Patient-Specific Variability in Interleukin-6 and Myeloperoxidase Responses in Osteoarthritis: Insights from Synthetic Data and Clustering Analysis
Abstract
1. Introduction
2. Methods
2.1. Patient Recruitment and Sample Preparation
2.1.1. Ethical Considerations
2.1.2. Patients
2.1.3. Synovial Biopsy Processing
2.2. Cell Isolation, Culturing, and Stimulation
2.2.1. Isolation and Culturing of Fibroblast-like Synoviocytes
2.2.2. Trypsinisation and Subculturing of Cultured Adherent Cells
2.2.3. Stimulation of Fibroblast-like Synoviocytes
2.2.4. Cryopreservation of Adherent Cells
2.2.5. Enzyme-Linked Immunosorbent Assay
2.3. Synthetic Data Generation and Validation
2.3.1. Synthetic Data Utilisation
2.3.2. k-Nearest Neighbour Analysis
2.3.3. Receiver Operating Characteristic Curves
2.4. Statistical Analysis and Clustering Analysis
2.4.1. Normality and Group Comparisons
2.4.2. Hierarchical Clustering
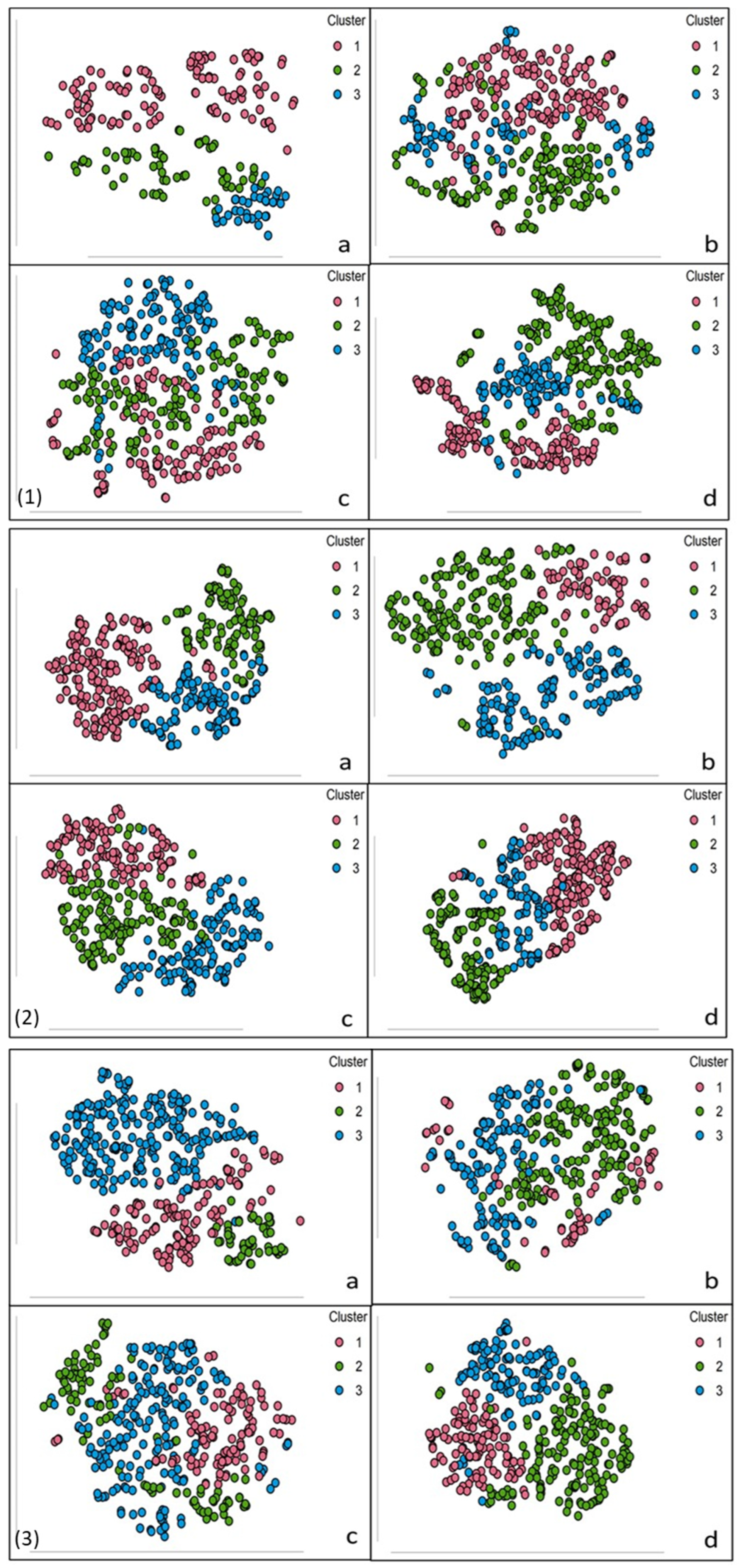
2.4.3. t-Distributed Stochastic Neighbour Embedding
3. Results
3.1. Variability in Inflammatory Responses
3.2. Dose–Response Patterns in IL-6 and MPO Production
3.2.1. Normality and Group Comparisons
3.2.2. Post Hoc Analysis for Group Differences
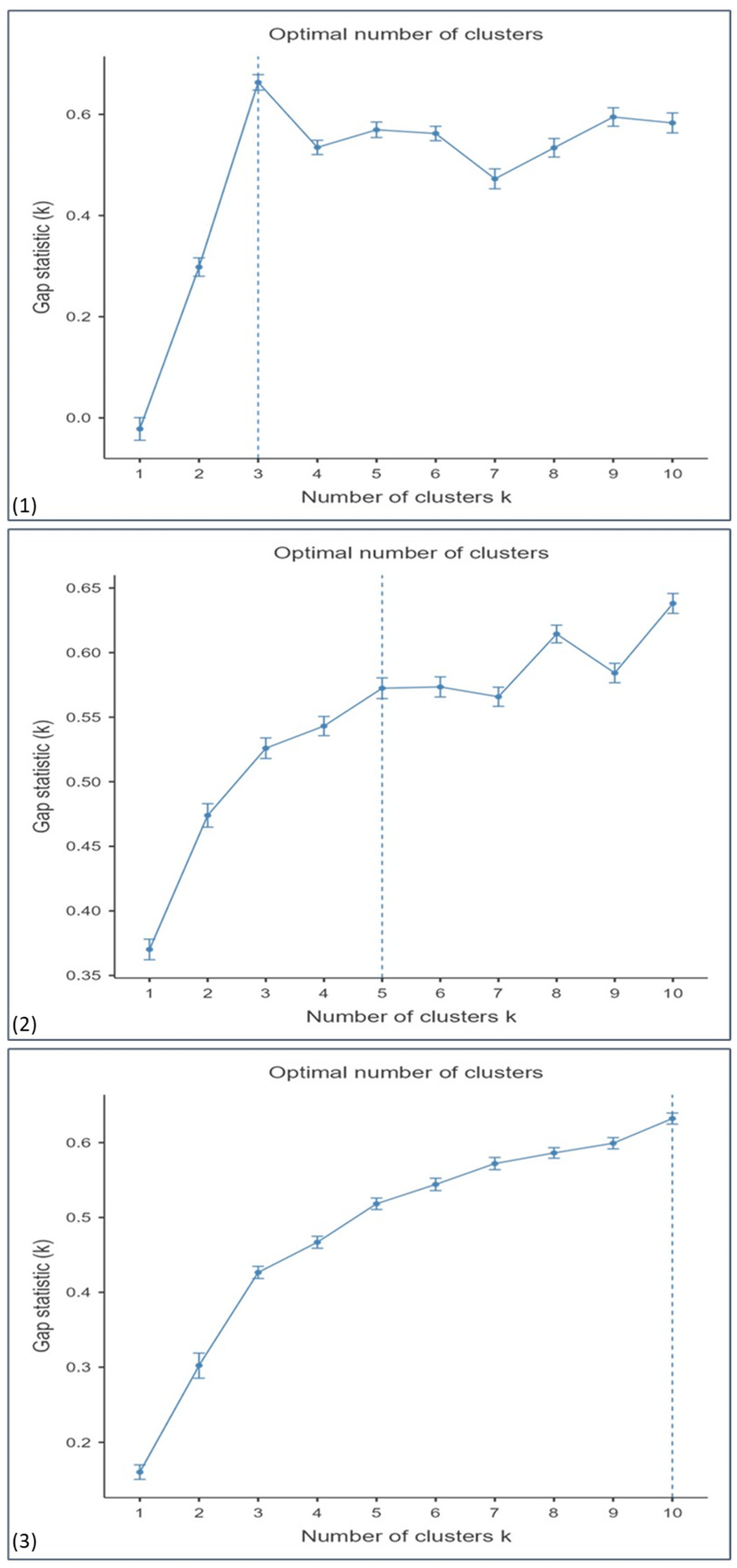
3.3. Identification of Patient Subgroups Through Clustering
3.4. Validation of Synthetic Data
3.4.1. kNN Classification
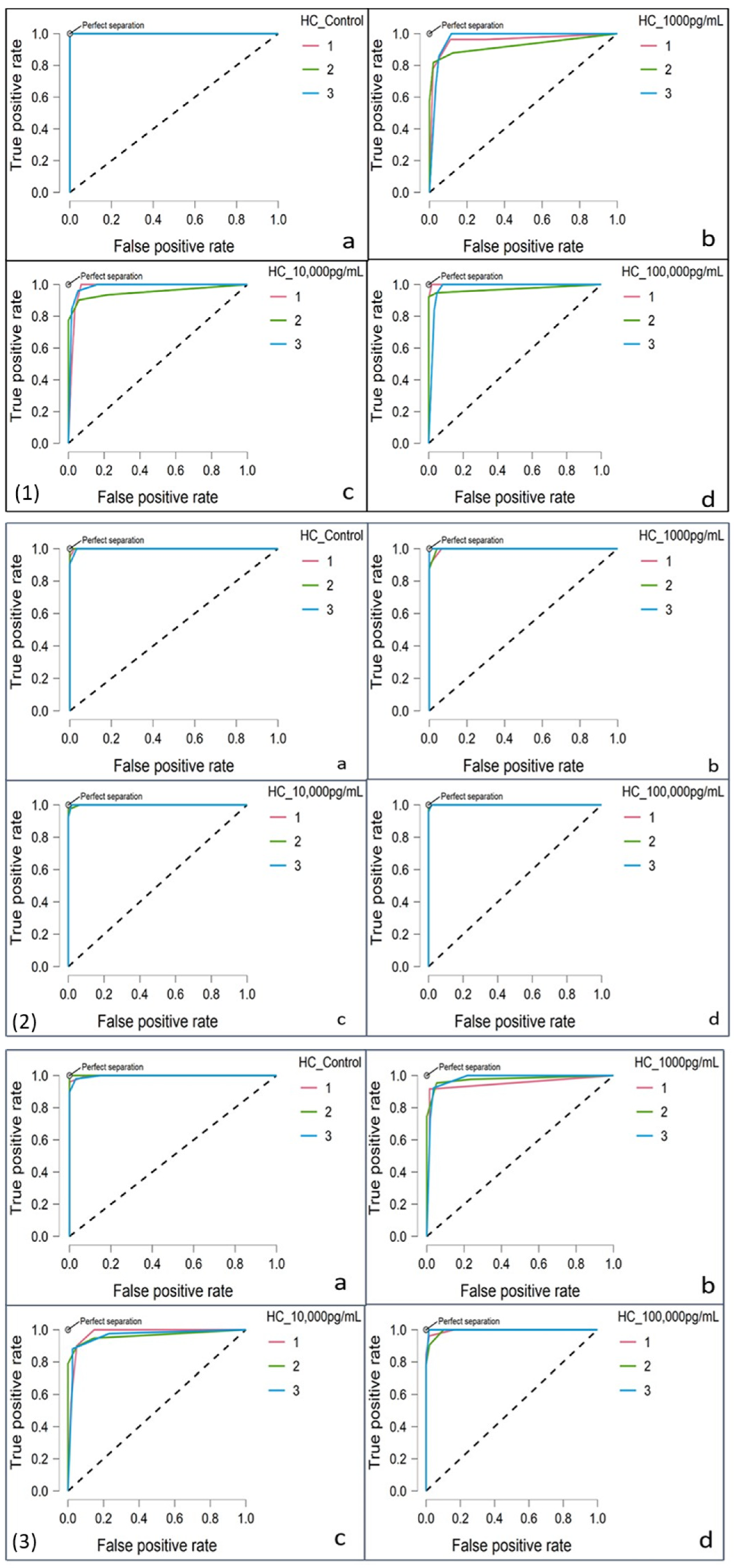
3.4.2. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, I.; Hundhausen, C.; Evanko, S.P.; Malapati, P.; Workman, G.; Chan, C.K.; Rims, C.; Firestein, G.S.; Boyle, D.L.; MacDonald, K.M.; et al. Crosstalk between CD4 T cells and synovial fibroblasts from human arthritic joints promotes hyaluronan-dependent leukocyte adhesion and inflammatory cytokine expression in vitro. Matrix Biol. Plus 2022, 14, 100110. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Fang, Y.; Tan, X.; Jiang, H.; Gong, X.; Wang, X.; Hong, W.; Tu, J.; Wei, W. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: An update. J. Cell. Mol. Med. 2020, 24, 9518–9532. [Google Scholar] [CrossRef] [PubMed]
