The Role of Artificial Intelligence and Emerging Technologies in Advancing Total Hip Arthroplasty
Abstract
1. Introduction
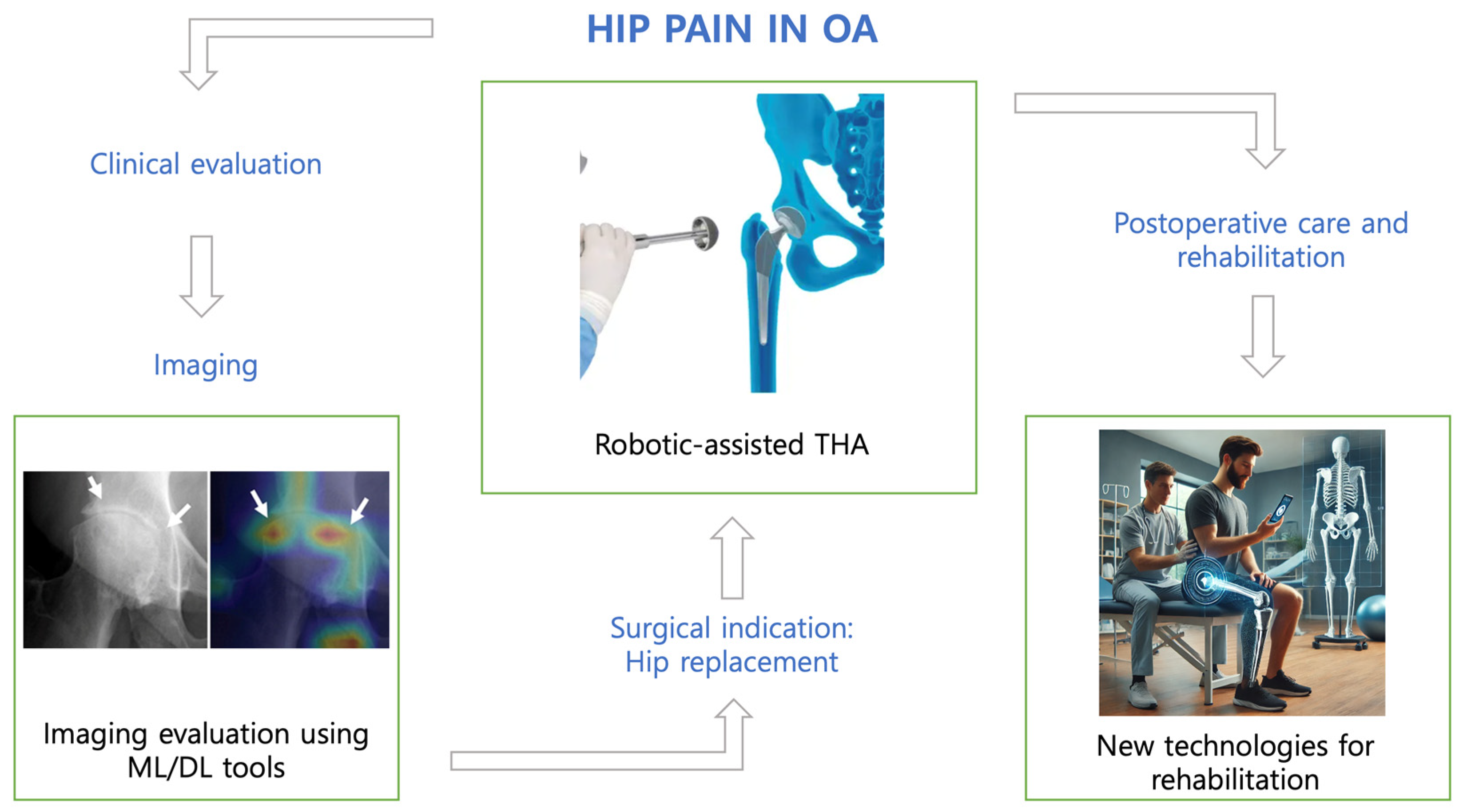
2. Hip Osteoarthritis Diagnosis, Preoperative Planning, and Risk Stratification
3. Implant Identification
4. The Impact of Robotics on Surgical Precision: Innovations and Outcomes
5. Virtual and Augmented Reality
6. Postoperative Care and Rehabilitation
7. Ethical Considerations and Critical Aspects
8. Current Barriers
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mickley, J.P.; Kaji, E.S.; Khosravi, B.; Mulford, K.L.; Taunton, M.J.; Wyles, C.C. Overview of Artificial Intelligence Research Within Hip and Knee Arthroplasty. Arthroplast. Today 2024, 27, 101396. [Google Scholar] [CrossRef] [PubMed]
- Hossain, E.; Rana, R.; Higgins, N.; Soar, J.; Barua, P.D.; Pisani, A.R.; Turner, K. Natural Language Processing in Electronic Health Records in Relation to Healthcare Decision-Making: A Systematic Review. Comput. Biol. Med. 2023, 155, 106649. [Google Scholar] [CrossRef] [PubMed]
- Deckey, D.G.; Rosenow, C.S.; Verhey, J.T.; Brinkman, J.C.; Mayfield, C.K.; Clarke, H.D.; Bingham, J.S. Robotic-Assisted Total Knee Arthroplasty Improves Accuracy and Precision Compared to Conventional Techniques. Bone Jt. J. 2021, 103-B, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.S.; Patrinely, J.R.; Osterman, T.; Wheless, L.; Johnson, D.B. On the Cusp: Considering the Impact of Artificial Intelligence Language Models in Healthcare. Med 2023, 4, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Al-Masri, A.A.; Alotaibi, M.; Meo, M.Z.S.; Meo, M.O.S. ChatGPT Knowledge Evaluation in Basic and Clinical Medical Sciences: Multiple Choice Question Examination-Based Performance. Healthcare 2023, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Needham, K.; Adams, C.; Coppolecchia, A.; Lavernia, C. Robotic-Assisted Total Hip Arthroplasty: An Economic Analysis. J. Comp. Eff. Res. 2021, 10, 1225–1234. [Google Scholar] [CrossRef]
- Venosa, M.; Calvisi, V.; Iademarco, G.; Romanini, E.; Ciminello, E.; Cerciello, S.; Logroscino, G. Evaluation of the Quality of ChatGPT’s Responses to Top 20 Questions about Robotic Hip and Knee Arthroplasty: Findings, Perspectives and Critical Remarks on Healthcare Education. Prosthesis 2024, 6, 913–922. [Google Scholar] [CrossRef]
- Suarez-Ahedo, C.; Lopez-Reyes, A.; Martinez-Armenta, C.; Martinez-Gomez, L.E.; Martinez-Nava, G.A.; Pineda, C.; Vanegas-Contla, D.R.; Domb, B. Revolutionizing Orthopedics: A Comprehensive Review of Robot-Assisted Surgery, Clinical Outcomes, and the Future of Patient Care. J. Robot. Surg. 2023, 17, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Soufi, M.; Otake, Y.; Uemura, K.; Kono, S.; Takashima, K.; Hamada, H.; Gu, Y.; Takao, M.; Okada, S.; et al. Automatic Hip Osteoarthritis Grading with Uncertainty Estimation from Computed Tomography Using Digitally-Reconstructed Radiographs. Int. J. Comput. Assist. Radiol. Surg. 2024, 19, 903–915. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, J.; Liu, J.; Ma, B.; Zhang, C.; Zhang, C.; Xia, T.; Shen, J. Effectiveness analysis of revision surgery after total hip arthroplasty assisted by artificial intelligence preoperative planning system. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2024, 38, 455–460. [Google Scholar] [CrossRef]
