Complement Inhibitors for Geographic Atrophy in Age-Related Macular Degeneration—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
- P—patients aged 50 years or over with GA due to dry AMD.
- I—a complement inhibitor agent was administered (any type, dose, and method of administration).
- C—comparison with GA and dry AMD in the placebo group.
- O—differences in rate of change between study groups and sham groups in terms of structural and/or functional parameters in a follow-up period of at least 52 weeks.
- S—prospective, interventional, and controlled trials were included.
2.2. Data Collection and Quality Appraisal of the Studies Included in the Review
Data Analysis
3. Results
3.1. Demographic and Inclusion Criteria of the Patients in the Quantitative Analysis
3.2. Functional Outcomes of the Complement Inhibitor Therapy for GA in AMD
3.3. Structural Outcomes of the Complement Inhibitor Therapy for GA in AMD
3.4. Other Imagistic Biomarkers
3.5. Factors Correlated with GA Progression and Response to Therapy
3.6. Genetic Analysis
3.7. Safety of Intravitreal Complement Inhibitors
4. Discussion
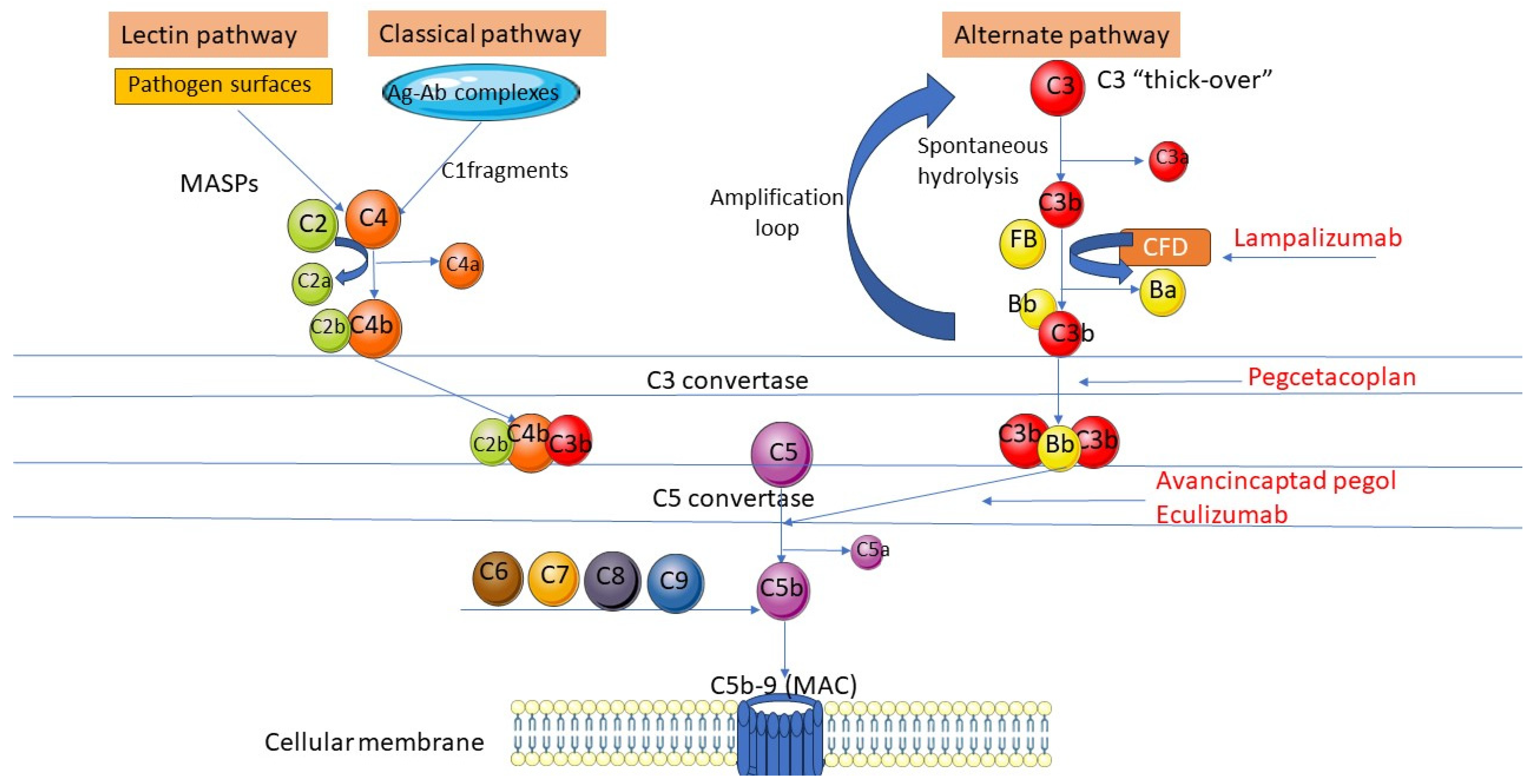
4.1. Complement System in AMD Pathology and Potential Limitations of Complement Inhibitor Therapy
4.2. Current Challenges in Complement Inhibitor Therapy for GA Due to AMD
4.3. Future Directions of Research
4.3.1. Complement Inhibitors in Neovascular AMD
4.3.2. Novel Biomarkers and a Personalized Therapeutic Approach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Vision Loss Expert Group of the Global Burden of Disease Study; The GBD 2019 Blindness and Vision Impairment Collaborators. Global estimates on the number of people blind or visually impaired by age-related macular degeneration: A meta-analysis from 2000 to 2020. Eye 2024, 38, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Bektas, M.; Sharp, D.; Luo, R.; Sarda, S.P.; Khan, S. Geographic atrophy: Mechanism of disease, pathophysiology, and role of the complement system. J. Manag. Care Spec. Pharm. 2023, 29 (Suppl. 5-a), S2–S11. [Google Scholar] [CrossRef]
- Varma, R.; Souied, E.H.; Tufail, A.; Tschosik, E.; Ferrara, D.; Zhang, J.; Silverman, D.; Dolan, C.; Bressler, N.M. Maximum Reading Speed in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD195–AMD201. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, CD000254. [Google Scholar] [CrossRef] [PubMed]
- Pameijer, E.M.; Heus, P.; Damen, J.A.A.; Spijker, R.; Hooft, L.; Ringens, P.J.; Imhof, S.M.; van Leeuwen, R. What did we learn in 35 years of research on nutrition and supplements for age-related macular degeneration: A systematic review. Acta Ophthalmol. 2022, 100, e1541–e1552. [Google Scholar] [CrossRef]
- Dascalu, A.M.; Anghelache, A.; Stana, D.; Costea, A.C.; Nicolae, V.A.; Tanasescu, D.; Costea, D.O.; Tribus, L.C.; Zgura, A.; Serban, D.; et al. Serum levels of copper and zinc in diabetic retinopathy: Potential new therapeutic targets (Review). Exp. Ther. Med. 2022, 23, 324. [Google Scholar] [CrossRef]
- Kim, B.J.; Mastellos, D.C.; Li, Y.; Dunaief, J.L.; Lambris, J.D. Targeting complement components C3 and C5 for the retina: Key concepts and lingering questions. Prog. Retin. Eye Res. 2021, 83, 100936. [Google Scholar] [CrossRef] [PubMed]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Piri, N.; Kaplan, H.J. Role of Complement in the Onset of Age-Related Macular Degeneration. Biomolecules 2023, 13, 832. [Google Scholar] [CrossRef]
- Park, Y.G.; Park, Y.S.; Kim, I.B. Complement System and Potential Therapeutics in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 6851. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahn, S.J.; Woo, S.J.; Hong, H.K.; Suh, E.J.; Ahn, J.; Park, J.H.; Ryoo, N.K.; Lee, J.E.; Kim, K.W.; et al. Proteomics-based identification and validation of novel plasma biomarkers phospholipid transfer protein and mannan-binding lectin serine protease-1 in age-related macular degeneration. Sci. Rep. 2016, 6, 32548. [Google Scholar] [CrossRef] [PubMed]
- Schick, T.; Steinhauer, M.; Aslanidis, A.; Altay, L.; Karlstetter, M.; Langmann, T.; Kirschfink, M.; Fauser, S. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye 2017, 31, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, T.; Jia, H.; Gao, M.; Li, Y.; Wan, X.; Huang, Z.; Li, M.; Zhai, Y.; Li, X.; et al. Targeting C3b/C4b and VEGF with a bispecific fusion protein optimized for neovascular age-related macular degeneration therapy. Sci. Transl. Med. 2022, 14, eabj2177. [Google Scholar] [CrossRef]