- José Alcaraz, M. New potential therapeutic approaches targeting synovial fibroblasts in rheumatoid arthritis. Biochem. Pharmacol. 2021, 194, 114815. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chang, W.A.; Wu, L.Y.; Huang, C.F.; Chen, C.H.; Kuo, P.L. Identification of Novel Genes in Osteoarthritic Fibroblast-Like Synoviocytes Using Next-Generation Sequencing and Bioinformatics Approaches. Int. J. Med Sci. 2019, 16, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, L.; Cheng, C.; Shan, W.; Ma, R.; Yin, Z.; Zhu, C. Parallel comparison of fibroblast-like synoviocytes from the surgically removed hyperplastic synovial tissues of rheumatoid arthritis and osteoarthritis patients. BMC Musculoskelet. Disord. 2019, 20, 591. [Google Scholar] [CrossRef]
- Kengla, C.; Kidiyoor, A.; Murphy, S.V. Chapter 68—Bioprinting Complex 3D Tissue and Organs. In Kidney Transplantation, Bioengineering and Regeneration; Orlando, G., Remuzzi, G., Williams, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 957–971. [Google Scholar]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the use of cell lines in biomedical research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T.; Balls, M.; Bardouille, C.; Blanck, O.; Coecke, S.; Gstraunthaler, G.; Lewis, D. Good Cell Culture Practice. ECVAM Good Cell Culture Practice Task Force Report 1. Altern. Lab. Anim. 2002, 30, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Coecke, S.; Balls, M.; Bowe, G.; Davis, J.; Gstraunthaler, G.; Hartung, T.; Hay, R.; Merten, O.W.; Price, A.; Schechtman, L.; et al. Guidance on good cell culture practice. A report of the second ECVAM task force on good cell culture practice. Altern. Lab. Anim. 2005, 33, 261–287. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B.; Attur, M. Developments in the scientific understanding of osteoarthritis. Arthritis Res. Ther. 2009, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Qazi, A.A.; Jørgensen, D.R.; Lillholm, M.; Loog, M.; Nielsen, M.; Dam, E.B. A framework for optimizing measurement weight maps to minimize the required sample size. Med. Image Anal. 2010, 14, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kasthurirathne, S.N.; Dexter, G.; Grannis, S.J. Generative Adversarial Networks for Creating Synthetic Free-Text Medical Data: A Proposal for Collaborative Research and Re-use of Machine Learning Models. AMIA Jt. Summits Transl. Sci. Proc. 2021, 2021, 335–344. [Google Scholar]
