Galectin-3 Predicts Long-Term Risk of Cerebral Disability and Mortality in Out-of-Hospital Cardiac Arrest Survivors
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Patient Enrollment
2.3. Clinical Data Abstraction
2.4. Biomarkers Measurement
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Biomarkers and Mortality
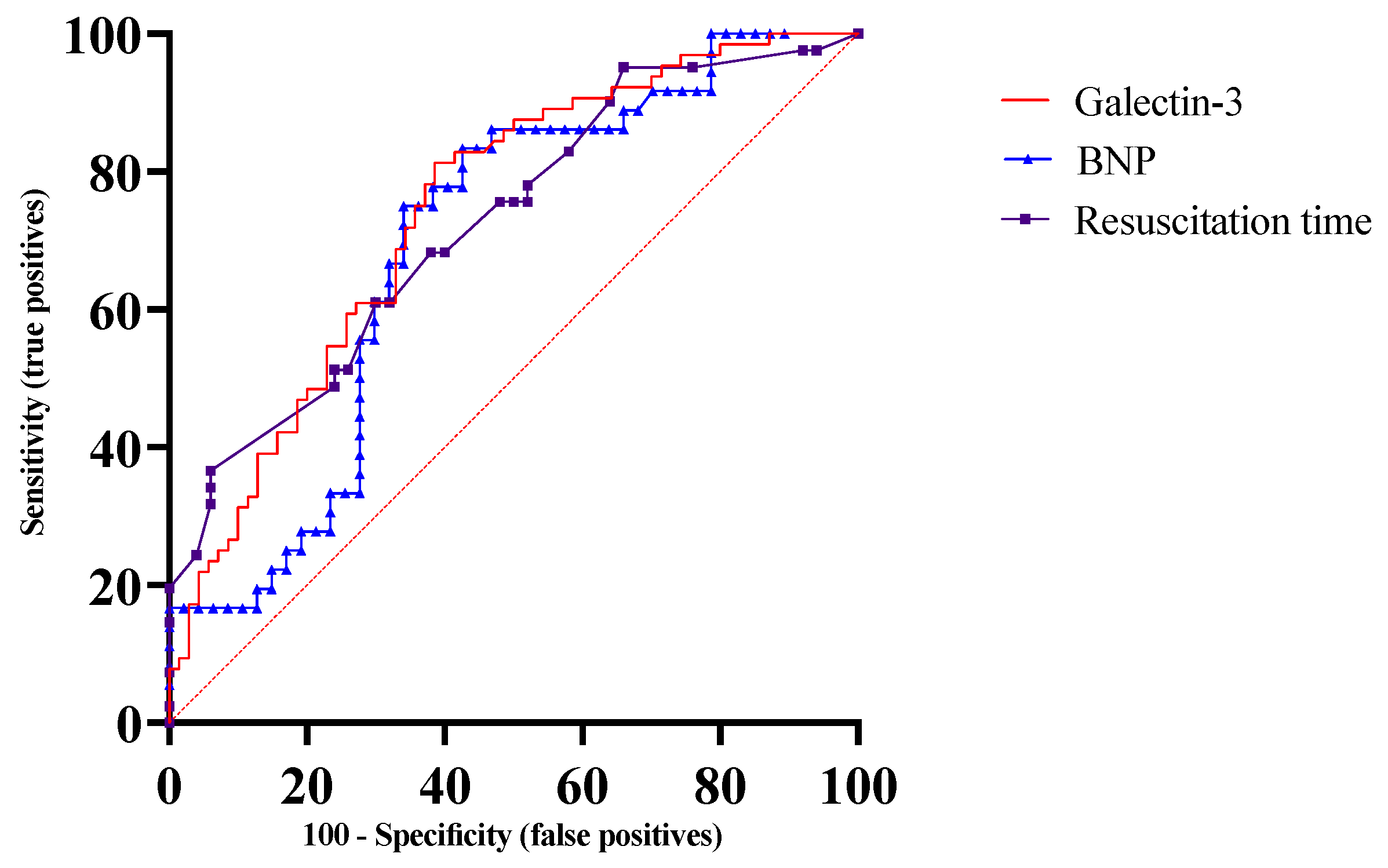
3.3. Biomarkers and Severe Cerebral Disability
3.4. Incremental Prognostic Value of Biomarkers
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360, Erratum in Circulation 2016, 133, e599. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Girotra, S.; Jones, P.G.; McNally, B.; Spertus, J.A.; Chan, P.S.; CARES Surveillance Group. Variation in Out-of-Hospital Cardiac Arrest Survival Across Emergency Medical Service Agencies. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geocadin, R.G.; Agarwal, S.; Goss, A.L.; Callaway, C.W.; Richie, M. Cardiac Arrest and Neurologic Recovery: Insights from the Case of Mr. Damar Hamlin. Ann. Neurol. 2023, 93, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Fugate, J.E.; Brinjikji, W.; Mandrekar, J.N.; Cloft, H.J.; White, R.D.; Wijdicks, E.F.; Rabinstein, A.A. Post-cardiac arrest mortality is declining: A study of the US National Inpatient Sample 2001 to 2009. Circulation 2012, 126, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Buick, J.E.; Drennan, I.R.; Scales, D.C.; Brooks, S.C.; Byers, A.; Cheskes, S.; Dainty, K.N.; Feldman, M.; Verbeek, P.R.; Zhan, C.; et al. Improving Temporal Trends in Survival and Neurological Outcomes After Out-of-Hospital Cardiac Arrest. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e003561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, P.S.; McNally, B.; Tang, F.; Kellermann, A.; CARES Surveillance Group. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation 2014, 130, 1876–1882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.H.; Cho, I.S.; Lee, S.H.; Min, Y.I.; Min, J.H.; Kim, S.H.; Lee, Y.H.; Korean Hypothermia Network Investigators. Correlation between initial serum levels of lactate after return of spontaneous circulation and survival and neurological outcomes in patients who undergo therapeutic hypothermia after cardiac arrest. Resuscitation 2015, 88, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sodeck, G.H.; Domanovits, H.; Sterz, F.; Schillinger, M.; Losert, H.; Havel, C.; Kliegel, A.; Vlcek, M.; Frossard, M.; Laggner, A.N. Can brain natriuretic peptide predict outcome after cardiac arrest? An observational study. Resuscitation 2007, 74, 439–445. [Google Scholar] [CrossRef] [PubMed]