- Winkler, T.W.; Grassmann, F.; Brandl, C.; Kiel, C.; Günther, F.; Strunz, T.; Weidner, L.; Zimmermann, M.E.; Korb, C.A.; Poplawski, A.; et al. Genome-wide association meta-analysis for early age-related macular degeneration highlights novel loci and insights for advanced disease. BMC Med. Genom. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Black, J.R.; Clark, S.J. Age-related macular degeneration: Genome-wide association studies to translation. Genet. Med. 2016, 18, 283–289. [Google Scholar] [CrossRef]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Hankinson, S.E.; Guo, Q.; Rimm, E.; Hunter, D.J. A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch. Ophthalmol. 2007, 125, 55–62. [Google Scholar] [CrossRef]
- Li, M.; Atmaca-Sonmez, P.; Othman, M.; Branham, K.E.; Khanna, R.; Wade, M.S.; Li, Y.; Liang, L.; Zareparsi, S.; Swaroop, A.; et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat. Genet. 2006, 38, 1049–1054. [Google Scholar] [CrossRef]
- Fakhouri, F.; Schwotzer, N.; Golshayan, D.; Frémeaux-Bacchi, V. The Rational Use of Complement Inhibitors in Kidney Diseases. Kidney Int. Rep. 2022, 7, 1165–1178. [Google Scholar] [CrossRef]
- Zadeh, S.; Price, H.; Drews, R.; Bouffard, M.A.; Young, L.H.; Narayanaswami, P. Novel uses of complement inhibitors in myasthenia gravis-Two case reports. Muscle Nerve 2024, 69, 368–372. [Google Scholar] [CrossRef]
- West, E.E.; Woodruff, T.; Fremeaux-Bacchi, V.; Kemper, C. Complement in human disease: Approved and up-and-coming therapeutics. Lancet 2024, 403, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Dong, N.; Yang, M.; Wang, J.; Feng, X.; Wang, Y. Complement Inhibitors in Age-Related Macular Degeneration: A Potential Therapeutic Option. J. Immunol. Res. 2021, 2021, 9945725. [Google Scholar] [CrossRef]
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 July 2024).
- Yehoshua, Z.; de Amorim Garcia Filho, C.A.; Nunes, R.P.; Gregori, G.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Sadda, S.; Feuer, W.; Rosenfeld, P.J. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: The COMPLETE study. Ophthalmology 2014, 121, 693–701. [Google Scholar] [CrossRef]
- Garcia Filho, C.A.; Yehoshua, Z.; Gregori, G.; Nunes, R.P.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Feuer, W.; Rosenfeld, P.J. Change in drusen volume as a novel clinical trial endpoint for the study of complement inhibition in age-related macular degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 18–31. [Google Scholar] [CrossRef]
- Yaspan, B.L.; Williams, D.F.; Holz, F.G.; Regillo, C.D.; Li, Z.; Dressen, A.; van Lookeren Campagne, M.; Le, K.N.; Graham, R.R.; Beres, T.; et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci. Transl. Med. 2017, 9, eaaf1443. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Steinle, N.C.; Pearce, I.; Monés, J.; Metlapally, R.; Saroj, N.; Hamdani, M.; Ribeiro, R.; Rosenfeld, P.J.; Lad, E.M. Impact of Baseline Characteristics on Geographic Atrophy Progression in the FILLY Trial Evaluating the Complement C3 Inhibitor Pegcetacoplan. Am. J. Ophthalmol. 2021, 227, 116–124. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Rosenfeld, P.J.; Waheed, N.K.; Singh, R.P.; Ronca, N.; Slakter, J.S.; Staurenghi, G.; Monés, J.; Baumal, C.R.; Saroj, N.; et al. Characterizing New-Onset Exudation in the Randomized Phase 2 FILLY Trial of Complement Inhibitor Pegcetacoplan for Geographic Atrophy. Ophthalmology 2021, 128, 1325–1336. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Monés, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Nittala, M.G.; Metlapally, R.; Ip, M.; Chakravarthy, U.; Holz, F.G.; Staurenghi, G.; Waheed, N.; Velaga, S.B.; Lindenberg, S.; Karamat, A.; et al. Association of Pegcetacoplan with Progression of Incomplete Retinal Pigment Epithelium and Outer Retinal Atrophy in Age-Related Macular Degeneration: A Post Hoc Analysis of the FILLY Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; Schmitz-Valckenberg, S.; Ribeiro, R.; Safaei, R.; McKeown, A.; Fleckenstein, M.; Holz, F.G. Association of complement C3 inhibitor pegcetacoplan with reduced photoreceptor degeneration beyond areas of geographic atrophy. Sci. Rep. 2022, 12, 17870. [Google Scholar] [CrossRef] [PubMed]
- Vogl, W.D.; Riedl, S.; Mai, J.; Reiter, G.S.; Lachinov, D.; Bogunović, H.; Schmidt-Erfurth, U. Predicting Topographic Disease Progression and Treatment Response of Pegcetacoplan in Geographic Atrophy Quantified by Deep Learning. Ophthalmol. Retin. 2023, 7, 4–13. [Google Scholar] [CrossRef]