- Hirvasniemi, J.; Gielis, W.P.; Arbabi, S.; Agricola, R.; van Spil, W.E.; Arbabi, V.; Weinans, H. Bone Texture Analysis for Prediction of Incident Radiographic Hip Osteoarthritis Using Machine Learning: Data from the Cohort Hip and Cohort Knee (CHECK) Study. Osteoarthr. Cartil. 2019, 27, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, T.; Liu, X.; Shen, K.; Lin, F.; Weng, Y.; Lin, B.; Liang, D.; Feng, E.; Zhang, Y. Clinical Application of Artificial Intelligence-Assisted Three-Dimensional Planning in Direct Anterior Approach Hip Arthroplasty. Int. Orthop. 2024, 48, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yi, J.; Huang, Y.; Guo, R.; Liu, Y.; Kong, X.; Chai, W. Application and Evaluation of Artificial Intelligence 3D Preoperative Planning Software in Developmental Dysplasia of the Hip. J. Orthop. Surg. Res. 2024, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhao, X.; Lu, Z.-D.; Yang, Y.; Ma, L.; Li, P. Accuracy Analysis of Artificial Intelligence-Assisted Three-Dimensional Preoperative Planning in Total Hip Replacement. Jt. Dis. Relat. Surg. 2023, 34, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhi, X.; Liu, X.; Zhang, Y.; Chai, W. Utility of a Novel Integrated Deep Convolutional Neural Network for the Segmentation of Hip Joint from Computed Tomography Images in the Preoperative Planning of Total Hip Arthroplasty. J. Orthop. Surg. Res. 2022, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Tarwala, R.; Dorr, L.D. Robotic Assisted Total Hip Arthroplasty Using the MAKO Platform. Curr. Rev. Musculoskelet. Med. 2011, 4, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Llombart-Blanco, R.; Mariscal, G.; Barrios, C.; Vera, P.; Llombart-Ais, R. MAKO Robot-Assisted Total Hip Arthroplasty: A Comprehensive Meta-Analysis of Efficacy and Safety Outcomes. J. Orthop. Surg. Res. 2024, 19, 698. [Google Scholar] [CrossRef]
- Domb, B.G.; El Bitar, Y.F.; Sadik, A.Y.; Stake, C.E.; Botser, I.B. Comparison of Robotic-Assisted and Conventional Acetabular Cup Placement in THA: A Matched-Pair Controlled Study. Clin. Orthop. Relat. Res. 2014, 472, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Domb, B.G.; Chen, J.W.; Lall, A.C.; Perets, I.; Maldonado, D.R. Minimum 5-Year Outcomes of Robotic-Assisted Primary Total Hip Arthroplasty with a Nested Comparison Against Manual Primary Total Hip Arthroplasty: A Propensity Score-Matched Study. J. Am. Acad. Orthop. Surg. 2020, 28, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, D.R.; Go, C.C.; Kyin, C.; Rosinsky, P.J.; Shapira, J.; Lall, A.C.; Domb, B.G. Robotic Arm-Assisted Total Hip Arthroplasty Is More Cost-Effective Than Manual Total Hip Arthroplasty: A Markov Model Analysis. J. Am. Acad. Orthop. Surg. 2021, 29, e168–e177. [Google Scholar] [CrossRef]