- von Auenmueller, K.I.; Christ, M.; Sasko, B.M.; Trappe, H.J. The Value of Arterial Blood Gas Parameters for Prediction of Mortality in Survivors of Out-of-hospital Cardiac Arrest. J. Emerg. Trauma Shock 2017, 10, 134–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wibrandt, I.; Norsted, K.; Schmidt, H.; Schierbeck, J. Predictors for outcome among cardiac arrest patients: The importance of initial cardiac arrest rhythm versus time to return of spontaneous circulation, a retrospective cohort study. BMC Emerg. Med. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mørk, S.R.; Bøtker, M.T.; Christensen, S.; Tang, M.; Terkelsen, C.J. Survival and neurological outcome after out-of-hospital cardiac arrest treated with and without mechanical circulatory support. Resusc. Plus 2022, 10, 100230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reynolds, J.C.; Frisch, A.; Rittenberger, J.C.; Callaway, C.W. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: When should we change to novel therapies? Circulation 2013, 128, 2488–2494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chai, J.; Fordyce, C.B.; Guan, M.; Humphries, K.; Hutton, J.; Christenson, J.; Grunau, B. The association of duration of resuscitation and long-term survival and functional outcomes after out-of-hospital cardiac arrest. Resuscitation 2023, 182, 109654. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, E.L.; Amuthan, R.; Adams, M.P.; Love, T.E.; Enfield, K.B.; Gimple, L.W.; Cantillon, D.J.; Menon, V. Initial arterial pH as a predictor of neurologic outcome after out-of-hospital cardiac arrest: A propensity-adjusted analysis. Resuscitation 2019, 139, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Grafeneder, J.; Schoergenhofer, C.; Schwameis, M.; Schriefl, C.; Poppe, M.; Clodi, C.; Koch, M.; Sterz, F.; Holzer, M.; et al. Initial Blood pH, Lactate and Base Deficit Add No Value to Peri-Arrest Factors in Prognostication of Neurological Outcome After Out-of-Hospital Cardiac Arrest. Front. Med. 2021, 8, 697906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosleh, W.; Kattel, S.; Bhatt, H.; Al-Jebaje, Z.; Khan, S.; Shah, T.; Dahal, S.; Khalil, C.; Frodey, K.; Elibol, J.; et al. Galectin-3 as a Risk Predictor of Mortality in Survivors of Out-of-Hospital Cardiac Arrest. Circ. Arrhythmia Electrophysiol. 2019, 12, e007519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutta, A.; Alirhayim, Z.; Masmoudi, Y.; Azizian, J.; McDonald, L.; Jogu, H.R.; Qureshi, W.T.; Majeed, N. Brain Natriuretic Peptide as a Marker of Adverse Neurological Outcomes Among Survivors of Cardiac Arrest. J. Intensive Care Med. 2022, 37, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Tsai, M.-S.; Chien, K.-L.; Chang, W.-T.; Wang, T.-D.; Chen, S.-C.; Ma, M.H.-M.; Hsu, H.-Y.; Chen, W.-J. Predicting the outcomes for out-of-hospital cardiac arrest patients using multiple biomarkers and suspension microarray assays. Sci. Rep. 2016, 6, 27187. [Google Scholar] [CrossRef]