- van der Ende, E.L.; Bron, E.E.; Poos, J.A.-O.; Jiskoot, L.C.; Panman, J.L.; Papma, J.M.; Meeter, L.H.; Dopper, E.G.P.; Wilke, C.A.-O.; Synofzik, M.A.-O.; et al. A data-driven disease progression model of fluid biomarkers in genetic frontotemporal dementia. Brain 2022, 145, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Dindorf, C.A.-O.; Konradi, J.A.-O.; Wolf, C.A.-O.; Taetz, B.; Bleser, G.A.-O.; Huthwelker, J.; Werthmann, F.; Bartaguiz, E.; Kniepert, J.; Drees, P.; et al. Classification and Automated Interpretation of Spinal Posture Data Using a Pathology-Independent Classifier and Explainable Artificial Intelligence (XAI). Sensors 2021, 21, 6323. [Google Scholar] [CrossRef] [PubMed]
- Prezja, F.; Paloneva, J.; Pölönen, I.; Niinimäki, E.; Äyrämö, S. DeepFake knee osteoarthritis X-rays from generative adversarial neural networks deceive medical experts and offer augmentation potential to automatic classification. Sci. Rep. 2022, 12, 18573. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Zhang, H.; Pang, L.; Lam, K.; Hui, C.; Zhang, S. Using the K-nearest neighbor algorithm for the classification of lymph node metastasis in gastric cancer. Comput. Math. Methods Med. 2012, 2012, 876545. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, J.; Chen, Y.; Lv, Z.; He, S.; Mao, J.; Xu, J.; Shen, L. Automatic Screening and Identifying Myopic Maculopathy on Optical Coherence Tomography Images Using Deep Learning. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef]
- Xu, C.; Jackson, S.A.-O. Machine learning and complex biological data. Genome Biol. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Clim, A.; Zota, R.D.; TinicĂ, G. The Kullback-Leibler Divergence Used in Machine Learning Algorithms for Health Care Applications and Hypertension Prediction: A Literature Review. Procedia Comput. Sci. 2018, 141, 448–453. [Google Scholar] [CrossRef]