- Nakamura, N.; Sugano, N.; Nishii, T.; Kakimoto, A.; Miki, H. A Comparison between Robotic-Assisted and Manual Implantation of Cementless Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 1072–1081. [Google Scholar] [CrossRef]
- Shah, A.A.; Devana, S.K.; Lee, C.; Kianian, R.; van der Schaar, M.; SooHoo, N.F. Development of a Novel, Potentially Universal Machine Learning Algorithm for Prediction of Complications After Total Hip Arthroplasty. J. Arthroplast. 2021, 36, 1655–1662.e1. [Google Scholar] [CrossRef] [PubMed]
- Rouzrokh, P.; Ramazanian, T.; Wyles, C.C.; Philbrick, K.A.; Cai, J.C.; Taunton, M.J.; Maradit Kremers, H.; Lewallen, D.G.; Erickson, B.J. Deep Learning Artificial Intelligence Model for Assessment of Hip Dislocation Risk Following Primary Total Hip Arthroplasty from Postoperative Radiographs. J. Arthroplast. 2021, 36, 2197–2203.e3. [Google Scholar] [CrossRef] [PubMed]
- Karnuta, J.M.; Haeberle, H.S.; Luu, B.C.; Roth, A.L.; Molloy, R.M.; Nystrom, L.M.; Piuzzi, N.S.; Schaffer, J.L.; Chen, A.F.; Iorio, R.; et al. Artificial Intelligence to Identify Arthroplasty Implants from Radiographs of the Hip. J. Arthroplast. 2021, 36, S290–S294.e1. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Fu, Y.; He, M.; Fu, X. Automated Identification of Hip Arthroplasty Implants Using Artificial Intelligence. Sci. Rep. 2022, 12, 12179. [Google Scholar] [CrossRef] [PubMed]
- Karnuta, J.M.; Murphy, M.P.; Luu, B.C.; Ryan, M.J.; Haeberle, H.S.; Brown, N.M.; Iorio, R.; Chen, A.F.; Ramkumar, P.N. Artificial Intelligence for Automated Implant Identification in Total Hip Arthroplasty: A Multicenter External Validation Study Exceeding Two Million Plain Radiographs. J. Arthroplast. 2023, 38, 1998–2003.e1. [Google Scholar] [CrossRef] [PubMed]
- Rouzrokh, P.; Mickley, J.P.; Khosravi, B.; Faghani, S.; Moassefi, M.; Schulz, W.R.; Erickson, B.J.; Taunton, M.J.; Wyles, C.C. THA-AID: Deep Learning Tool for Total Hip Arthroplasty Automatic Implant Detection with Uncertainty and Outlier Quantification. J. Arthroplast. 2024, 39, 966–973.e17. [Google Scholar] [CrossRef] [PubMed]
- Klemt, C.; Uzosike, A.C.; Cohen-Levy, W.B.; Harvey, M.J.; Subih, M.A.; Kwon, Y.-M. The Ability of Deep Learning Models to Identify Total Hip and Knee Arthroplasty Implant Design from Plain Radiographs. J. Am. Acad. Orthop. Surg. 2022, 30, 409–415. [Google Scholar] [CrossRef]
- Murphy, M.; Killen, C.; Burnham, R.; Sarvari, F.; Wu, K.; Brown, N. Artificial Intelligence Accurately Identifies Total Hip Arthroplasty Implants: A Tool for Revision Surgery. Hip Int. 2022, 32, 766–770. [Google Scholar] [CrossRef]
- Fascio, E.; Vitale, J.A.; Sirtori, P.; Peretti, G.; Banfi, G.; Mangiavini, L. Early Virtual-Reality-Based Home Rehabilitation after Total Hip Arthroplasty: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 1766. [Google Scholar] [CrossRef] [PubMed]
- Berton, A.; Longo, U.G.; Candela, V.; Fioravanti, S.; Giannone, L.; Arcangeli, V.; Alciati, V.; Berton, C.; Facchinetti, G.; Marchetti, A.; et al. Virtual Reality, Augmented Reality, Gamification, and Telerehabilitation: Psychological Impact on Orthopedic Patients’ Rehabilitation. J. Clin. Med. 2020, 9, 2567. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Lau, K.M.K.; Cheng, A.S.K.; Lau, T.S.K.; Lau, F.O.T.; Lau, M.C.H.; Law, S.W. Use of Mobile App to Enhance Functional Outcomes and Adherence of Home-Based Rehabilitation Program for Elderly with Hip Fracture: A Randomized Controlled Trial. Hong Kong Physiother. J. 2022, 42, 99–110. [Google Scholar] [CrossRef]