- Spoormans, E.M.; Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.D.; Dubois, E.A.; Meuwissen, M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; et al. The Prognostic Value of Troponin-T in Out-of-Hospital Cardiac Arrest Without ST-Segment Elevation: A COACT Substudy. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101191. [Google Scholar] [CrossRef]
- Røsjø, H.; Vaahersalo, J.; Hagve, T.A.; Pettilä, V.; Kurola, J.; Omland, T.; FINNRESUSCI Laboratory Study Group. Prognostic value of high-sensitivity troponin T levels in patients with ventricular arrhythmias and out-of-hospital cardiac arrest: Data from the prospective FINNRESUSCI study. Crit. Care 2014, 18, 605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasson, C.; Rogers, M.A.; Dahl, J.; Kellermann, A.L. Predictors of survival from out-of-hospital cardiac arrest: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Berdowski, J.; Berg, R.A.; Tijssen, J.G.; Koster, R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation 2010, 81, 1479–1487. [Google Scholar] [CrossRef]
- Mosleh, W.; Chaudhari, M.R.; Sonkawade, S.; Mahajan, S.; Khalil, C.; Frodey, K.; Shah, T.; Dahal, S.; Karki, R.; Katkar, R.; et al. The Therapeutic Potential of Blocking Galectin-3 Expression in Acute Myocardial Infarction and Mitigating Inflammation of Infarct Region: A Clinical Outcome-Based Translational Study. Biomark. Insights 2018, 13, 1177271918771969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, P.S.; Berg, R.A.; Tang, Y.; Curtis, L.H.; Spertus, J.A.; American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Association Between Therapeutic Hypothermia and Survival After In-Hospital Cardiac Arrest. JAMA 2016, 316, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maupain, C.; Bougouin, W.; Lamhaut, L.; Deye, N.; Diehl, J.L.; Geri, G.; Perier, M.C.; Beganton, F.; Marijon, E.; Jouven, X.; et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest. Eur. Heart J. 2016, 37, 3222–3228. [Google Scholar] [CrossRef] [PubMed]
- Agusala, V.; Khera, R.; Cheeran, D.; Mody, P.; Reddy, P.P.; Link, M.S. Diagnostic and prognostic utility of cardiac troponin in post-cardiac arrest care. Resuscitation 2019, 141, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Geri, G.; Mongardon, N.; Dumas, F.; Chenevier-Gobeaux, C.; Varenne, O.; Jouven, X.; Vivien, B.; Mira, J.P.; Empana, J.P.; Spaulding, C.; et al. Diagnosis performance of high sensitivity troponin assay in out-of-hospital cardiac arrest patients. Int. J. Cardiol. 2013, 169, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Laver, S.; Farrow, C.; Turner, D.; Nolan, J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004, 30, 2126–2128. [Google Scholar] [CrossRef]
- Lemiale, V.; Dumas, F.; Mongardon, N.; Giovanetti, O.; Charpentier, J.; Chiche, J.D.; Carli, P.; Mira, J.P.; Nolan, J.; Cariou, A. Intensive care unit mortality after cardiac arrest: The relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013, 39, 1972–1980. [Google Scholar] [CrossRef]