- Mai, J.; Riedl, S.; Reiter, G.S.; Lachinov, D.; Vogl, W.D.; Bogunovic, H.; Schmidt-Erfurth, U. Comparison of Fundus Autofluorescence Versus Optical Coherence Tomography-based Evaluation of the Therapeutic Response to Pegcetacoplan in Geographic Atrophy. Am. J. Ophthalmol. 2022, 244, 175–182. [Google Scholar] [CrossRef]
- Riedl, S.; Vogl, W.D.; Mai, J.; Reiter, G.S.; Lachinov, D.; Grechenig, C.; McKeown, A.; Scheibler, L.; Bogunović, H.; Schmidt-Erfurth, U. The Effect of Pegcetacoplan Treatment on Photoreceptor Maintenance in Geographic Atrophy Monitored by Artificial Intelligence-Based OCT Analysis. Ophthalmology. Retina 2022, 6, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Lad, E.M.; Holz, F.G.; Rosenfeld, P.J.; Guymer, R.H.; Boyer, D.; Grossi, F.; Baumal, C.R.; Korobelnik, J.F.; Slakter, J.S.; et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): Two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet 2023, 402, 1434–1448. [Google Scholar] [CrossRef]
- Edmonds, R.; Steffen, V.; Honigberg, L.A.; Chang, M.C. Alternative Complement Pathway Inhibition by Lampalizumab: Analysis of Data From Chroma and Spectri Phase III Clinical Trials. Ophthalmol. Sci. 2023, 3, 100286. [Google Scholar] [CrossRef]
- Patel, S.S.; Lally, D.R.; Hsu, J.; Wykoff, C.C.; Eichenbaum, D.; Heier, J.S.; Jaffe, G.J.; Westby, K.; Desai, D.; Zhu, L.; et al. Correction: Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye 2023, 37, 3705. [Google Scholar] [CrossRef]
- Khanani, A.M.; Patel, S.S.; Staurenghi, G.; Tadayoni, R.; Danzig, C.J.; Eichenbaum, D.A.; Hsu, J.; Wykoff, C.C.; Heier, J.S.; Lally, D.R.; et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet 2023, 402, 1449–1458. [Google Scholar] [CrossRef]
- Fu, D.J.; Bagga, P.; Naik, G.; Glinton, S.; Faes, L.; Liefers, B.; Lima, R.; Wignall, G.; Keane, P.A.; Ioannidou, E.; et al. Pegcetacoplan Treatment and Consensus Features of Geographic Atrophy Over 24 Months. JAMA Ophthalmol. 2024, 142, 548–558. [Google Scholar] [CrossRef]
- Dalton, J.E.; Bolen, S.D.; Mascha, E.J. Publication Bias: The Elephant in the Review. Anesth. Analg. 2016, 123, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; Ramirez-Campillo, R.; Clemente, F.M.; Büttner, F.C.; Andrade, R. The Perils of Misinterpreting and Misusing “Publication Bias” in Meta-analyses: An Education Review on Funnel Plot-Based Methods. Sports Med. 2024, 54, 257–269. [Google Scholar] [CrossRef]
- SYFOVRE® (Pegcetacoplan Injection) Continued to Demonstrate Increasing Treatment Effects over 30 Months in Patients with Geographic Atrophy (GA). Available online: https://investors.apellis.com/news-releases/news-release-details/syfovrer-pegcetacoplan-injection-continued-demonstrate (accessed on 5 September 2024).
- INVESTOR CALL: IZERVAY™ (Avacincaptad Pegol Intravitreal Solution) GATHER2 2-Year Data Presented at AAO 2023. Available online: https://www.astellas.com/en/system/files/2c4120fd04/izervay_investor_call_20231106.pdf (accessed on 5 September 2024).
- Shughoury, A.; Sevgi, D.D.; Ciulla, T.A. Molecular Genetic Mechanisms in Age-Related Macular Degeneration. Genes 2022, 13, 1233. [Google Scholar] [CrossRef]
- Jia, H.; Li, T.; Sun, J.; Gong, Y.; Liu, H.; Wang, H.; Chen, J.; Liu, W.; Lu, S.; Feng, L.; et al. A Novel Bispecific Fusion Protein Targeting C3b/C4b and VEGF in Patients with nAMD: A Randomized, Open-Label, Phase 1b Study. Am. J. Ophthalmol. 2023, 248, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/study/NCT05972473 (accessed on 17 July 2024).
- Zarranz-Ventura, J.; Escobar-Barranco, J.J.; Gómez-Baldó, L.; Gallego-Pinazo, R.; Study Investigators. Reasons for Delayed Anti-VEGF Treatment During COVID-19 Lockdown and Clinical Impact in Neovascular Age-Related Macular Degeneration. Ophthalmol. Ther. 2023, 12, 2537–2555. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, Y.C.; Kang, K.T. Two-Year Follow-Up Study of Patients with Neovascular Age-Related Macular Degeneration Undergoing Anti-VEGF Treatment during the COVID-19 Pandemic. J. Clin. Med. 2024, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, A.M.; Tudosie, M.S.; Smarandache, G.C.; Serban, D. Impact of COVID-19 pandemic upon ophthalmological clinical practice. Rom. J. Leg. Med. 2020, 28, 96–100. [Google Scholar] [CrossRef]
- Rego-Lorca, D.; Valverde-Megías, A.; Fernández-Vigo, J.I.; Oribio-Quinto, C.; Murciano-Cespedosa, A.; Sánchez-Quirós, J.; Donate-López, J.; García-Feijóo, J. Long-Term Consequences of COVID-19 Lockdown in Neovascular AMD Patients in Spain: Structural and Functional Outcomes after 1 Year of Standard Follow-Up and Treatment. J. Clin. Med. 2022, 11, 5063. [Google Scholar] [CrossRef]