- Stone, A.V.; Loeser, R.F.; Vanderman, K.S.; Long, D.L.; Clark, S.C.; Ferguson, C.M. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthr. Cartil. 2014, 22, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Wallace, C.S.; Anamelechi, C.C.; Clermont, E.; Reichert, W.M.; Truskey, G.A. The use of mild trypsinization conditions in the detachment of endothelial cells to promote subsequent endothelialization on synthetic surfaces. Biomaterials 2007, 28, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Merten, O.-W. Advances in cell culture: Anchorage dependence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140040. [Google Scholar] [CrossRef] [PubMed]
- Nghakliana, F. How to Calculate the Total Number of Cells in a Flask from a Hemocytometer. Available online: https://www.researchgate.net/post/guys_how_do_i_calculate_the_total_number_of_cells_in_a_flask_from_a_hemocytometer (accessed on 24 November 2021).
- Connolly, M.; Mullan, R.H.; McCormick, J.; Matthews, C.; Sullivan, O.; Kennedy, A.; FitzGerald, O.; Poole, A.R.; Bresnihan, B.; Veale, D.J.; et al. Acute-phase serum amyloid A regulates tumor necrosis factor alpha and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 2012, 64, 1035–1045. [Google Scholar] [CrossRef]
- Xu, L.; Feng, X.; Tan, W.; Gu, W.; Guo, D.; Zhang, M.; Wang, F. IL-29 enhances Toll-like receptor-mediated IL-6 and IL-8 production by the synovial fibroblasts from rheumatoid arthritis patients. Arthritis Res. Ther. 2013, 15, R170. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Gao, D.; Baust, J.M. Cryopreservation: An emerging paradigm change. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef]
- Awan, M.; Buriak, I.; Fleck, R.; Fuller, B.; Goltsev, A.; Kerby, J.; Lowdell, M.; Mericka, P.; Petrenko, A.; Petrenko, Y.; et al. Dimethyl sulfoxide: A central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020, 15, 1463–1491. [Google Scholar] [CrossRef] [PubMed]
- Zellkulturen, T.; Freshney, R.I.; deGruyte, W. Culture of Animal Cells: A Manual of Basic Technique; Wiley-Liss Inc.: New York, NY, USA, 2000. [Google Scholar]
- Ren, Z.; Lee, Y.J. Cross-Domain Self-Supervised Multi-task Feature Learning Using Synthetic Imagery. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 762–771. [Google Scholar]
- Pfeifer, S.M.; Attaran, M.; Goldstein, J.; Lindheim, S.R.; Petrozza, J.C.; Rackow, B.W.; Siegelman, E.; Troiano, R.; Winter, T.; Zuckerman, A.; et al. ASRM müllerian anomalies classification 2021. Fertil. Steril. 2021, 116, 1238–1252. [Google Scholar] [CrossRef]