- Dragancea, I.; Rundgren, M.; Englund, E.; Friberg, H.; Cronberg, T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation 2013, 84, 337–342. [Google Scholar] [CrossRef]
- Omland, T.; de Lemos, J.A.; Sabatine, M.S.; Christophi, C.A.; Rice, M.M.; Jablonski, K.A.; Tjora, S.; Domanski, M.J.; Gersh, B.J.; Rouleau, J.L.; et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N. Engl. J. Med. 2009, 361, 2538–2547. [Google Scholar] [CrossRef]
- Grubb, N.R.; Fox, K.A.A.A.; Cawood, P. Resuscitation from out-of-hospital cardiac arrest: Implication for cardiac enzyme estimation. Resuscitation 1996, 33, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chiu, T.F.; Fang, J.Y.; Kuan, J.T.; Chen, J.C. The influence of cardiopulmonary resuscitation without defibrilliation on serum levels of cardiac enzymes: A time course study of out-of-hospital cardiac arrest survivors. Resuscitation 2006, 68, 343–349. [Google Scholar] [CrossRef]
- Frunza, O.; Russo, I.; Saxena, A.; Shinde, A.V.; Humeres, C.; Hanif, W.; Rai, V.; Su, Y.; Frangogiannis, N.G. Myocardial Galectin-3 Expression Is Associated with Remodeling of the Pressure-Overloaded Heart and May Delay the Hypertrophic Response without Affecting Survival, Dysfunction, and Cardiac Fibrosis. Am. J. Pathol. 2016, 186, 1114–1127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; André, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Lang, D.; Urban, D.; Rommel, K.P.; von Roeder, M.; Fengler, K.; Blazek, S.; Kandolf, R.; Klingel, K.; Thiele, H.; et al. Plasma and Cardiac Galectin-3 in Patients With Heart Failure Reflects Both Inflammation and Fibrosis: Implications for Its Use as a Biomarker. Circ. Heart Fail. 2017, 10, e003804. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N.; Kim, D.Y.; Boo, K.Y.; Kim, Y.G.; Roh, S.Y.; Baek, Y.S.; Kim, D.H.; Lee, D.I.; Shim, J.; Choi, J.I.; et al. Therapeutic implications of galectin-3 in patients with atrial fibrillation. Sci. Rep. 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Yuan, Y.; Guo, K.; Lao, Y.; Huang, X.; Feng, L. Value of Galectin-3 in Acute Myocardial Infarction. Am. J. Cardiovasc. Drugs 2020, 20, 333–342. [Google Scholar] [CrossRef] [PubMed]
- van Kimmenade, R.R.; Januzzi, J.L., Jr.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Onur, I.; Elitok, A.; Ademoglu, E.; Altun, I.; Bilge, A.K.; Adalet, K. Galectin-3 correlates with arrhythmogenic right ventricular cardiomyopathy and predicts the risk of ventricular -arrhythmias in patients with implantable defibrillators. Acta Cardiol. 2017, 72, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.C.; Al-Dalahmah, O.; Hillis, J.; Young, C.C.; Asbed, I.; Sakaguchi, M.; O’Neill, E.; Szele, F.G. Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases. Cells 2021, 10, 3047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Revilla, J.; Boza-Serrano, A.; Espinosa-Oliva, A.M.; Soto, M.S.; Deierborg, T.; Ruiz, R.; de Pablos, R.M.; Burguillos, M.A.; Venero, J.L. Galectin-3, a rising star in modulating microglia activation under conditions of neurodegeneration. Cell Death Dis. 2022, 13, 628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhuang, J.J.; Zhou, L.; Zheng, Y.H.; Ding, Y.S. The serum galectin-3 levels are associated with the severity and prognosis of ischemic stroke. Aging 2021, 13, 7454–7464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makimoto, H.; Müller, P.; Denise, K.; Clasen, L.; Lin, T.; Angendohr, S.; Schmidt, J.; Brinkmeyer, C.; Kelm, M.; Bejinariu, A. Clinical Impact of Circulating Galectin-3 on Ventricular Arrhythmias and Heart Failure Hospitalization Independent of Prior Ventricular Arrhythmic Events in Patients with Implantable Cardioverter-defibrillators. Intern. Med. 2022, 61, 969–977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pricci, F.; Leto, G.; Amadio, L.; Iacobini, C.; Romeo, G.; Cordone, S.; Gradini, R.; Barsotti, P.; Liu, F.-T.; Di Mario, U.; et al. Role of galectin-3 as a receptor for advanced glycosylation end products. Kidney Int. 2000, 58, S31–S39. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-L. Regulation of ion channels by secreted Klotho: Mechanisms and implications. Kidney Int. 2010, 77, 855–860. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ramirez, R.J.; Yokokawa, M.; Kaur, K.; Ponce-Balbuena, D.; Sinno, M.C.; Willis, B.C.; Ghanbari, H.; Ennis, S.R.; Guerrero-Serna, G.; et al. Galectin-3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. JACC Basic Transl. Sci. 2016, 1, 143–154. [Google Scholar] [CrossRef]
- Humaloja, J.; Ashton, N.J.; Skrifvars, M.B. Brain Injury Biomarkers for Predicting Outcome After Cardiac Arrest. Crit. Care 2022, 26, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoiland, R.L.; Rikhraj, K.J.K.; Thiara, S.; Fordyce, C.; Kramer, A.H.; Skrifvars, M.B.; Wellington, C.L.; Griesdale, D.E.; Fergusson, N.A.; Sekhon, M.S. Neurologic Prognostication after Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 390–398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kattel, S.; Bhatt, H.; Xu, S.; Gurung, S.; Pokharel, S.; Sharma, U.C. Macrophage-specific protein perforin-2 is associated with poor neurological recovery and reduced survival after sudden cardiac arrest. Resuscitation 2020, 155, 180–188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goto, Y.; Funada, A.; Goto, Y. Relationship Between the Duration of Cardiopulmonary Resuscitation and Favorable Neurological Outcomes After Out-of-Hospital Cardiac Arrest: A Prospective, Nationwide, Population-Based Cohort Study. J. Am. Heart Assoc. 2016, 5, e002819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| AMI (n = 30) | OHCA (n = 144) | p Value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age, years | 64.4 ± 11.7 | 60.8 ± 14.4 | 0.28 |
| Gender, % females | 40 | 34.7 | 0.67 |
