The 3-Steps Approach for Lumbar Stenosis with Anatomical Insights, Tailored for Young Spine Surgeons
Abstract
1. Introduction
Epidemiology and Pathophysiology
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Follow-Up
2.4. Additional Data Collection
3. Surgical Technique
3.1. Anatomical Consideration
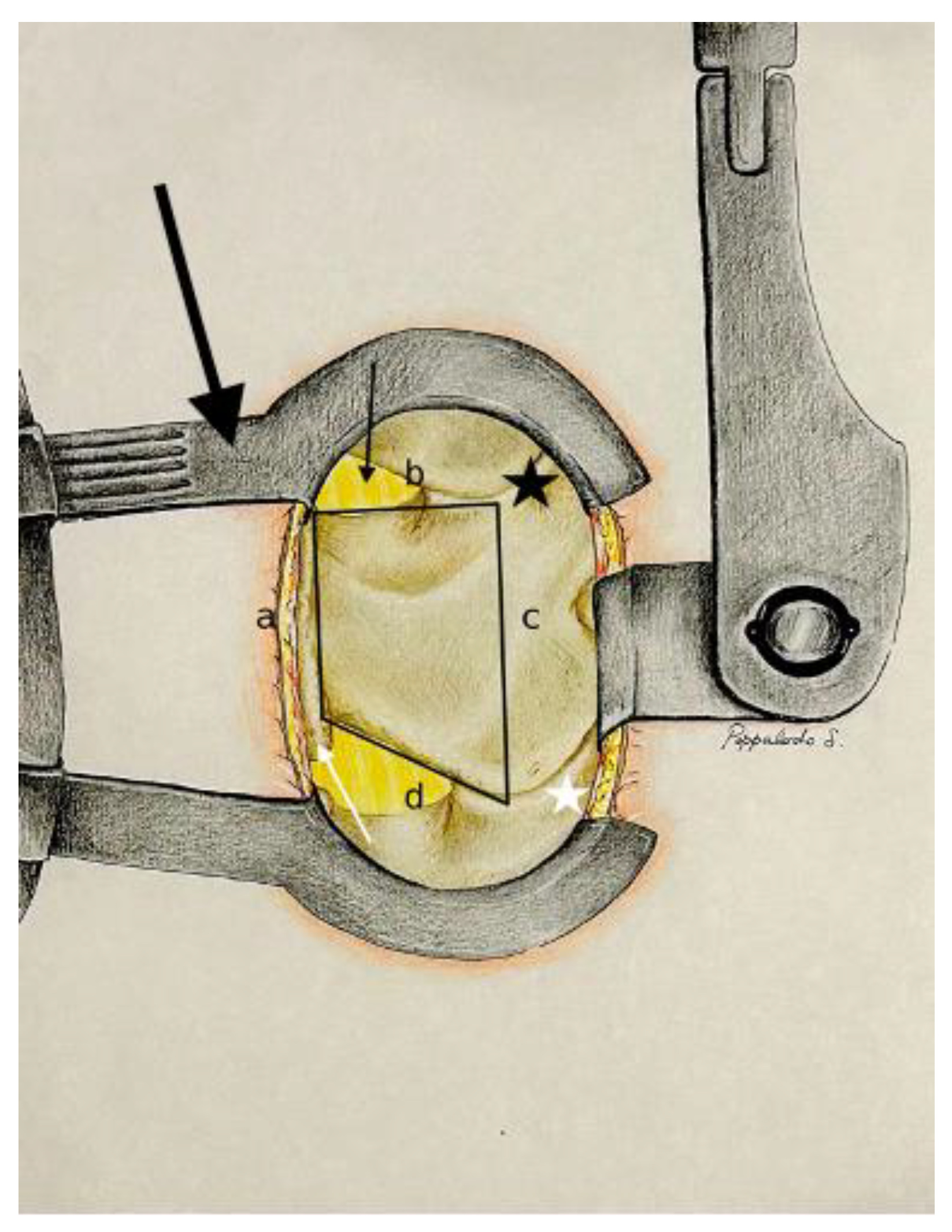
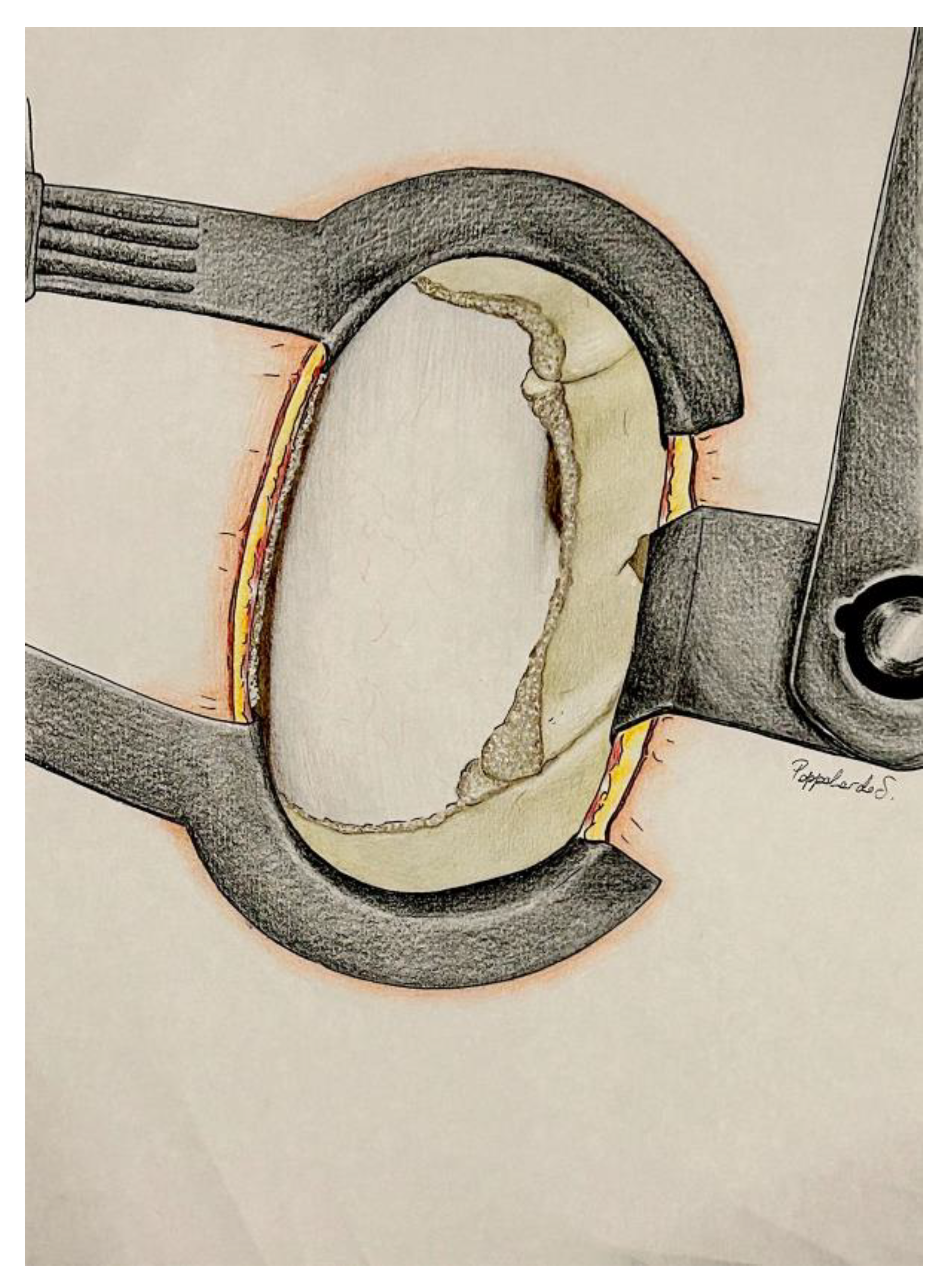
3.2. Posterior Surgical Lumbar Trapezoid: Anatomical Landmarks
- -
- The caudal margin of the spinous process base is situated at the lower base of the spinous process, and marks the inferior limit of the trapezoidal space.
- -
- The cranial margin of the spinous process base is located at the upper base of the spinous process, and establishes the superior boundary of the trapezoid.
- -
- The medial margin of the superior articular process is defined by the inner edge of the superior articular process, and marks the medial limit on the superior aspect of the trapezoid.
- -
- The medial margin of the inferior articular process is found along the inner edge of the inferior articular process, and represents the medial boundary on the inferior aspect of the trapezoid.
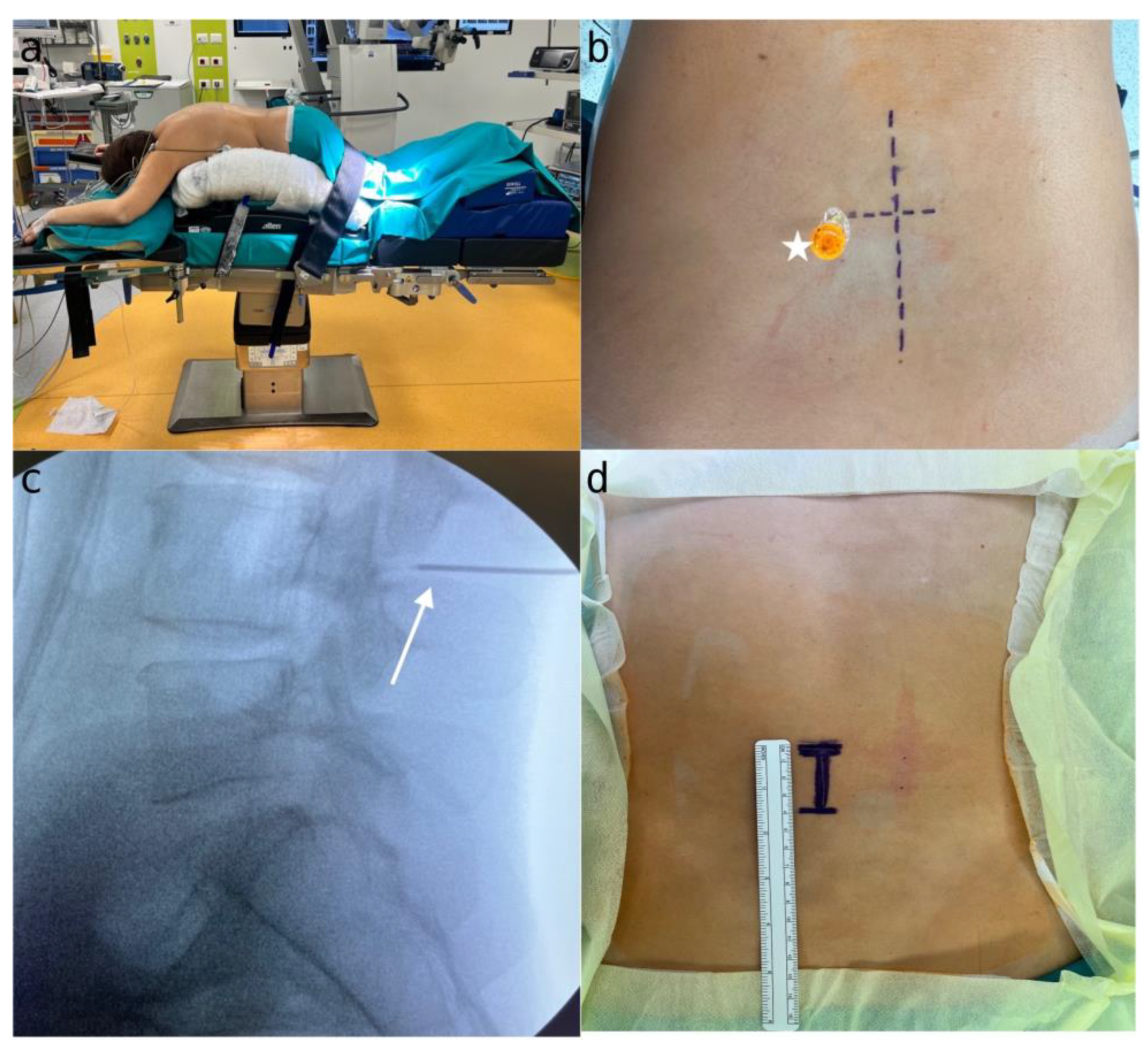
3.3. Pre-Operative Evaluation, Positioning, and Surgical Level Localization
4. Three Step Approach for Lumbar Spinal Stenosis
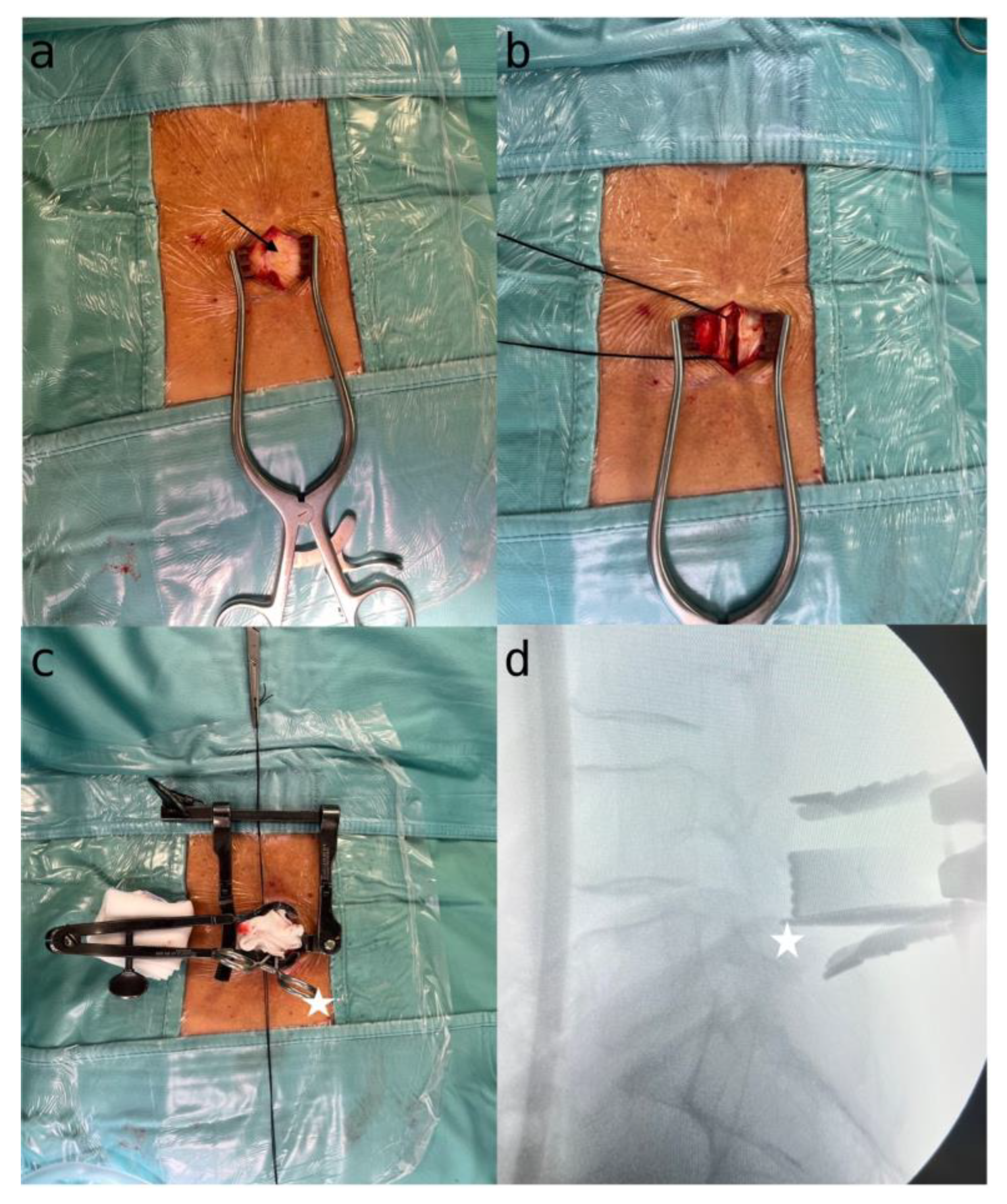
4.1. Step 1: Exposure and Skeletal Visualization
4.2. Step 2: Microscopic Identification and Decompression
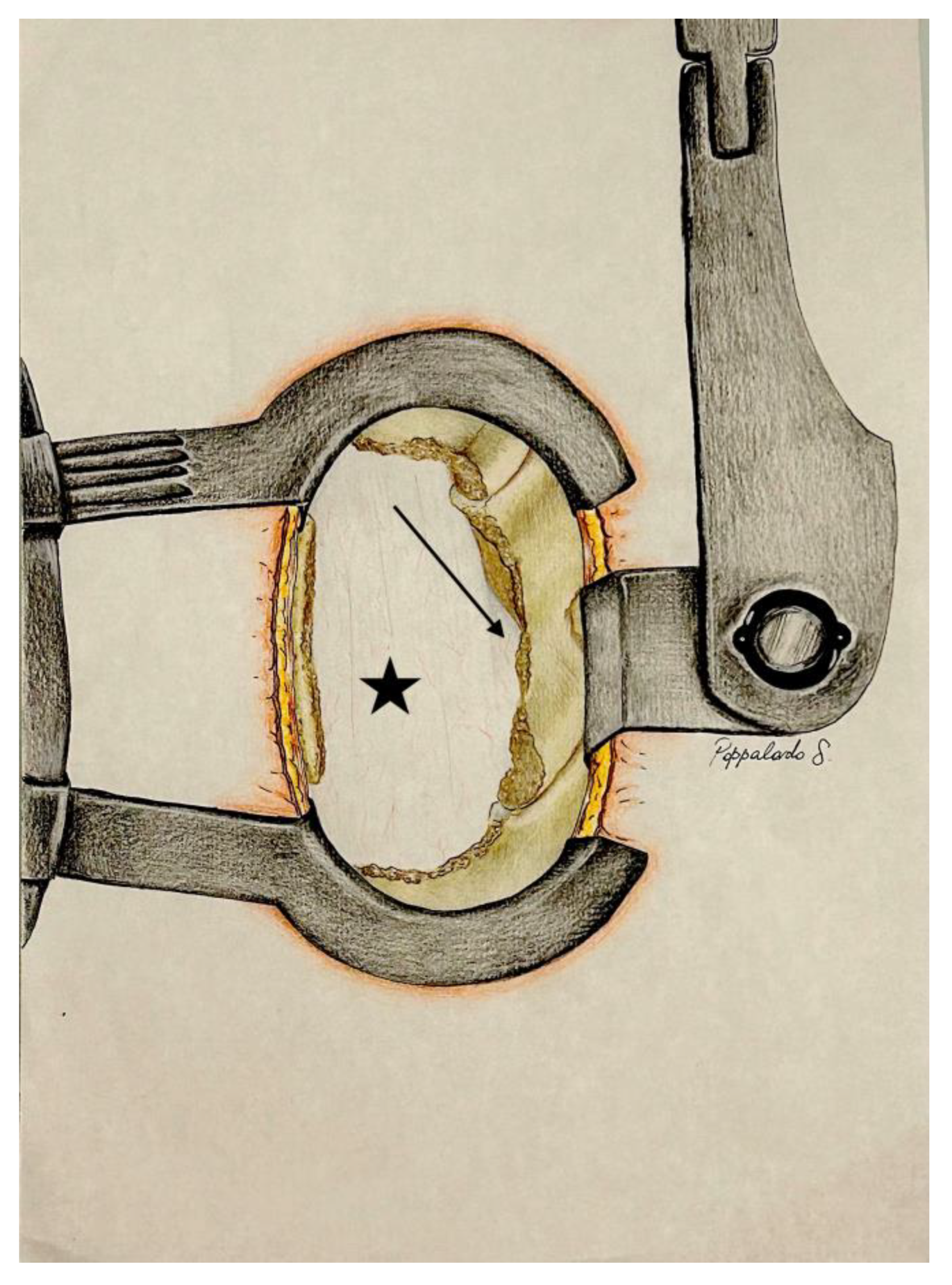
4.3. Step 3: Undermining of the Spinous Process Base and Contralateral Decompression
5. Results
5.1. Study Population
5.2. Operative Details
5.3. Complications
5.4. Pre and Postoperative Clinical Assessment
5.5. Measurement
6. Discussion
6.1. Impact of Surgical Approach
6.2. Comparation with Others Approachs
6.2.1. Comparison with Open and Traditional Approaches
6.2.2. Microscopic Unilateral vs. Bilateral Decompression
6.2.3. Endoscopic and Microendoscopic Techniques
6.3. Predictor of Outcome
6.4. Surgical Complications
6.5. Training of Young Surgeons and Progressive Improvement