- Künzel, S.H.; Lindner, M.; Sassen, J.; Möller, P.T.; Goerdt, L.; Schmid, M.; Schmitz-Valckenberg, S.; Holz, F.G.; Fleckenstein, M.; Pfau, M. Association of Reading Performance in Geographic Atrophy Secondary to Age-Related Macular Degeneration with Visual Function and Structural Biomarkers. JAMA Ophthalmol. 2021, 139, 1191–1199. [Google Scholar] [CrossRef]
- Pfau, M.; Jolly, J.K.; Wu, Z.; Denniss, J.; Lad, E.M.; Guymer, R.H.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Fundus-controlled perimetry (microperimetry): Application as outcome measure in clinical trials. Prog. Retin. Eye Res. 2021, 82, 100907. [Google Scholar] [CrossRef]
- Foote, K.G.; Loumou, P.; Griffin, S.; Qin, J.; Ratnam, K.; Porco, T.C.; Roorda, A.; Duncan, J.L. Relationship Between Foveal Cone Structure and Visual Acuity Measured with Adaptive Optics Scanning Laser Ophthalmoscopy in Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3385–3393. [Google Scholar] [CrossRef]
- Witkin, A.J.; Jaffe, G.J.; Srivastava, S.K.; Davis, J.L.; Kim, J.E. Retinal Vasculitis After Intravitreal Pegcetacoplan: Report From the ASRS Research and Safety in Therapeutics (ReST) Committee. J. Vitreoretin. Dis. 2023, 8, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Kusunoki, K.; Anders, H.J. Balancing efficacy and safety of complement inhibitors. J. Autoimmun. 2024, 145, 103216. [Google Scholar] [CrossRef]
- Abidi, M.; Karrer, E.; Csaky, K.; Handa, J.T. A Clinical and Preclinical Assessment of Clinical Trials for Dry Age-Related Macular Degeneration. Ophthalmol. Sci. 2022, 2, 100213. [Google Scholar] [CrossRef]
- Nebbioso, M.; Lambiase, A.; Cerini, A.; Limoli, P.G.; La Cava, M.; Greco, A. Therapeutic Approaches with Intravitreal Injections in Geographic Atrophy Secondary to Age-Related Macular Degeneration: Current Drugs and Potential Molecules. Int. J. Mol. Sci. 2019, 20, 1693. [Google Scholar] [CrossRef] [PubMed]
- Csaky, K.G.; Miller, J.M.L.; Martin, D.F.; Johnson, M.W. Drug Approval for the Treatment of Geographic Atrophy: How We Got Here and Where We Need to Go. Am. J. Ophthalmol. 2024, 263, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.; Brănescu, C.M.; Smarandache, G.C.; Tudor, C.; Tănăsescu, C.; Tudosie, M.S.; Stana, D.; Costea, D.O.; Dascalu, A.M.; Spătaru, R.I. Safe surgery in day care centers: Focus on preventing medical legal issues. Rom. J. Leg. Med. 2021, 29, 60–64. [Google Scholar] [CrossRef]
- Biarnés, M.; Garrell-Salat, X.; Gómez-Benlloch, A.; Guarro, M.; Londoño, G.; López, E.; Ruiz, S.; Vázquez, M.; Sararols, L. Methodological Appraisal of Phase 3 Clinical Trials in Geographic Atrophy. Biomedicines 2023, 11, 1548. [Google Scholar] [CrossRef]
- Complement Inhibitors for Age-Related Macular Degeneration. Available online: https://www.cochrane.org/CD009300/EYES_complement-inhibitors-age-related-macular-degeneration (accessed on 17 July 2024).
- Serban, D.; Spataru, R.I.; Vancea, G.; Balasescu, S.A.; Socea, B.; Tudor, C.; Dascalu, A.M. Informed consent in all surgical specialties: From legal obligation to patient satisfaction. Rom. J. Leg. Med. 2020, 28, 317–321. [Google Scholar] [CrossRef]
- Flores, R.; Carneiro, Â.; Tenreiro, S.; Seabra, M.C. Retinal Progression Biomarkers of Early and Intermediate Age-Related Macular Degeneration. Life 2022, 12, 36. [Google Scholar] [CrossRef]
- Serban, D.; Smarandache, A.M.; Cristian, D.; Tudor, C.; Duta, L.; Dascalu, A.M. Medical errors and patient safety culture-shifting the healthcare paradigm in Romanian hospitals. Rom. J. Leg. Med. 2020, 28, 195–201. [Google Scholar] [CrossRef]
- Spaide, R.F.; Vavvas, D.G. Complement inhibition for geographic atrophy: Review of Salient Functional Outcomes and Perspective. Retina 2023, 43, 1064–1069. [Google Scholar] [CrossRef] [PubMed]



| Yehoshua et al., 2014 [25] | Garcia Filho et al., 2014 [26] | Yaspan et al., 2017 [27] | Holz et al., 2018 [28] | Liao et al., 2020 [29] | Steinle et al., 2021 [30] | Wykoff et al., 2021 [31] | Jaffe et al., 2021 [32] | Nittalla et al., 2022 [33] | Pfau et al., 2022 [34] | Vogl et al., 2023 [35] | Mai et al., 2022 [36] | Riedl et al., 2022 [37] | Heier et al., 2023 [38] | Edmonds et al., 2023 [39] | Patel et al., 2023 [40] | Khanani et al., 2023 [41] | Fu et al., 2024 [42] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection (≤4) | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 4 |