| Risk Factors | |||
| HTN, % | 77.8 | 59.3 | 0.19 |
| DM, % | 22.2 | 30.0 | 0.59 |
| CKD, % | 0 | 18.6 | 0.04 |
| Smoking, % | 50 | 51.8 | 1.0 |
| Atrial fibrillation, % | 22.2 | 11.4 | 0.25 |
| Family history of cardiac arrest, % | 11.1 | 0.72 | 0.03 |
| Prior AMI, % | 38.9 | 16.4 | 0.04 |
| Prior coronary revascularization, % | 44.4 | 17.2 | 0.012 |
| Medications | |||
| Aspirin, % | 20.0 | 30.0 | 0.3 |
| Statin, % | 55.6 | 42.2 | 0.31 |
| Beta Blocker, % | 61.1 | 39.3 | 0.13 |
| Aldosterone blocker, % | 0.0 | 3.6 | 1.00 |
| ACE/ARBi, % | 50.0 | 32.9 | 0.18 |
| Diuretics, % | 16.7 | 25.9 | 0.56 |
| Laboratory Values | |||
| Hemoglobin, g/dL | 13.3 ± 1.5 | 12.9 ± 2.3 | 0.47 |
| Creatinine, mg/dL | 1.0 ± 0.3 | 1.9 ± 1.9 | 0.0005 |
| eGFR. mL/min/1.73 m2 | 56.4 ± 5.7 | 48.0 ± 20.4 | 0.02 |
| BNP, ng/mL | 259.6 ± 246.1 | 695.6 ± 1213.8 | 0.47 |
| Initial CK, U/L | 448.0 ± 538.6 | 1121.5 ± 1649.2 | 0.10 |
| CK, U/L | 76.6 ± 151.9 | 95.1 ± 218.3 | 0.90 |
| Peak troponin, ng/mL | 79.3 ± 104.2 | 28.3 ± 114.4 | 0.02 |
| Serum galectin-3, ng/mL, median (IQR) | 12.68 (9.61–17.39) | 31.48 (17.25–52.60) | <0.0001 |
| Echo and ECG Parameters | |||
| LVEF post-arrest, % | 50.2 ± 12.3 | 48.1 ± 14.1 | 0.62 |
| LVEF < 35%, % | 11.8 | 24.1 | 0.36 |
| QTc on initial ECG, ms | 409.4 ± 40.6 | 446.0 ± 53.6 | 0.005 |
| Outcomes | |||
| All-cause mortality, % | 10.0 | 60.4 | <0.0001 |
| Survivors (n = 57) | Non-Survivors (n = 87) | p-Value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age, years | 56.5 ± 13.5 | 63.6 ± 14.3 | 0.003 |
| Female gender, % | 31.5 | 36.7 | 0.59 |
| Medications on Presentation | |||
| Aspirin, % | 32.4 | 26.1 | 0.46 |
| Statin, % | 32.4 | 52.2 | 0.03 |
| Beta Blocker, % | 38.0 | 40.6 | 0.86 |
| ACEI/ARB, % | 33.8 | 31.9 | 0.86 |
| Diuretic, % | 11.4 | 40.6 | <0.0001 |
| Aldosterone blocker, % | 3.6 | 5.8 | 0.21 |
| Risk Factors | |||
| HTN, % | 59.2 | 59.4 | 1.00 |
| DM, % | 21.1 | 39.1 | 0.02 |
| Smoking, % | 47.9 | 55.9 | 0.39 |
| Prior AMI, % | 12.7 | 20.3 | 0.26 |
| CKD, % | 9.9 | 27.5 | 0.008 |
| Atrial fibrillation, % | 9.9 | 13.0 | 0.60 |
| Prior coronary revascularization, % | 16.9 | 17.4 | 1.00 |
| Family history of SCA, % | 1.4 | 0 | 1.00 |
| Laboratory Values | |||
| Hemoglobin, g/dL | 13.7 ± 1.8 | 12.0 ± 2.5 | <0.0001 |
| Creatinine, mg/dL | 1.5 ± 1.1 | 2.2 ± 2.5 | 0.04 |
| eGFR, mL/min/1.73 m2 | 54.16 ± 12.93 | 44.1 ± 22.2 | 0.008 |
| BNP, pg/mL | 231 ± 347 | 1090 ± 1481 | <0.0001 |
| CK, U/L | 1160.6 ± 1539.3 | 1080.1 ± 1773.5 | 0.64 |
| Peak troponin, ng/mL | 19.7 ± 34.8 | 33.9 ± 143.7 | 0.17 |
| Serum galectin-3, ng/mL, median (IQR) | 20.3 (9.2–34.7) | 40.1 (26.6–58.7) | <0.0001 |
| Time from event to galectin 3 sample, mins | 295 ± 412.9 | 213.1 ± 297.1 | 0.56 |
| Echo and EKG Parameters | |||
| LVEF post-arrest, % | 48. 2 ± 12.6 | 48.1 ± 15.5 | 0.57 |
| LVEF, <35%, % | 21.4 | 26.9 | 0.54 |