- Kalron, A.; Tawil, H.; Peleg-Shani, S.; Vatine, J.-J. Effect of Telerehabilitation on Mobility in People after Hip Surgery: A Pilot Feasibility Study. Int. J. Rehabil. Res. 2018, 41, 244–250. [Google Scholar] [CrossRef]
- Zanghelini, F.; Ponzo, A.; Xydopoulos, G.; Fordham, R.; Khanal, S. Cost-Effectiveness of GaitSmart and an Artificial Intelligence Solution for Rehabilitation of Patients Undergoing Total Hip Arthroplasty (THA) and Total Knee Arthroplasty (TKA) in Older Population in the United Kingdom. Geriatrics 2024, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Doiron-Cadrin, P.; Kairy, D.; Vendittoli, P.-A.; Lowry, V.; Poitras, S.; Desmeules, F. Feasibility and Preliminary Effects of a Tele-Prehabilitation Program and an in-Person Prehablitation Program Compared to Usual Care for Total Hip or Knee Arthroplasty Candidates: A Pilot Randomized Controlled Trial. Disabil. Rehabil. 2020, 42, 989–998. [Google Scholar] [CrossRef]
- McKeon, J.F.; Alvarez, P.M.; Vajapey, A.S.; Sarac, N.; Spitzer, A.I.; Vajapey, S.P. Expanding Role of Technology in Rehabilitation After Lower-Extremity Joint Replacement: A Systematic Review. JBJS Rev. 2021, 9, e21. [Google Scholar] [CrossRef] [PubMed]
- Innmann, M.M.; Reichel, F.; Schaper, B.; Merle, C.; Beaulé, P.E.; Grammatopoulos, G. How Does Spinopelvic Mobility and Sagittal Functional Cup Orientation Affect Patient-Reported Outcome 1 Year after THA?—A Prospective Diagnostic Cohort Study. J. Arthroplast. 2021, 36, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, M.; Oakden-Rayner, L.; Beam, A.L. The False Hope of Current Approaches to Explainable Artificial Intelligence in Health Care. Lancet Digit. Health 2021, 3, e745–e750. [Google Scholar] [CrossRef] [PubMed]
- Rainey, J.; Sodhi, N.; Gililland, J.M.; Mont, M.A. The Growing Role of Artificial Intelligence and Technology in Hip and Knee Arthroplasty. Surg. Technol. Int. 2024, 44, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Cirimele, V.; D’Amone, G.; Stellato, L.; Ferrini, A.; Gregori, P.; Faiella, E. Magnetic Resonance Imaging in the Evaluation of Avulsion Injuries of the Pelvis and Hip in Adolescent Professional Footballers: A Case Series. J. Orthop. Case Rep. 2024, 14, 147–152. [Google Scholar] [CrossRef] [PubMed]
- von Schacky, C.E.; Sohn, J.H.; Liu, F.; Ozhinsky, E.; Jungmann, P.M.; Nardo, L.; Posadzy, M.; Foreman, S.C.; Nevitt, M.C.; Link, T.M.; et al. Development and Validation of a Multitask Deep Learning Model for Severity Grading of Hip Osteoarthritis Features on Radiographs. Radiology 2020, 295, 136–145. [Google Scholar] [CrossRef]
- Jang, S.J.; Kunze, K.N.; Vigdorchik, J.M.; Jerabek, S.A.; Mayman, D.J.; Sculco, P.K. John Charnley Award: Deep Learning Prediction of Hip Joint Center on Standard Pelvis Radiographs. J. Arthroplast. 2022, 37, S400–S407.e1. [Google Scholar] [CrossRef] [PubMed]