7. Strength and Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sobański, D.; Staszkiewicz, R.; Stachura, M.; Gadzieliński, M.; Grabarek, B.O. Presentation, Diagnosis, and Management of Lower Back Pain Associated with Spinal Stenosis: A Narrative Review. Med. Sci. Monit. 2023, 29, e939237-e1. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, N.D.; Lambrechts, M.J.; Trenchfield, D.; Sherman, M.; Karamian, B.A.; Fredericks, D.J.; Boere, P.; Siegel, N.; Tran, K.; Canseco, J.A.; et al. Patient-Specific Risk Factors Increase Episode of Care Costs After Lumbar Decompression. Clin. Spine Surg. A Spine Publ. 2023, 36, E339–E344. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Zimmerman, Z.E.; Mass, H.; Makhni, M.C. Diagnosis and Management of Lumbar Spinal Stenosis: A Review. JAMA 2022, 327, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Witiw, C.D.; O’Toole, E. Cervical, Thoracic, and Lumbar Stenosis. In Youmans and Winn Neurological Surgery; Richard, W.H., Ed.; Elsevier: Philadelphia, PA, USA, 2022; Volume 3, pp. 2497–2509. [Google Scholar]
- Jang, J.N.; Song, Y.; Kim, J.W.; Kim, Y.U. Comparison of Ligamentum Flavum Thickness between Central and Lateral Lesions in a Patient with Central Lumbar Spinal Canal Stenosis. Medicine 2023, 102, E34873. [Google Scholar] [CrossRef]
- Sudhir, G.; Vignesh Jayabalan, S.; Gadde, S.; Venkatesh Kumar, G.; Karthik Kailash, K. Analysis of Factors Influencing Ligamentum Flavum Thickness in Lumbar Spine—A Radiological Study of 1070 Disc Levels in 214 Patients. Clin. Neurol. Neurosurg. 2019, 182, 19–24. [Google Scholar] [CrossRef]
- Quattrocchi, C.C.; Alexandre, A.M.; Pepa, G.M.D.; Altavilla, R.; Zobel, B.B. Modic Changes: Anatomy, Pathophysiology and Clinical Correlation. Acta Neurochir. Suppl. 2011, 108, 49–53. [Google Scholar] [CrossRef]
- Aleksić, V.; Todorović, J.; Miladinović, N.; Aleksić, N.; Bogosavljević, V.; Đurović, M.; Kocić, S.; Aleksić, R.; Joković, M. Ligamentum Flavum Analysis in Patients with Lumbar Discus Hernia and Lumbar Spinal Stenosis. Sci. Rep. 2023, 13, 3804. [Google Scholar] [CrossRef]
- Porter, R.W.; Ward, D. Cauda Equina Dysfunction. The Significance of Two-Level Pathology. Spine (Phila Pa 1976) 1992, 17, 9–15. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Wang, X.; Liu, X. Ligamentum Flavum Fibrosis and Hypertrophy: Molecular Pathways, Cellular Mechanisms, and Future Directions. FASEB J. 2020, 34, 9854–9868. [Google Scholar] [CrossRef]