- Anagnostou, P.; Barbas, P.; Vrahatis, A.G.; Tasoulis, S.K. Approximate kNN Classification for Biomedical Data. In Proceedings of the 2020 IEEE International Conference on Big Data (Big Data), Atlanta, GA, USA, 10–13 December 2020; pp. 3602–3607. [Google Scholar]
- O’Brien, S.F.; Yi, Q.L. How do I interpret a confidence interval? Transfusion 2016, 56, 1680–1683. [Google Scholar] [CrossRef]
- Ren, G.; Lutz, I.; Railton, P.; Wiley, J.P.; McAllister, J.; Powell, J.; Krawetz, R.J. Serum and synovial fluid cytokine profiling in hip osteoarthritis: Distinct from knee osteoarthritis and correlated with pain. BMC Musculoskelet. Disord. 2018, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Shatz, I. Assumption-checking rather than (just) testing: The importance of visualization and effect size in statistical diagnostics. Behav. Res. Methods 2024, 56, 826–845. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, R.A.K.; Shakeel, H.; Awan, W.B.; Faheem, M.; Aslam, M. Analysis of COVID-19 data using neutrosophic Kruskal Wallis H test. BMC Med. Res. Methodol. 2021, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Boonhoh, W.; Sontigun, N.; Fungwithaya, P.; Wongtawan, T. Hematological analysis of naturally infecting blood parasites in dogs. Vet. World 2023, 16, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.J.; Gaitskell, K.; Turnbull, I.; Cairns, B.J.; Clarke, R. Characterisation, identification, clustering, and classification of disease. Sci. Rep. 2021, 11, 5405. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.H.M.; Machado, A.R.P.; Andrade, A.O. On the Use of t-Distributed Stochastic Neighbor Embedding for Data Visualization and Classification of Individuals with Parkinson’s Disease. Comput. Math. Methods Med. 2018, 2018, 8019232. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, A.M.; Alhowimel, A.A.-O.; Alshehri, M.A.-O.; Alqahtani, B.A.; Alhwoaimel, N.A.; Segal, N.A.-O.X.; Kluding, P.M. Osteoarthritis and Diabetes: Where Are We and Where Should We Go? Diagnostics 2023, 13, 1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Ma, J.; Qin, H.; Zeng, Q.; Hu, F.; Jiang, T.; Mao, W.; Zhao, Y.; Chen, X.; et al. Identification of Distinct Immune Cell Subsets Associated With Asymptomatic Infection, Disease Severity, and Viral Persistence in COVID-19 Patients. Front. Immunol. 