- Nich, C.; Behr, J.; Crenn, V.; Normand, N.; Mouchère, H.; d’Assignies, G. Applications of Artificial Intelligence and Machine Learning for the Hip and Knee Surgeon: Current State and Implications for the Future. Int. Orthop. 2022, 46, 937–944. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, J.-J.; Kang, K.-H.; Lee, J.-H.; In, Y. Detection of Prosthetic Loosening in Hip and Knee Arthroplasty Using Machine Learning: A Systematic Review and Meta-Analysis. Medicina 2023, 59, 782. [Google Scholar] [CrossRef]
- Hidaka, R.; Matsuda, K.; Igari, T.; Takeuchi, S.; Imoto, Y.; Yagi, S.; Kawano, H. Development and Accuracy of an Artificial Intelligence Model for Predicting the Progression of Hip Osteoarthritis Using Plain Radiographs and Clinical Data: A Retrospective Study. BMC Musculoskelet. Disord. 2024, 25, 893. [Google Scholar] [CrossRef]
- Bulloni, M.; Gambaro, F.M.; Chiappetta, K.; Grappiolo, G.; Corino, V.; Loppini, M. AI-Based Hip Prosthesis Failure Prediction through Evolutional Radiological Indices. Arch. Orthop. Trauma. Surg. 2024, 144, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Borjali, A.; Chen, A.F.; Bedair, H.S.; Melnic, C.M.; Muratoglu, O.K.; Morid, M.A.; Varadarajan, K.M. Comparing the Performance of a Deep Convolutional Neural Network with Orthopedic Surgeons on the Identification of Total Hip Prosthesis Design from Plain Radiographs. Med. Phys. 2021, 48, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, H.S.; Helm, J.M.; Navarro, S.M.; Karnuta, J.M.; Schaffer, J.L.; Callaghan, J.J.; Mont, M.A.; Kamath, A.F.; Krebs, V.E.; Ramkumar, P.N. Artificial Intelligence and Machine Learning in Lower Extremity Arthroplasty: A Review. J. Arthroplast. 2019, 34, 2201–2203. [Google Scholar] [CrossRef] [PubMed]
- Borjali, A.; Chen, A.F.; Muratoglu, O.K.; Morid, M.A.; Varadarajan, K.M. Detecting Total Hip Replacement Prosthesis Design on Plain Radiographs Using Deep Convolutional Neural Network. J. Orthop. Res. 2020, 38, 1465–1471. [Google Scholar] [CrossRef]
- Shah, A.K.; Lavu, M.S.; Hecht, C.J.; Burkhart, R.J.; Kamath, A.F. Understanding the Use of Artificial Intelligence for Implant Analysis in Total Joint Arthroplasty: A Systematic Review. Arthroplasty 2023, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Gurung, B.; Liu, P.; Harris, P.D.R.; Sagi, A.; Field, R.E.; Sochart, D.H.; Tucker, K.; Asopa, V. Artificial Intelligence for Image Analysis in Total Hip and Total Knee Arthroplasty: A Scoping Review. Bone Jt. J. 2022, 104-B, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Yi, P.H. Artificial Intelligence in Orthopedic Implant Model Classification: A Systematic Review. Skelet. Radiol. 2022, 51, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Abdel, M.P.; Carender, C.N.; Berry, D.J. Current Practice Trends in Primary Hip and Knee Arthroplasties Among Members of the American Association of Hip and Knee Surgeons. J. Arthroplast. 2023, 38, 1921–1927.e3. [Google Scholar] [CrossRef] [PubMed]
