organisms. World J. Urol. 2022, 40, 529–535. [Google Scholar] [CrossRef]
- Qi, T.; Lai, C.; Li, Y.; Chen, X.; Jin, X. The predictive and diagnostic ability of IL-6 for postoperative urosepsis in patients undergoing percutaneous nephrolithotomy. Urolithiasis 2021, 49, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Kriplani, A.; Pandit, S.; Chawla, A.; de la Rosette, J.; Laguna, P.; Jayadeva Reddy, S.; Somani, B.K. Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and lymphocyte-monocyte ratio (LMR) in predicting systemic inflammatory response syndrome (SIRS) and sepsis after percutaneous nephrolithotomy (PNL). Urolithiasis 2022, 50, 341–348. [Google Scholar] [CrossRef]
- Peng, C.; Li, J.; Xu, G.; Jin, J.; Chen, J.; Pan, S. Significance of preoperative systemic immune-inflammation (SII) in predicting postoperative systemic inflammatory response syndrome after percutaneous nephrolithotomy. Urolithiasis 2021, 49, 513–519. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, K.; Chen, X.; Zeng, G.; Sun, F. The Predictive Value of Preoperative Albumin-Globulin Ratio for Systemic Inflammatory Response Syndrome After Percutaneous Nephrolithotomy. Int. J. Gen. Med. 2022, 15, 7407–7415. [Google Scholar] [CrossRef]
- Xu, H.; Hu, L.; Wei, X.; Niu, J.; Gao, Y.; He, J.; Hou, J. The Predictive Value of Preoperative High-Sensitive C-Reactive Protein/Albumin Ratio in Systemic Inflammatory Response Syndrome After Percutaneous Nephrolithotomy. J. Endourol. 2019, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Opondo, D.; Tefekli, A.; Esen, T.; Labate, G.; Sangam, K.; De Lisa, A.; Shah, H.; de la Rosette, J. Impact of case volumes on the outcomes of percutaneous nephrolithotomy. Eur. Urol. 2012, 62, 1181–1187. [Google Scholar] [CrossRef][Green Version]
- Schilling, D.; Gakis, G.; Walcher, U.; Stenzl, A.; Nagele, U. The learning curve in minimally invasive percutaneous nephrolitholapaxy: A 1-year retrospective evaluation of a novice and an expert. World J. Urol. 2011, 29, 749–753. [Google Scholar] [CrossRef]
- Tanriverdi, O.; Boylu, U.; Kendirci, M.; Kadihasanoglu, M.; Horasanli, K.; Miroglu, C. The learning curve in the training of percutaneous nephrolithotomy. Eur. Urol. 2007, 52, 206–211. [Google Scholar] [CrossRef]
- Farcas, M.; Reynolds, L.F.; Lee, J.Y. Simulation-Based Percutaneous Renal Access Training: Evaluating a Novel 3D Immersive Virtual Reality Platform. J. Endourol. 2021, 35, 695–699. [Google Scholar] [CrossRef]
- Sainsbury, B.; Łącki, M.; Shahait, M.; Goldenberg, M.; Baghdadi, A.; Cavuoto, L.; Ren, J.; Green, M.; Lee, J.; Averch, T.D.; et al. Evaluation of a Virtual Reality Percutaneous Nephrolithotomy (PCNL) Surgical Simulator. Front. Robot. AI 2019, 6, 145. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, I.; Kallidonis, P.; Vasilas, M.; Panagopoulos, V.; Kamal, W.; Liatsikos, E. Challenging the wisdom of puncture at the calyceal fornix in percutaneous nephrolithotripsy: Feasibility and safety study with 137 patients operated via a non-calyceal percutaneous track. World J. Urol. 2017, 35, 795–801. [Google Scholar] [CrossRef]
- Kallidonis, P.; Kyriazis, I.; Kotsiris, D.; Koutava, A.; Kamal, W.; Liatsikos, E. Papillary vs Nonpapillary Puncture in Percutaneous Nephrolithotomy: A Prospective Randomized Trial. J. Endourol. 2017, 31, S4–S9. [Google Scholar] [CrossRef]
- Oo, M.M.; Gandhi, H.R.; Chong, K.T.; Goh, J.Q.; Ng, K.W.; Hein, A.T.; Tan, Y.K. Automated Needle Targeting with X-ray (ANT-X)—Robot-assisted device for percutaneous nephrolithotomy (PCNL) with its first successful use in human. J. Endourol. 2021, 35, e919. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, P.; Luo, X.; Hao, Z.; Lu, C.; Zhang, H.; Zhou, T.; Xu, S. Novel laser positioning navigation to aid puncture during percutaneous nephrolithotomy: A preliminary report. World J. Urol. 2019, 37, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Howlett, J.; Lazarus, J.; Kaestner, L. SabreSource™: A novel percutaneous nephrolithotomy apparatus to aid collecting system puncture—A preliminary report. S. Afr. J. Surg. 2020, 58, 105. [Google Scholar] [PubMed]
- Borofsky, M.S.; Rivera, M.E.; Dauw, C.A.; Krambeck, A.E.; Lingeman, J.E. Electromagnetic Guided Percutaneous Renal Access Outcomes Among Surgeons and Trainees of Different Experience Levels: A Pilot Study. Urology 2020, 136, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Ruhayel, Y.; Tepeler, A.; Dabestani, S.; MacLennan, S.; Petřík, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Türk, C.; et al. Tract Sizes in Miniaturized Percutaneous Nephrolithotomy: A Systematic Review from the European Association of Urology Urolithiasis Guidelines Panel. Eur. Urol. 2017, 72, 220–235. [Google Scholar] [CrossRef]