| Adequate case definition | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Representativeness of cases | ? | ? | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Selection of controls | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ? | ★ | ★ | ★ |
| Control definition | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Comparability (≤2) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Considers the effect of age | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Considers the effect of any additional factor | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Outcome (≤3) | 3 | 3 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 1 | 3 | 3 | 2 |
| Objective, validated laboratory method | ★ | ★ | ★ | ★ | ★ | ? | ? | ★ | ? | ? | ? | ? | ? | ★ | ? | ★ | ★ | ★ |
| Blinded assessment | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ? | ★ | ★ | ? |
| Appropriate statistical analysis | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Total Score (≤9) | 8 | 8 | 9 | 9 | 9 | 8 | 8 | 9 | 8 | 8 | 8 | 8 | 8 | 9 | 7 | 9 | 9 | 8 |
| Study, Year | Agent, Dose | Action | Type, Design | No. Patients | Follow-Up (w) | Primary Outcomes | Secondary Outcomes | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Yehoshua Z [25], 2014 | iv Eculizumab 600 mg/w (low dose)/900 mg/w (high dose), 4 w, then 900 mg/w (low dose)/1200 mg/w (high dose) every 2 w until w24 | C5 inhibitor | COMPLETE study, phase 2, RCT | 30 (low-dose group; high-dose group; placebo: 10;10;10) | 52 w | Change in mean GA area (CSLO and FAF) | Change in BCVA and LLD (ETDRS) | No difference in structural and functional outcomes at w26 and w52 between treated groups and controls; initial LLD correlates with GA progression at 6 months |
| Garcia Filho CA [26], 2014 | iv Eculizumab 600 mg/w (low dose)/900 mg/w (high dose), 4 w, then 900 mg/w (low dose)/1200 mg/w (high dose) every 2 w until w24 | C5 inhibitor | COMPLETE study, phase 2, RCT | 30 (low-dose group; high-dose group; placebo: 10;10;10) | 52 w | Change in mean square root Drusen volume in central 3 mm and 5 mm area measured using SD-OCT | - | No differences between treated groups and placebo at 26 w and 52 w (0.02 vs. −0.05, p = 0.17) |
| Yaspan BL [27], 2017 | Intravitreal lampalizumab, 10 mg | Factor D inhibitor | MAHALO phase 2 study (42 L M; 41 LEOM, 40 SM) | 123 | 78 w | Change in mean GA area (FAF) | Change in BCVA (ETDRS) | Reduction rate of GA area change: 20% ↓ LM vs. SM; 7.7% ↓ LEOM vs. SM (no effect) |
| Holz FG [28], 2018 | Intravitreal lampalizumab, 10 mg | Factor D inhibitor | CHROMA (906) and SPECTRI (975) phase 3, RCT (628 q4w; 627 q6w; 626 SM) | 1881 | 96 w | Change in mean GA area (FAF) | Change in BCVA and LLD (ETDRS) | No effect in treating GA (↓ by −3.6; −3.5 vs. SM) |
| Liao D [29], 2020 | Intravitreal pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | FILLY trial, phase 2, RCT | 246 (84: PM; 78: PEOM; 80: SM) | 78 w | Square root of the change in the GA area | Change in BCVA and LLD (ETDRS); untransformed GA area; foveal encroachment (FAF) | ↓ Growth of square root GA in treated arms (29% PM vs. SM; 20% PEOM vs. SM); 30% and 20% ↓ in untransformed GA area growth at month 12 in the PM and PEOM vs. SM; no effect on changes in foveal encroachment, BCVA, LLD |
| Steinle N [30], 2021 | Intravitreal Pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | post hoc, FILLY trial | 192 (67 PM; 58 PEOM; 67 SM) | 52 w | Risk factors for GA progression | Extrafoveal lesions and larger LLD: potential risk factors for GA progression; ↓ GA progression in treated groups after adjusting these risk factors | |
| Wykoff CC [31], 2021 | Intravitreal Pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | post hoc, FILLY trial | 246 (26 with new eAMD) | 78 w | Risk factors for new-onset exudative AMD (eAMD) in the study eye | Baseline eAMD (18; 69%) in the fellow eye and DLS in the study eye (19; 73.1%) are associated with new eAMD, in a dose-dependent manner | |
| Jaffe GJ [32], 2021 | Intravitreal avacincaptad pegol 2 mg (0.1 mL)/4 mg (2 injections of 0.1 mL) | C5 inhibitor | GATHER 1 phase 2/3 | 286, two phases; 67 (2 mg); 110 (sham); phase 2: 85 (4 mg); 86 (sham) | 52 w | Mean rate of change in GA (FAF and SD-OCT) | Change in NL and LL-BCVA (ETDRS) | ↓ Growth in GA area by 27.4% (2 mg group vs. sham) and 27.8% (% (4 mg group vs. sham); no difference for NL BCVA and LLD |
| Nittala MG [33], 2022 | Intravitreal pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | post hoc, FILLY trial | 167 (41 PM; 56 PEOM; 70 SM) | 52 w | Progression from iRORA to cRORA outside 500 µm surrounding GA, using the Zeiss Cirrus/Heidelberg Spectralis OCT | - | Risk of progression to cRORA in treated arms vs. sham: 48% ↓ (PM) and 24% ↓ (PEOM) |