- Haffer, H.; Adl Amini, D.; Perka, C.; Pumberger, M. The Impact of Spinopelvic Mobility on Arthroplasty: Implications for Hip and Spine Surgeons. J. Clin. Med. 2020, 9, 2569. [Google Scholar] [CrossRef] [PubMed]
- Callanan, M.C.; Jarrett, B.; Bragdon, C.R.; Zurakowski, D.; Rubash, H.E.; Freiberg, A.A.; Malchau, H. The John Charnley Award: Risk Factors for Cup Malpositioning: Quality Improvement through a Joint Registry at a Tertiary Hospital. Clin. Orthop. Relat. Res. 2011, 469, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bohl, D.D.; Nolte, M.T.; Ong, K.; Lau, E.; Calkins, T.E.; Della Valle, C.J. Computer-Assisted Navigation Is Associated with Reductions in the Rates of Dislocation and Acetabular Component Revision Following Primary Total Hip Arthroplasty. J. Bone Jt. Surg. Am. 2019, 101, 250–256. [Google Scholar] [CrossRef]
- Bendich, I.; Vigdorchik, J.M.; Sharma, A.K.; Mayman, D.J.; Sculco, P.K.; Anderson, C.; Della Valle, A.G.; Su, E.P.; Jerabek, S.A. Robotic Assistance for Posterior Approach Total Hip Arthroplasty Is Associated with Lower Risk of Revision for Dislocation When Compared to Manual Techniques. J. Arthroplast. 2022, 37, 1124–1129. [Google Scholar] [CrossRef]
- Kim, K.; Kwon, S.; Kwon, J.; Hwang, J. A Review of Robotic-Assisted Total Hip Arthroplasty. Biomed. Eng. Lett. 2023, 13, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Realyvasquez, J.; Simcox, T.; Rozell, J.C.; Schwarzkopf, R.; Davidovitch, R.I. Robotics Versus Navigation Versus Conventional Total Hip Arthroplasty: Does the Use of Technology Yield Superior Outcomes? J. Arthroplast. 2021, 36, 2801–2807. [Google Scholar] [CrossRef]
- Redmond, J.M.; Gupta, A.; Hammarstedt, J.E.; Petrakos, A.E.; Finch, N.A.; Domb, B.G. The Learning Curve Associated with Robotic-Assisted Total Hip Arthroplasty. J. Arthroplast. 2015, 30, 50–54. [Google Scholar] [CrossRef]
- Korber, S.; Antonios, J.K.; Sivasundaram, L.; Mayfield, C.K.; Kang, H.P.; Chung, B.C.; Oakes, D.A.; Heckmann, N.D. Utilization of Technology-Assisted Total Hip Arthroplasty in the United States from 2005 to 2018. Arthroplast. Today 2021, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Eckhard, L.; Walter, W.L.; Peng, A.; Hatton, A.; Donnelly, B.; de Steiger, R. The Use of Computer Navigation in Total Hip Arthroplasty Is Associated with a Reduced Rate of Revision for Dislocation: A Study of 6,912 Navigated THA Procedures from the Australian Orthopaedic Association National Joint Replacement Registry. J. Bone Jt. Surg. Am. 2021, 103, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J.C.; Christopher, Z.K.; Moore, M.L.; Pollock, J.R.; Haglin, J.M.; Bingham, J.S. Patient Interest in Robotic Total Joint Arthroplasty Is Exponential: A 10-Year Google Trends Analysis. Arthroplast. Today 2022, 15, 13–18. [Google Scholar] [CrossRef]
- Simcox, T.; Singh, V.; Oakley, C.T.; Koenig, J.A.; Schwarzkopf, R.; Rozell, J.C. Comparison of Utilization and Short-Term Complications Between Technology-Assisted and Conventional Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2022, 30, e673–e682. [Google Scholar] [CrossRef]