- Adhikari, M.B.; Karna, S.; Adhikari, K.; Kasaju, A.; Baidya, J.L. Impact of Miniaturization on Early Outcome of Percutaneous Nephrolithotomy. J. Nepal Health Res. Counc. 2019, 17, 320–324. [Google Scholar] [CrossRef]
- Croghan, S.M.; Skolarikos, A.; Jack, G.S.; Manecksha, R.P.; Walsh, M.T.; O’Brien, F.J.; Davis, N.F. Upper urinary tract pressures in endourology: A systematic review of range, variables and implications. BJU Int. 2023, 131, 267–279. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
| Reference | Sample Size (n) | Type of Study | Technology | Diminished Complications |
|---|---|---|---|---|
| [21] | 45 | Prospective randomized | Printed 3D model | Yes |
| [22] | 20 | Prospective non-randomized | Printed 3D model | Yes |
| [23] | 72 | Prospective randomized | Printed 3D model | No |
| [24] | 139 | Retrospective | Virtual 3D model | Yes |
| [25] | 120 | Prospective randomized | Virtual 3D model | Less intrasurgical bleeding |
| [26] | 140 | Prospective randomized | Virtual 3D model | Less Hb drop |
| [27] | 48 | Prospective randomized | Virtual 3D model | No |
| [28] | 60 | Retrospective | Virtual 3D model | No |
| [29] | 22 | Prospective randomized | Printed 3D puncture guide access plate | Less intrasurgical bleeding |
| [30] | 141 | Retrospective | 3D volume segmentation | No Prediction of complications |
| Reference, First Author, Date | Sample Size (n) | Type of Study | Technology | Diminished Complications |
|---|---|---|---|---|
| [31] | 44 | Prospective non-randomized | Augmented reality (iPad-assisted puncture) | No |
| [32] | 10 | Prospective non-randomized | Augmented reality (three-dimensional mixed reality holograms) | No |
| [33] | 61 | Prospective | Augmented reality (three-dimensional mixed reality holograms) | No |
| [34] | 160 | Prospective randomized | Contrast US vs. standard US | Less Hb drop |
| [35] | 72 | Prospective randomized | Contrast US vs. standard US | Less Hb drop |
| [36] | 56 | Prospective randomized | Contrast US vs. standard US | Less Hb drop |
| [37] | 228 | Retrospective | Dopper colour US vs. standard US | Less Hb drop Less transfusion rate Less LOS |
| [38] | 348 | Prospective non-randomized | Dopper colour US vs. Standard US | Less tract bleeding Less Hb drop Less LOS |
| [39] | 33 | Retrospective | C-arm CT-guided with 3D virtual navigation after standard failed access. | No |
| [40] | 71 | Prospective randomized | Robotic-assisted fluoroscopic-guided vs. Standard US | No |
| Reference, First Author, Date | Sample Size (n) | Type of Study | Biomarker | Prediction of Complications |
|---|---|---|---|---|
| [41] | 200 | Prospective non-randomized | Bacterial virulence genes: hlb, pvl, fnbB, can and seb (stone culture and PCR assay) | Sepsis |
| [42] | 156 | Retrospective | NOD2 gen | Sepsis |
| [43] | 387 | Prospective non-randomized | HMGB1/HLA-DR | Sepsis |
| [44] | 154 | Retrospective | Low CD3+ cell/high IL2r | SIRS |
| [45] | 407 | Retrospective | Procalcitonin | Postoperative fever |
| [46] | 356 | Retrospective | Procalcitonin | Sepsis |
| [47] | 90 | Retrospective | Interleukin 6 | Sepsis |
| [48] | 517 | Prospective non-randomized | NLR, PLR, LMR | SIRS/Sepsis |
| [49] | 356 | Retrospective | NLR, PLR, LMR | SIRS |
| [50] | 354 | Retrospective | Preoperative AGR | SIRS |
| [51] | 556 | Retrospective | hs-CRP/Alb ratio | SIRS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Baltar, C.; Martínez Corral, M.E.; Pérez Fentes, D. Predicting and Avoiding Complications in Percutaneous Nephrolithotomy in the Era of Personalized Medicine: A Scoping Review. J. Pers. Med. 2024, 14, 962. https://doi.org/10.3390/jpm14090962
Fernández Baltar C, Martínez Corral ME, Pérez Fentes D. Predicting and Avoiding Complications in Percutaneous Nephrolithotomy in the Era of Personalized Medicine: A Scoping Review. Journal of Personalized Medicine. 2024; 14(9):962. https://doi.org/10.3390/jpm14090962
Chicago/Turabian StyleFernández Baltar, Carlos, María Elena Martínez Corral, and Daniel Pérez Fentes. 2024. "Predicting and Avoiding Complications in Percutaneous Nephrolithotomy in the Era of Personalized Medicine: A Scoping Review" Journal of Personalized Medicine 14, no. 9: 962. https://doi.org/10.3390/jpm14090962
APA StyleFernández Baltar, C., Martínez Corral, M. E., & Pérez Fentes, D. (2024). Predicting and Avoiding Complications in Percutaneous Nephrolithotomy in the Era of Personalized Medicine: A Scoping Review. Journal of Personalized Medicine, 14(9), 962. https://doi.org/10.3390/jpm14090962