- La Rocca, G.; Galieri, G.; Mazzucchi, E.; Pignotti, F.; Orlando, V.; Pappalardo, S.; Olivi, A.; Sabatino, G. The Three-Step Approach for Lumbar Disk Herniation with Anatomical Insights Tailored for the Next Generation of Young Spine Surgeons. J. Clin. Med. 2024, 13, 3571. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Moss, N.; Virk, M.S.; Fu, K.-M.G. Spinal Anatomy. In Youmans and Winn Neurological Surgery; Winn, H.R., Ed.; Elsevier: Philadelphia, PA, USA, 2022; Volume 3, pp. 2390–2401. [Google Scholar]
- Galieri, G.; Mazzucchi, E.; Pignotti, F.; Rinaldi, P.; De Santis, V.; La Rocca, G.; Sabatino, G. Lumbo-Sacral Pedicular Aplasia Diagnosis and Treatment: A Systematic Literature Review and Case Report. Br. J. Neurosurg. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yoshiki, T.; Shuichi, M.; James, D.; Disk, K. Degeneration and Regeneration. In Youmans and Winn Neurological Surgery; Winn, H.R., Ed.; Elsevier: Philadelphia, PA, USA, 2022; Volume 3, pp. 2408–2413. [Google Scholar]
- Hermansen, E.; Austevoll, I.M.; Hellum, C.; Storheim, K.; Myklebust, T.Å.; Aaen, J.; Banitalebi, H.; Anvar, M.; Rekeland, F.; Brox, J.I.; et al. Comparison of 3 Different Minimally Invasive Surgical Techniques for Lumbar Spinal Stenosis: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, E224291. [Google Scholar] [CrossRef]
- Overdevest, G.M.; Jacobs, W.; Vleggeert-Lankamp, C.; Thomé, C.; Gunzburg, R.; Peul, W. Effectiveness of Posterior Decompression Techniques Compared with Conventional Laminectomy for Lumbar Stenosis. Cochrane Database Syst. Rev. 2015, 2015, CD010036. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Thomas, A.; Kanna, R.M.; Prasad Shetty, A. Lumbar Spinous Process Splitting Decompression Provides Equivalent Outcomes to Conventional Midline Decompression in Degenerative Lumbar Canal Stenosis: A Prospective, Randomized Controlled Study of 51 Patients. Spine (Phila Pa 1976) 2013, 38, 1737–1743. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Surgical Treatment for Lumbar Lateral Recess Stenosis with the Full-Endoscopic Interlaminar Approach versus Conventional Microsurgical Technique: A Prospective, Randomized, Controlled Study. J. Neurosurg. Spine 2009, 10, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Alves, O.L.; Anania, C.D.; Zileli, M.; Fornari, M. Decompressive Surgery for Lumbar Spinal Stenosis: WFNS Spine Committee Recommendations. World Neurosurg. X 2020, 7, 100076. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; El Chamaa, A.; Mehta, S.; Rushton, A.; Battié, M.C. Depression as a Prognostic Factor for Lumbar Spinal Stenosis Outcomes: A Systematic Review. Eur. Spine J. 2024, 33, 851–871. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E.; Gilder, H.; Maloney, P.R.; McCutcheon, B.A.; Rinaldo, L.; Shepherd, D.; Kerezoudis, P.; Ubl, D.S.; Crowson, C.S.; Krauss, W.E.; et al. Lumbar Decompression in the Elderly: Increased Age as a Risk Factor for Complications and Nonhome Discharge. J. Neurosurg. Spine 2017, 26, 353–362. [Google Scholar] [CrossRef]
- Knutsson, B.; Michaëlsson, K.; Sandén, B. Obesity Is Associated with Inferior Results after Surgery for Lumbar Spinal Stenosis: A Study of 2633 Patients from the Swedish Spine Register. Spine (Phila Pa 1976) 2013, 38, 435–441. [Google Scholar] [CrossRef]
- Ghobrial, J.; Gadjradj, P.; Harhangi, B.; Dammers, R.; Vleggeert-Lankamp, C. Outcome of Non-Instrumented Lumbar Spinal Surgery in Obese Patients: A Systematic Review. Br. J. Neurosurg. 2022, 36, 447–456. [Google Scholar] [CrossRef]
- Merrill, R.K.; Zebala, L.P.; Peters, C.; Qureshi, S.A.; McAnany, S.J. Impact of Depression on Patient-Reported Outcome Measures After Lumbar Spine Decompression. Spine (Phila Pa 1976) 2018, 43, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchi, E.; La Rocca, G.; Cusumano, D.; Bazzu, P.; Pignotti, F.; Galieri, G.; Rinaldi, P.; De Santis, V.; Sabatino, G. The Role of Psychopathological Symptoms in Lumbar Stenosis: A Prediction Model of Disability after Lumbar Decompression and Fusion. Front. Psychol. 2023, 14, 1070205. [Google Scholar] [CrossRef] [PubMed]
- Canseco, J.A.; Karamian, B.A.; Minetos, P.D.; Paziuk, T.M.; Gabay, A.; Reyes, A.A.; Bechay, J.; Xiao, K.B.; Nourie, B.O.; Kaye, I.D.; et al. Risk Factors for 30-Day and 90-Day Readmission After Lumbar Decompression. Spine (Phila Pa 1976) 2022, 47, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Zhao, X.W.; Ma, J.X.; Li, F.; Wang, Y.; Lu, B. Effectiveness of Surgery versus Conservative Treatment for Lumbar Spinal Stenosis: A System Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Surg. 2017, 44, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.L.; Zhou, C.P.; Liu, R.; Zhu, K.L.; Du, M.R.; Gao, H.R.; Wu, S.D.; Sun, L.L.; Yan, X.D.; Liu, Y.; et al. Management for Lumbar Spinal Stenosis: A Network Meta-Analysis and Systematic Review. Int. J. Surg. 2021, 85, 19–28. [Google Scholar] [CrossRef]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I.; Kreuter, W.; Goodman, D.C.; Jarvik, J.G. Trends, Major Medical Complications, and Charges Associated with Surgery for Lumbar Spinal Stenosis in Older Adults. JAMA 2010, 303, 1259–1265. [Google Scholar] [CrossRef]
- Li, G.; Patil, C.G.; Lad, S.P.; Ho, C.; Tian, W.; Boakye, M. Effects of Age and Comorbidities on Complication Rates and Adverse Outcomes after Lumbar Laminectomy in Elderly Patients. Spine (Phila Pa 1976) 2008, 33, 1250–1255. [Google Scholar] [CrossRef]
- Sobottke, R.; Aghayev, E.; Röder, C.; Eysel, P.; Delank, S.K.; Zweig, T. Predictors of Surgical, General and Follow-up Complications in Lumbar Spinal Stenosis Relative to Patient Age as Emerged from the Spine Tango Registry. Eur. Spine J. 2012, 21, 411–417. [Google Scholar] [CrossRef]
- Suzuki, A.; Nakamura, H. Microendoscopic Lumbar Posterior Decompression Surgery for Lumbar Spinal Stenosis: Literature Review. Medicina 2022, 58, 384. [Google Scholar] [CrossRef]
- Zhuang, H.X.; Guo, S.J.; Meng, H.; Lin, J.S.; Yang, Y.; Fei, Q. Unilateral Biportal Endoscopic Spine Surgery for Lumbar Spinal Stenosis: A Systematic Review and Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4998–5012. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.F.; Shan, H.; Wan, Z.Y.; Wang, Z.; Viswanath, O.; Paladini, A.; Varrassi, G.; Wang, H.Q. Decompression Using Minimally Invasive Surgery for Lumbar Spinal Stenosis Associated with Degenerative Spondylolisthesis: A Review. Pain. Ther. 2021, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Fourney, D.R.; Dettori, J.R.; Norvell, D.C.; Dekutoski, M.B. Does Minimal Access Tubular Assisted Spine Surgery Increase or Decrease Complications in Spinal Decompression or Fusion? Spine (Phila Pa 1976) 2010, 35, S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Alhaug, O.K.; Dolatowski, F.; Austevoll, I.; Mjønes, S.; Lønne, G. Incidental Dural Tears Associated with Worse Clinical Outcomes in Patients Operated for Lumbar Spinal Stenosis. Acta Neurochir. 