| QTc on initial EKG, ms | 432.8 ± 51.9 | 460.1 ± 52.1 | 0.001 |
| Event Variables | |||
| Duration of resuscitation, mins | 8.8 ± 8.6 | 19.2 ± 18.6 | 0.0001 |
| Initial Asystole/PEA rhythm, % | 19.7 | 64.2 | 0.001 |
| Variable | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Age | 1.02 (1.004–1.037) | 0.010 |
| Galectin-3 | 5.79 (2.88–12.04) | <0.0001 |
| BNP | 2.53 (1.67–3.93) | <0.0001 |
| Hemoglobin | 0.83 (0.75–0.91) | <0.0001 |
| eGFR | 0.987 (0.97–0.99) | 0.030 |
| Arterial pH | 0.19 (0.05–0.77) | 0.010 |
| Initial rhythm asystole | 4.21 (2.59–7.06) | <0.0001 |
| Resuscitation time | 1.022 (1.009–1.032) | <0.0001 |
| QTc | 1.004 (1.001–1.007) | 0.009 |
| History of diabetes | 1.83 (1.17–2.82) | 0.006 |
| History of CKD | 1.716 (1.032–2.74) | 0.029 |
| History of diuretic use | 2.42 (1.55–3.75) | <0.0001 |
| History of statin use | 1.74 (1.13–2.68) | 0.010 |
| Predictor | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Galectin-3 | 15.93 | 1.49–191.4 | p = 0.022 |
| Resuscitation time | 1.02 | 0.97–1.08 | p = 0.372 |
| BNP | 1.17 | 0.43–3.27 | p = 0.745 |
| Arterial pH | 0.37 | 0.001–17.58 | p = 0.629 |
| Reference Model | Reference + Risk Factor(s) | Reference Model + BNP | Reference Model + pH | Reference Model + Resuscitation Time | Reference Model + Galectin-3 | [Reference Model + Risk Factor(s)] + BNP | [Reference Model + Risk Factor(s)] + Galectin-3 | |
|---|---|---|---|---|---|---|---|---|
| Log-likelihood ratio | - | p = 0.009 | p = 0.01 | p = 0.014 | p = 0.003 | p < 0.0001 | p = 0.005 | p < 0.0001 |
| AIC | 701.3 | 697.8 | 698.6 | 698.7 | 695.3 | 679.8 | 696.4 | 676.4 |
| Partial LL | −348.7 | −345.9 | −346.3 | −346.3 | −343.6 | −336.9 | −344.2 | −334.2 |
| Discrimination | ||||||||
| C-statistic | 0.59 (0.50–0.67) | 0.61 (0.53–0.70) | 0.62 (0.54–0.71) | 0.62 (0.54–0.71) | 0.65 (0.56–0.73) | 0.73 (0.65–0.80) | 0.63 (0.55–0.72) | 0.74 (0.67–0.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelradi, A.; Mosleh, W.; Kattel, S.; Al-Jebaje, Z.; Tajlil, A.; Pokharel, S.; Sharma, U.C. Galectin-3 Predicts Long-Term Risk of Cerebral Disability and Mortality in Out-of-Hospital Cardiac Arrest Survivors. J. Pers. Med. 2024, 14, 994. https://doi.org/10.3390/jpm14090994
Abdelradi A, Mosleh W, Kattel S, Al-Jebaje Z, Tajlil A, Pokharel S, Sharma UC. Galectin-3 Predicts Long-Term Risk of Cerebral Disability and Mortality in Out-of-Hospital Cardiac Arrest Survivors. Journal of Personalized Medicine. 2024; 14(9):994. https://doi.org/10.3390/jpm14090994
Chicago/Turabian StyleAbdelradi, Amr, Wasim Mosleh, Sharma Kattel, Zaid Al-Jebaje, Arezou Tajlil, Saraswati Pokharel, and Umesh C. Sharma. 2024. "Galectin-3 Predicts Long-Term Risk of Cerebral Disability and Mortality in Out-of-Hospital Cardiac Arrest Survivors" Journal of Personalized Medicine 14, no. 9: 994. https://doi.org/10.3390/jpm14090994