| Pfau M [34], 2022 | Intravitreal Pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | post hoc, FILLY trial | 192 (67 PM; 61 PEOM; 64 SM) | 78 w | PRD along the 5.16° and 2.58° contour-line of GA by Heidelberg Spectralis OCT | - | ONL, IS, and OS thickness ↑ in treated groups vs. control (p < 0.001); the effect is ↑ in PM vs. PEOM |
| Vogl WD [35], 2022 | Intravitreal pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | post hoc, FILLY trial | 146 (57 PM; 46 PEOM; 53 SM) | 52 w | AI-based GAMM fit for LPR | - | Highly nonuniform LPR related to eccentricity to the fovea, progression direction, PR integrity, and HRF; when controlling co-factors, LPR ↓ by −28.0% (monthly) and −23.9% (EOM) vs. sham |
| Mai J [36], 2022 | Intravitreal pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | post hoc, FILLY trial | 113 patients (38 PM, 36 PEOM, and 39 SM) | 52 w | Correlation of GA (FAF vs. SD-OCT); characterization of other OCT biomarkers | - | Excellent agreement in GA area evaluation; ↓ RPE atrophy progression and ↓ in EZ impairment (SD-OCT) in treated groups, potentially more sensitive in monitoring GA therapy |
| Riedl S [37], 2022 | Intravitreal pegcetacoplan (APL-2) 15 mg (0.1 mL) | C3 inhibitor | post hoc, FILLY trial | 162 (52: PM; 54: PEOM; 56: SM) | 52 w | Inhibition of PR loss and thinning in GA on SD-OCT by deep-learning-based automated PR quantification | - | PR loss and thinning ↓ in treated arms; higher ↓ in the PR loss/RPE loss ratio in PM vs. SM (C3 inhibition prevents more PR loss than RPE loss) |
| Heier JS [38], 2023 | Intravitreal pegcetacoplan (APL-2) 15 mg per 0.1 mL | C3 inhibitor | OAKS/DERBY studies, phase 3, RCT | 1258 OAKS 614 (202 PM; 205 PEOM; 207 SM); DERBY 597 (201 PM; 201 PEOM; 195 SM) | 104 w | Change in mean GA area (FAF) | Change in monocular maximum speed reading (MNREAD/Radner chart), mean functional reading independence index score, BVCA, LLD (ETDRS); change from baseline in the mean threshold sensitivity of all points in the study eye by mesopic microperimetry (OAKS only) | ↓ in GA area change in treated vs. SM: At 12 m: –21% (PM); –16% (PEOM) for OAKS; –12% (PM); –11% (PEOM) for DERBY (endpoint not met) At 24 m: −22% (PM); −18% (PEOM) for OAKS; ↓by 19% (PM); 16% (PEOM) in DERBY The effect was higher in non-subfoveal GA vs. subfoveal GA; no difference in functional endpoints |
| Edmonds R [39], 2023 | Intravitreal lampalizumab, 10 mg | Factor D inhibitor | CHROMA (906) and SPECTRI (975) Phase 3, RCT | 97(35-q4w; 32-q6w; 30-SM) | 96 w | Changes in Complement fractions in aqueous humor: CFD, full-length CFB; the Bb fragment; full-length C3; the C3c, iC3b, C3b; full-length C4; the C4c, C4b | a 92% to 124% median ↑ in CFD levels; 41% to 43% median ↓ in the Bb: CFB ratio no effect on C3, C3 processing; C4, C4 processing; | |
| Pattel SS [40], 2023 | Intravitreal avacincaptad pegol 2 mg (0.1 mL)/4 mg (2 injections of 0.1 mL) | C5 inhibitor | GATHER 1 phase 2/3 | 201, two phases: 67 (2 mg); 110 (SM); phase 2: 83 (4 mg); 84 (SM) | 78 w | Mean rate of change in GA (FAF and SD-OCT) | Change in NL and LL-BCVA (ETDRS) | ↓ growth in GA area by 28.1% (2 mg group vs. sham) and 30.0% (% (4 mg group vs. sham); no difference in NL BCVA and LLD |
| Khanani MA [41], 2023 | Intravitreal avacincaptad pegol 2 mg (0.1 mL) | C5 inhibitor | GATHER 2 phase 3 | 448; (225 treated; 223 SM) | 104 w | Mean rate of change in GA (FAF) at 6, 12, 24 m | Change in BCVA and LL-BCVA at 12 m (ETDRS letters) | ↓ growth in GA area by 18% (2 mg group vs. sham) at 12 m; no difference in change in BCVA and LL-BCVA |
| Fu DJ [42], 2024 | Intravitreal pegcetacoplan (APL-2) 15 mg per 0·1 mL | C3 inhibitor | post hoc for OAKS/DERBY studies | 936 | 104 w | Change in GA area, PRD, RPE loss, RORA (SD-OCT) | Change in NL and LL-BCVA (ETDRS) | Delay in atrophy of both the RPE and PR in treated arms vs. sham, starting from month 2; no correlation with BCVA |
| Study | Age (yrs, Mean ± SD, for Each Study Arm) | Sex F (n, %) | Race (White n, %) | CNV in Fellow Eye (n, %) | Initial BCVA (Letters, Mean ± SD) | LLD (Letters, Mean ± SD) | Initial GA Area (Mean ± SD, mm2) | Initial GA Area (Mean Square Root ± SD, mm) | Change in Square Root GA Area (Mean ± SD, mm) | Change in GA Area (mm2) | Change in BCVA (Letters) | Change in LLD (Letters) | Targeted Genetic Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yehoshua Z [25], 2014 (COMPLETE study) | 79 ± 7; 81 ± 6; | No info | No info | No info | 71.3 ± 7.8; 78.6 ± 5.2; | No info | 7.3 ± 4.8; 4.6 ± 3.6 | 2.5 ± 0.9; 2.02 ± 0.74 | 0.37 ± 0.21; 0.37 ± 0.22 | No info | 0.7 ± 7.2; 2.9 ± 7.0 | No info | No correlation with tested alleles |