2023, 165, 99. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sato, T.; Hyodo, H.; Kawamata, T.; Takahashi, E.; Miyatake, N.; Tokunaga, M. Incidental Durotomy during Lumbar Spine Surgery: Risk Factors and Anatomic Locations: Clinical Article. J. Neurosurg. Spine 2013, 18, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Tenhoeve, S.A.; Karsy, M. Lumbar Epidural Hematoma as a Rare Complication From Minimally Invasive Lumbar Decompression. Cureus 2023, 15, e51083. [Google Scholar] [CrossRef]
- Soejima, Y.; Arizono, T.; Bekki, H.; Inokuchi, A.; Izumi, T.; Imamura, R.; Hamada, T.; Nakamura, K.; Sakai, M.; Yoshimoto, M.; et al. Factors Affecting Postoperative Spinal Epidural Hematoma and the Optimal Order of Vertebral Body Decompression in Multivertebral Microendoscopic Laminectomy. Cureus 2022, 14, e25404. [Google Scholar] [CrossRef]
- Hohenberger, C.; Zeman, F.; Höhne, J.; Ullrich, O.W.; Brawanski, A.; Schebesch, K.M. Symptomatic Postoperative Spinal Epidural Hematoma after Spinal Decompression Surgery: Prevalence, Risk Factors, and Functional Outcome. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 290–296. [Google Scholar] [CrossRef]
- Leonardi, M.A.; Zanetti, M.; Saupe, N.; Min, K. Early Postoperative MRI in Detecting Hematoma and Dural Compression after Lumbar Spinal Decompression: Prospective Study of Asymptomatic Patients in Comparison to Patients Requiring Surgical Revision. Eur. Spine J. 2010, 19, 2216–2222. [Google Scholar] [CrossRef]
- Bekki, H.; Arizono, T.; Inokuchi, A.; Imamura, R.; Hamada, T.; Oyama, R.; Hyodo, Y.; Kinoshita, E.; Kido, M. Risk Factors for Incidence of Postoperative Spinal Epidural Hematoma Following Multilevel Microendoscopic Laminectomy. Spine Surg. Relat. Res. 2021, 6, 45–50. [Google Scholar] [CrossRef]
- Deyo, R.A.; Martin, B.I.; Kreuter, W.; Jarvik, J.G.; Angier, H.; Mirza, S.K. Revision Surgery Following Operations for Lumbar Stenosis. J. Bone Jt. Surg. Am. 2011, 93, 1979–1986. [Google Scholar] [CrossRef]