- Dretakis, K.; Koutserimpas, C. Pitfalls with the MAKO Robotic-Arm-Assisted Total Knee Arthroplasty. Medicina 2024, 60, 262. [Google Scholar] [CrossRef]
- Sharma, A.K.; Cizmic, Z.; Carroll, K.M.; Jerabek, S.A.; Paprosky, W.G.; Sculco, P.K.; Gonzalez Della Valle, A.; Schwarzkopf, R.; Mayman, D.J.; Vigdorchik, J.M. Computer Navigation for Revision Total Hip Arthroplasty Reduces Dislocation Rates. Indian. J. Orthop. 2022, 56, 1061–1065. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Thomas, G.E.R.; Garfjeld-Roberts, P.; Gundle, R.; Taylor, A.; McLardy-Smith, P.; Murray, D.W. The John Charnley Award: Highly Crosslinked Polyethylene in Total Hip Arthroplasty Decreases Long-Term Wear: A Double-Blind Randomized Trial. Clin. Orthop. Relat. Res. 2015, 473, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.A.; Somerville, L.; McCalden, R.W.; Naudie, D.D.; MacDonald, S.J. Highly Cross-Linked Polyethylene Decreases the Rate of Revision of Total Hip Arthroplasty Compared with Conventional Polyethylene at 13 Years’ Follow-Up. Bone Jt. J. 2016, 98-B, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Borsinger, T.M.; Chandi, S.K.; Puri, S.; Debbi, E.M.; Blevins, J.L.; Chalmers, B.P. Total Hip Arthroplasty: An Update on Navigation, Robotics, and Contemporary Advancements. HSS J. 2023, 19, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Andriollo, L.; Montagna, A.; Mazzella, G.G.; Sangaletti, R.; Benazzo, F.; Rossi, S.M.P. Navigated versus Conventional Medial Unicompartmental Knee Arthroplasty: Minimum 18 Years Clinical Outcomes and Survivorship of the Original Cartier Design. Knee 2024, 49, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhao, Y.; Men, J.; Ma, Z.-R.; Jiang, H.-Z.; Liu, C.-Y.; Feng, W. Application of Virtual and Augmented Reality Technology in Hip Surgery: Systematic Review. J. Med. Internet Res. 2023, 25, e37599. [Google Scholar] [CrossRef]
- Su, S.; Wang, R.; Chen, Z.; Zhou, F.; Zhang, Y. Augmented Reality-Assisted versus Conventional Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2023, 18, 920. [Google Scholar] [CrossRef] [PubMed]
- DiGioia, A.M.; Jaramaz, B.; Blackwell, M.; Simon, D.A.; Morgan, F.; Moody, J.E.; Nikou, C.; Colgan, B.D.; Aston, C.A.; Labarca, R.S.; et al. The Otto Aufranc Award. Image Guided Navigation System to Measure Intraoperatively Acetabular Implant Alignment. Clin. Orthop. Relat. Res. 1998, 355, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Hasegawa, S.; Tsukada, S.; Matsubara, M. A Pilot Study of Augmented Reality Technology Applied to the Acetabular Cup Placement During Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Logishetty, K.; Western, L.; Morgan, R.; Iranpour, F.; Cobb, J.P.; Auvinet, E. Can an Augmented Reality Headset Improve Accuracy of Acetabular Cup Orientation in Simulated THA? A Randomized Trial. Clin. Orthop. Relat. Res. 2019, 477, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Tanino, H.; Mitsutake, R.; Takagi, K.; Ito, H. Does a Commercially Available Augmented Reality-Based Portable Hip Navigation System Improve Cup Positioning During THA Compared with the Conventional Technique? A Randomized Controlled Study. Clin. Orthop. Relat. Res. 