| Yaspan BL [27], 2017 (MAHALO Study) | 80.4 ± 7.2; 77.1 ± 7.3; 78.5 ± 7.39 | 28 (66.7); 18 (43.9); 24 (60.0) | 40 (95.2); 41 (100.0); 40 (100.0) | No info | 47.6 ± 12.8; 49.5 ± 11; 45.9 ± 13.4 | No info | 8.5 ± 3.8; 8.5 ± 4.9; 8.8 ± 4.1 | 3.3 ± 1.5; 3.3 ± 1.9; 3.4 ± 1.6 | 2.3 ± 0.1 3.1 ± 0.1 2.9 ± 0.08 | 2.2 ± 1.3; 3 ± 1.9; 2.8 ± 2 | −3.3 ± 1.9; −1.4 ± 1.9; −4.9 ± 1.9; | No info | CFH, C2/CFB- no correlation; carriers of the CFI risk allele associated with GA progression and response to IVL |
| Holz FG [28], 2018 (SPECTRI and CHROMA studies) | 78.0 ± 8.0; 77.4 ± 7.9; 78.5 ± 8.3 | 379 (60.4); 375 (59.8); 377 (60.2) | 608 (97.1); 608 (96.8); 611 (97.4) | No info | 66.1 ± 9.8; 66.0 ± 9.9; 66.0 ± 9.9 | 30.1 ± 15.7; 29.7 ± 16; 29.6 ± 16.3 | 8 ± 4 8.1 ± 3.9 8.3 ±4.2 | No info | 0.3 ± 0.0 0.3 ± 0.0 0.3 ± 0.0 | 1.9 ± 0.0 2.05 ±0.0 2.05 ± 0.0 | −4.1 ± 0.5 −4.9 ± 0.5 −4.9 ± 0.5 | No info | CFI, CFH, C2/CFB not related to GA progression and response to IVL |
| Liao DS [29], 2020 (FILLY study) | 79.6 ± 7.5; 80.9 ± 7.5; 78.4 ± 7.4 | 55 (64.0); 50 (63.3); 49 (60.5) | 84 (97.7); 76 (96.2); 81(100.0) | 36(41.9); 28(35.4); 29(35.8) | 59.8 ± 15.7; 58.4 ± 16.0; 59.8 ± 17.2 | 23.5 ± 14.5; 27.1 ± 15.7; 26.2 ± 17.1 | 8 ± 3.8; 9 ± 4.47; 8.2 ± 4 | 2.7 ± 0.6; 2.9 ± 0.7; 2.8 ± 0.7 | 0.2 ± 0.02; 0.2 ± 0.02; 0.3 ± 0.02 | 2.35 ± 0.02; 2.6 ± 0.02; 3.05 ± 0.02 | −7.7 ± 0.5; −8.8 ± 0.6; −6.4 ± 0.5 | −2.7 * −4.6 −4.2 | rs2230199 in C3 and rs3750846 in ARMS2 correlated with GA growth independent of the treatment arm |
| Heier JS [38], 2023 (OAKS and DERBY Studies) | OAKS: 78.8 ± 7.2 78.1 ± 7.7 7 8.6 ± 7.3 DERBY: 78.7 ± 6.9 79.2 ± 7.1 78.6 ± 7.3 | OAKS: 125 (62%) 117 (57%) 133 (64%) DERBY: 118 (59%) 120 (60%) 123 (63%) | OAKS: 185 (92%) 189 (92%) 188 (91%) DERBY: 187 (93%) 186 (93%) 188 (96%) | No info | OAKS 61 ± 15.3 58.2 ± 17 57.6 ± 16.6 DERBY: 59.5 ± 17.4 58.7 ± 16.1 59 ± 16.9 | OAKS: 26.7 ± 16.8 25.7 ± 17.6 24.9 ± 17.4 DERBY: 27.3 ± 17.7 25.6 ± 16.4 25.7 ± 16.5 | OAKS: 8.1 ± 3.9 8.3 ± 3.9 8.2 ± 3.7 DERBY: 8.3 ± 4.1 8.2 ± 3.9 8.2 ± 4.2 | No info | OAKS: 1.56 ± 0.08 1.65 ± 0.08 1.97 ± 0.08 DERBY: 1.73 ± 0.08 1.76 ± 0.07 1.96 + 0.1 | OAKS: 3.1 ± 0.1 3.3 ± 0.1 4·0 ± 0.15 DERBY: 3.2 ± 0.1 3.3 ± 0.1 3.9 ± 0.2 | −7.9 ± 0.7 −8.8 ± 0.7 −6.9 ± 0.7 | No info | Not assessed |
| Pattel SS [40], 2021 (GATHER 1 study) | 78.8 ± 10.2 78.2 ± 8.8 79.2 ± 8.3 78.2 ± 9.0 | 45 (67.2) 79 (71.8) 58 (69.9) 61 (72.6) | 67 (100) 107 (97.3) 82 (98.8) 82 (97.6) | No info | 70.2 ± 10 69 ± 10.4 69.5 ± 9.8 68.3 ± 11.0 | 33.5 34.5 32.7 34.4 | 7.3 ± 3.8 7.4 ± 3.8 7.9 ± 4.2 7.4 ± 3.9 | 2.6 ± 0.7 2.6 ± 0.7 2.7 ± 0.7 2.6 ± 0.7 | 0.3 ± 0.07 0.4 ± 0.07 0.3 ± 0.07 0.4 ± 0.07 | No info | −7.9 ± 2.6 −9.3 ± 2.6 −3.8 ± 3.1 −3.5 ± 3 | No info | Not assessed |
| Khanani MA [41], 2023 (GATHER 2 study) | 77; 77 | 154 (68%) (treated arm) 156 (70%, sham) | 182 (81%) 186 (84%) | No info | 70.9 ± 8.9 71.6 ± 9.4 | 29.1 ± 19.7 32.6 ± 19.6 | 7.5 ± 4 7.8 ± 3.9 | 2.6 ± 0.7 2.7 ± 0.7 | 1.6 ± 0.1 2.3 ± 0.1 | 1.7 ± 0·2 2.1 ± 0·2 | 1.3 ± 1·4 0.9 ± 1·5 | −3.0 ± 2.1 −1.7 ± 1.4 | Not assessed |
| Complement Inhibitor Treated Arm (n = 2684) | Sham Arm (n = 1476) | p-Value * | |
|---|---|---|---|
| Age (years) | 78.1 ± 7.8 | 77.8 ± 7.9 | 0.67 |
| White (n, %) ** | 2642 (96.8%) | 1376 (91.4%) | 0.23 |
| Female ** | 1524 (56.7%) | 942 (63.8%) | 0.38 |
| GA area (mm2) | 8.0 ± 3.9 | 8.1 ± 4 | 0.69 |
| Square root GA area (mm) | 2.7 ± 0.9 | 2.7 ± 0.8 | 1.00 |
| NL-BCVA | 63.7 ± 13.4 | 64.2 ± 13.7 | 0.24 |
| LLD | 28.6 ± 16.6 | 29.1 ± 17.1 | 0.35 |
| Change in GA area (mm2) | 2.4 ± 0.7 | 2.7 ± 0.8 | <0.0001 |
| Change in square root GA area (mm) | 0.29 ± 0.05 | 0.32 ± 0.06 | <0.0001 |
| Change in NL-BCVA | −5.07 ± 3.03 | −4.9 ± 3.1 | 0.08 |
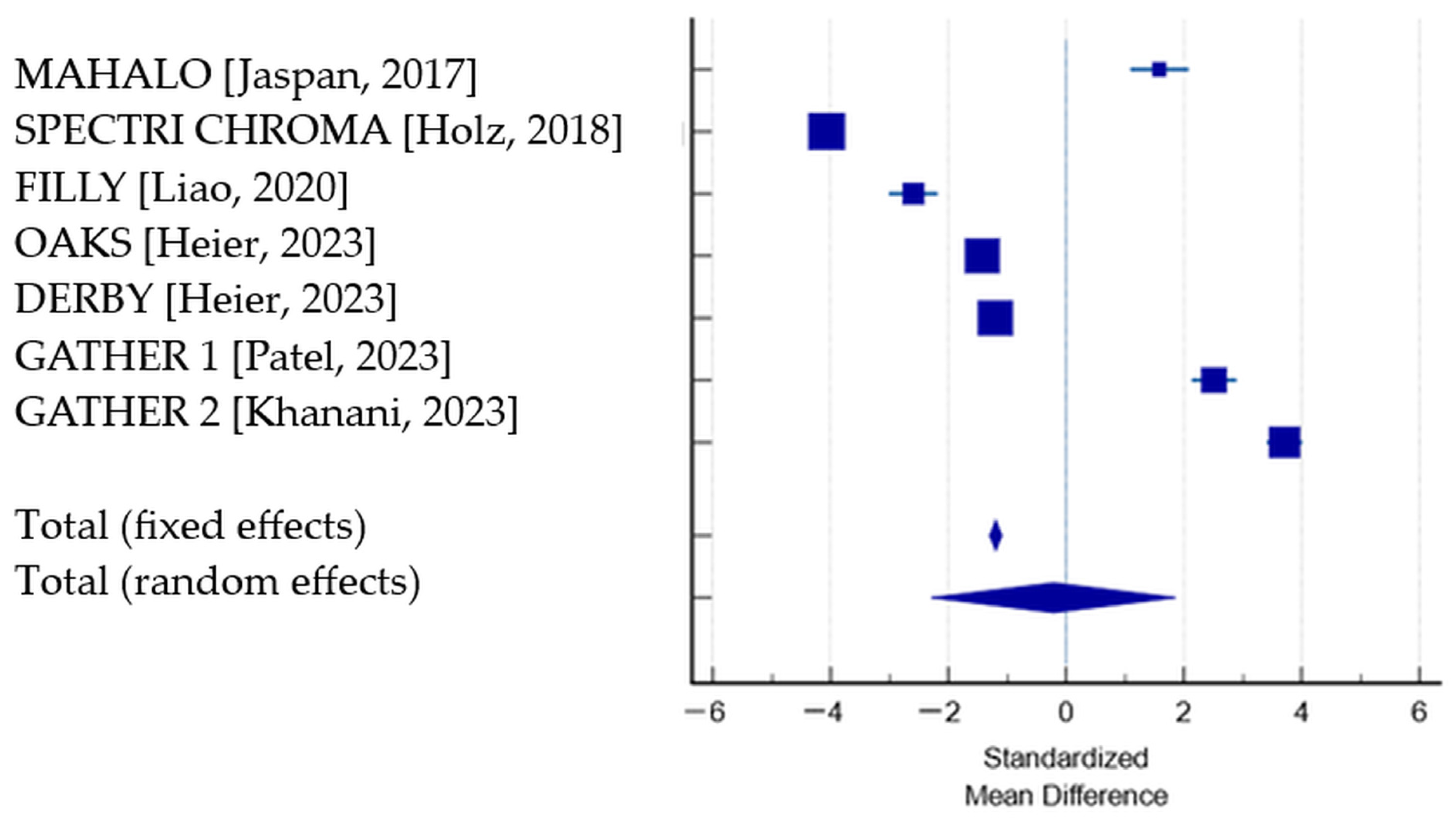
| Study | N1 (Treated) | BCVA Change Treated Group, Mean (SD) | N2 (Sham) | BCVA Change Sham Group, Mean (SD) | SMD | SE | 95% CI | p | Weight (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed | Random | |||||||||
| MAHALO [27] | 42 | −4.1 (0.5) | 40 | −4.9 (0.5) | 1.585 | 0.251 | 1.085 to 2.085 | 4.23 | 14.22 | |
| SPECTRI CHROMA [28] | 628 | −7.9 (2.6) | 626 | 0.9 (1.5) | −4.071 | 0.0990 | −4.265 to −3.877 | 27.30 | 14.32 | |
| FILLY [29] | 84 | −7.7 (0.5) | 80 | −6.4 (0.5) | −2.588 | 0.211 | −3.005 to −2.171 | 6.00 | 14.26 | |