- Radcliff, K.; Curry, P.; Hilibrand, A.; Kepler, C.; Lurie, J.; Zhao, W.; Albert, T.J.; Weinstein, J. Risk for Adjacent Segment and Same Segment Reoperation after Surgery for Lumbar Stenosis: A Subgroup Analysis of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976) 2013, 38, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Lang, Z.; Li, J.S.; Yang, F.; Yu, Y.; Khan, K.; Jenis, L.G.; Cha, T.D.; Kang, J.D.; Li, G. Reoperation of Decompression Alone or Decompression plus Fusion Surgeries for Degenerative Lumbar Diseases: A Systematic Review. Eur. Spine J. 2018, 28, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Chung, C.K.; Kim, C.H.; Choi, Y.; Kim, M.J.; Yim, D.; Yang, S.H.; Lee, C.H.; Hwang, S.H.; Kim, D.H.; et al. The Long-Term Reoperation Rate Following Surgery for Lumbar Stenosis: A Nationwide Sample Cohort Study With a 10-Year Follow-Up. Spine (Phila Pa 1976) 2020, 45, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.F.; Mroz, T.; Hsu, W.; Chutkan, N. Management of Degenerative Lumbar Spinal Stenosis in the Elderly. Neurosurgery 2015, 77 (Suppl. S4), S68–S74. [Google Scholar] [CrossRef]
| Demographic Data | N. of Patients | Other Data | |
|---|---|---|---|
| Patients | 530 | Average Age | 67 years (±9.04) |
| Employed/non-employed | 262/268 | Male/Female Ratio | 262/268 |
| Smokers/Non-smokers | 162/368 | Mean BMI | 26.88 (±4.3) |
| Arterial Hypertension | 273 | Mean Follow-up | 12–48 months |
| Previous orthopedic surgery | 95 | ||
| Fibromyalgia | 109 |
| Lumbar Stenosis Treated | N. of Levels | Lumbar Stenosis Treated | N. of Levels | Surgical Data | |
|---|---|---|---|---|---|
| Total lumbar patients | 530 | L5S1 | 87 | Average wound size | 4.61 cm (±1.68) |
| Total lumbar levels | L4L5 | 360 | Mean surgical time | 47 min (±19.4) | |
| Single level | 325 | L3L4 | 234 | Average hospital stay | 2.64 days (±0.78) |
| Double level | 157 | L2L3 | 92 | Average wound size | 4.51 cm (±1.58) |
| Triple level | 39 | L2L1 | 13 | ||
| Quadruple level | 9 | D12D11 | 1 | ||
| D11D10 | 1 | ||||
| D10D9 | 1 |
| Intraoperative Complications | N. of Complications | % | Postoperative Complications | N. of Complications | % |
|---|---|---|---|---|---|
| Dural tear | 32 | 6.04 | Recurrence | 0 | 0 |
| Atrial fibrillation | 1 | 0.19 | Instability | 0 | 0 |
| 0.11 | Subcutaneous hematoma | 3 | 0.57 | ||
| 1.96 | Thromboembolism | 1 | 0.19 | ||
| Anemia | 1 | 0.19 | |||
| Amaurosis left eye | 1 | 0.19 | |||
| Wound infection | 8 | 1.52 |
| Patient Assessment | Pre-Operative | Post-Operative |
|---|---|---|