2022, 13, 812514. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Wu, R.; Ding, T.; Xue, H.; Gao, C.; Li, X.; Wang, C. New Insights From Single-Cell Sequencing Data: Synovial Fibroblasts and Synovial Macrophages in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 709178. [Google Scholar] [CrossRef]
| Kruskal–Wallis | ||||
|---|---|---|---|---|
| χ2 | df | p | ε2 | |
| IL-6 Response to LPS | 3049 | 3 | <0.001 | 0.268 |
| MPO Response to LPS | 435 | 5 | <0.001 | 0.0397 |
| MPO Response to IL-6 | 656 | 4 | <0.001 | 0.0577 |
| Kruskal–Wallis | ||||
|---|---|---|---|---|
| χ2 | df | p | ε2 | |
| IL-6/LPS Response to Patients | 6332 | 6 | <0.001 | 0.557 |
| MPO/LPS Response to Patients | 9756 | 7 | <0.001 | 0.890 |
| MPO/IL-6 Response to Patients | 10,149 | 6 | <0.001 | 0.893 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, L.J.; Byrne, J.L.; Edwards, S.; O’Hara, R. Patient-Specific Variability in Interleukin-6 and Myeloperoxidase Responses in Osteoarthritis: Insights from Synthetic Data and Clustering Analysis. J. Pers. Med. 2025, 15, 17. https://doi.org/10.3390/jpm15010017
Coleman LJ, Byrne JL, Edwards S, O’Hara R. Patient-Specific Variability in Interleukin-6 and Myeloperoxidase Responses in Osteoarthritis: Insights from Synthetic Data and Clustering Analysis. Journal of Personalized Medicine. 2025; 15(1):17. https://doi.org/10.3390/jpm15010017
Chicago/Turabian StyleColeman, Laura Jane, John L. Byrne, Stuart Edwards, and Rosemary O’Hara. 2025. "Patient-Specific Variability in Interleukin-6 and Myeloperoxidase Responses in Osteoarthritis: Insights from Synthetic Data and Clustering Analysis" Journal of Personalized Medicine 15, no. 1: 17. https://doi.org/10.3390/jpm15010017
APA StyleColeman, L. J., Byrne, J. L., Edwards, S., & O’Hara, R. (2025). Patient-Specific Variability in Interleukin-6 and Myeloperoxidase Responses in Osteoarthritis: Insights from Synthetic Data and Clustering Analysis. Journal of Personalized Medicine, 15(1), 17. https://doi.org/10.3390/jpm15010017