| OAKS [38] | 202 | −7.9 (0.7) | 207 | −6.9 (0.7) | −1.426 | 0.111 | −1.643 to −1.209 | 21.86 | 14.32 | |
| DERBY [38] | 201 | −7.7 (0.8) | 195 | −6.8 (0.7) | −1.194 | 0.109 | −1.408 to −0.980 | 22.54 | 14.32 | |
| GATHER 1 [40] | 67 | −3.3 (1.9) | 134 | −9.3 (2.6) | 2.508 | 0.195 | 2.124 to 2.892 | 7.06 | 14.27 | |
| GATHER 2 [41] | 225 | 1.3 (1.4) | 223 | −4.9 (1.9) | 3.711 | 0.156 | 3.405 to 4.018 | 11.02 | 14.29 | |
| Total (fixed effects) | 1449 | 1505 | −1.194 | 0.0517 | −1.296 to −1.093 | <0.001 | 100.00 | 100.00 | ||
| Total (random effects) | 1449 | 1505 | −0.213 | 1.061 | −2.294 to 1.867 | 0.841 | 100.00 | 100.00 | ||
| Study | N1 (Treated) | N2 (Sham) | Root Square GA Change: Treated vs. Sham | SMD | SE | 95% CI | t | p | Weight (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed | Random | |||||||||
| MAHALO [27] | 42 | 40 | 2.3 (0.08) vs. 2.9 (0.08) | −7.429 | 0.620 | −8.663 to −6.195 | 0.49 | 13.19 | ||
| SPECTRI CHROMA [28] | 628 | 626 | 0.3 (0.0) vs. 0.3 (0.0) | 0.000 | 0.0564 | −0.111 to 0.111 | 59.19 | 14.59 | ||
| FILLY [29] | 84 | 80 | 0.2 (0.02) vs 0.3 (0.02) | −4.977 | 0.316 | −5.600 to −4.353 | 1.89 | 14.21 | ||
| OAKS [38] | 202 | 207 | 1.56 (0.08) vs. 1.97 (0.08) | −5.116 | 0.204 | −5.517 to −4.714 | 4.52 | 14.44 | ||
| DERBY [38] | 201 | 195 | 1.73 (0.08) vs. 1.97 (0.1) | −2.650 | 0.138 | −2.920 to −2.379 | 9.96 | 14.53 | ||
| GATHER 1 [40] | 67 | 134 | 0.3 (0.07) vs. 0.4 (0.07) | −1.423 | 0.165 | −1.749 to −1.098 | 6.92 | 14.49 | ||
| GATHER 2 [41] | 225 | 223 | 1.6(0.7) vs. 2.3(0.1) | −1.395 | 0.105 | −1.601 to −1.188 | 17.03 | 14.56 | ||
| Total (fixed effects) | 1449 | 1505 | −0.962 | 0.0434 | −1.047 to −0.877 | −22.146 | <0.001 | 100.00 | 100.00 | |
| Total (random effects) | 1449 | 1505 | 2954 | −3.219 | 0.722 | −4.636 to −1.803 | −4.457 | <0.001 | 100.00 | 100.00 |
| Study, Year | Systemic SAE (n,%) | Ocular SAE (n,%) | IOP > 30 mmHg (n,%) | Endophthalmitis (n, %) | Non-Infectious Ocular Inflammation | De Novo CNV in the Study Eye |
|---|---|---|---|---|---|---|
| Yaspan BL [27], 2017 | 11 (25.6) 10 (22.7) 15 (35.7) | 0 3 (6.8) 1 (2.4) | Not reported | None | Not reported | Not reported |
| Holz FG [28], 2018 | 120 (19); 84 (13.9); 103 (16.6) | 39 (6.2); 38 (6.1); 17 (2.7) | 52 (8.3); 35 (5.6); 2 (0.3%) | 5 (0.04% per no. of injections) 5 (0.4% per no. of patients) | Not reported | Study eye: 7 (1.1); 12 (1.9); 12 (1.9); Fellow eye: 8 (1.3); 10 (1.9); 11 (1.9) |
| Liao DS [29], 2020 | 19 (22.1); | 4 (4.7); 2 (2.5); 1 (1.2) | 1 (1.2); 1 (1.3); 0 | 2 (2.3); 1 (1.3); 0 | Not reported | 18 (20.9); 7 (8.9); 1 (1.2) |
| Heier JS [38], 2023 | No info | 11 (2.6) 8 (1.9) 3 (0.7) | No info | OAKS: 2 (1); 2 (1); 0; DERBY: 0; 0; 0 | OAKS: 11 (5); 3 (1); 1 (0) DERBY: 5 (2); 6 (3); 0 | OAKS: 24 (11); 16 (8); 4 (2) DERBY: 27 (13); 12 (6); 9 (4) |
| Pattel SS [40], 2023 | No info | 1 (1.5) 1 (1.2) 0 | No info | 0; 0; 0 | 1 (1.5) in 2 mg arm | No info |
| Khanani AM [41], 2023 | 29 (13) 35 (16) | 5 (2) 1 (<1) | No info | 0; 0 | No info | 2 (1) 1 (0.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dascalu, A.M.; Grigorescu, C.C.; Serban, D.; Tudor, C.; Alexandrescu, C.; Stana, D.; Jurja, S.; Costea, A.C.; Alius, C.; Tribus, L.C.; et al. Complement Inhibitors for Geographic Atrophy in Age-Related Macular Degeneration—A Systematic Review. J. Pers. Med. 2024, 14, 990. https://doi.org/10.3390/jpm14090990
Dascalu AM, Grigorescu CC, Serban D, Tudor C, Alexandrescu C, Stana D, Jurja S, Costea AC, Alius C, Tribus LC, et al. Complement Inhibitors for Geographic Atrophy in Age-Related Macular Degeneration—A Systematic Review. Journal of Personalized Medicine. 2024; 14(9):990. https://doi.org/10.3390/jpm14090990
Chicago/Turabian StyleDascalu, Ana Maria, Catalin Cicerone Grigorescu, Dragos Serban, Corneliu Tudor, Cristina Alexandrescu, Daniela Stana, Sanda Jurja, Andreea Cristina Costea, Catalin Alius, Laura Carina Tribus, and et al. 2024. "Complement Inhibitors for Geographic Atrophy in Age-Related Macular Degeneration—A Systematic Review" Journal of Personalized Medicine 14, no. 9: 990. https://doi.org/10.3390/jpm14090990
APA StyleDascalu, A. M., Grigorescu, C. C., Serban, D., Tudor, C., Alexandrescu, C., Stana, D., Jurja, S., Costea, A. C., Alius, C., Tribus, L. C., Dumitrescu, D., Bratu, D., & Cristea, B. M. (2024). Complement Inhibitors for Geographic Atrophy in Age-Related Macular Degeneration—A Systematic Review. Journal of Personalized Medicine, 14(9), 990. https://doi.org/10.3390/jpm14090990