| ODI (Oswestry Disability Index) | 52.2% (±18.6) | 24.7% (±18.7) |
| VAS (Visual Analog Scale) Leg Pain | 8.01 (±1.4) | 3.1 (±2.5) |
| VAS (Visual Analog Scale) Back Pain | 8.01 (±1.37) | 2.2 (±2.3) |
| EQ-5D (EuroQuality of life5 Dimensions) | 0.377 (±0.211) | 0.684 (±0.233) |
| Surgical Triangle Dimension | Characteristics/Borders | Average Length (mm) | Standard Deviation |
|---|---|---|---|
| First side | from the caudal to the cranial point of the base of the spinous process | 16.6 | 2.02 |
| Second Side | from the cranial point of the base of the spinous process to the medial margin of the superior articular process | 20.3 | 1.28 |
| Third Side | from the medial margin of the superior articular process to the medial margin of the inferior articular process | 26.5 | 2.06 |
| Fourth Side | from the caudal point of the base of the spinous process to the medial margin of the inferior articular process | 21.5 | 1.86 |
| Area of drilled bone | Trapezoidal area | 437.4 mm2 | 40.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocca, G.L.; Galieri, G.; Mazzucchi, E.; Pignotti, F.; Orlando, V.; Pappalardo, S.; Olivi, A.; Sabatino, G. The 3-Steps Approach for Lumbar Stenosis with Anatomical Insights, Tailored for Young Spine Surgeons. J. Pers. Med. 2024, 14, 985. https://doi.org/10.3390/jpm14090985
Rocca GL, Galieri G, Mazzucchi E, Pignotti F, Orlando V, Pappalardo S, Olivi A, Sabatino G. The 3-Steps Approach for Lumbar Stenosis with Anatomical Insights, Tailored for Young Spine Surgeons. Journal of Personalized Medicine. 2024; 14(9):985. https://doi.org/10.3390/jpm14090985
Chicago/Turabian StyleRocca, Giuseppe La, Gianluca Galieri, Edoardo Mazzucchi, Fabrizio Pignotti, Vittorio Orlando, Simona Pappalardo, Alessandro Olivi, and Giovanni Sabatino. 2024. "The 3-Steps Approach for Lumbar Stenosis with Anatomical Insights, Tailored for Young Spine Surgeons" Journal of Personalized Medicine 14, no. 9: 985. https://doi.org/10.3390/jpm14090985
APA StyleRocca, G. L., Galieri, G., Mazzucchi, E., Pignotti, F., Orlando, V., Pappalardo, S., Olivi, A., & Sabatino, G. (2024). The 3-Steps Approach for Lumbar Stenosis with Anatomical Insights, Tailored for Young Spine Surgeons. Journal of Personalized Medicine, 14(9), 985. https://doi.org/10.3390/jpm14090985