2024, 482, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, J.; Vadcard, L.; Girard, P.; Dubois, M.; Merloz, P.; Troccaz, J. Assessment of a Percutaneous Iliosacral Screw Insertion Simulator. Orthop. Traumatol. Surg. Res. 2009, 95, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, F.; Leong, A.P.Y.; Xu, L.; Chen, X.; Wang, Q. Precision Insertion of Percutaneous Sacroiliac Screws Using a Novel Augmented Reality-Based Navigation System: A Pilot Study. Int. Orthop. 2016, 40, 1941–1947. [Google Scholar] [CrossRef]
- Ezzet, K.A.; McCauley, J.C. Use of Intraoperative X-Rays to Optimize Component Position and Leg Length during Total Hip Arthroplasty. J. Arthroplast. 2014, 29, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Zavala-González, J.; Martínez, D.; Gutiérrez-Espinoza, H. Effectiveness of Adding Virtual Reality to Physiotherapeutic Treatment in Patients with Total Hip Arthroplasty. A Randomized Controlled Trial. Clin. Rehabil. 2022, 36, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, L.M.; Middleton, P.F.; Anthony, A.; Hamdorf, J.; Cregan, P.; Scott, D.; Maddern, G.J. Surgical Simulation: A Systematic Review. Ann. Surg. 2006, 243, 291–300. [Google Scholar] [CrossRef]
- Tsukada, S.; Ogawa, H.; Hirasawa, N.; Nishino, M.; Aoyama, H.; Kurosaka, K. Augmented Reality- vs Accelerometer-Based Portable Navigation System to Improve the Accuracy of Acetabular Cup Placement During Total Hip Arthroplasty in the Lateral Decubitus Position. J. Arthroplast. 2022, 37, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Dennler, C.; Bauer, D.E.; Scheibler, A.-G.; Spirig, J.; Götschi, T.; Fürnstahl, P.; Farshad, M. Augmented Reality in the Operating Room: A Clinical Feasibility Study. BMC Musculoskelet. Disord. 2021, 22, 451. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.M.P.; Panzera, R.M.; Sangaletti, R.; Andriollo, L.; Giudice, L.; Lecci, F.; Benazzo, F. Problems and Opportunities of a Smartphone-Based Care Management Platform: Application of the Wald Principles to a Survey-Based Analysis of Patients’ Perception in a Pilot Center. Healthcare 2024, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Andriollo, L.; Picchi, A.; Sangaletti, R.; Perticarini, L.; Rossi, S.M.P.; Logroscino, G.; Benazzo, F. The Role of Artificial Intelligence in Anterior Cruciate Ligament Injuries: Current Concepts and Future Perspectives. Healthcare 2024, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, B.; Papalia, G.F.; Parisi, F.R.; Luciano, C.; Gregori, P.; Vorini, F.; Marinozzi, A.; Farsetti, P.; Papalia, R. Early Return to Activity of Daily Living after Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. Hip Int. 2023, 33, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, P.; Ciatti, C.; Gattoni, S.; Puma Pagliarello, C.; Moretti, G.; Cauteruccio, M.; Carpaneto, D.; Capelli, P.; Gurrieri, L.; Banchini, F.; et al. The Impact of COVID-19 Pandemic on the Emergency Room and Orthopedic Departments in Piacenza: A Retrospective Analysis. Acta Biomed. 2020, 91, e2020028. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, D.; Hou, S.; Liu, W.; Gao, F.; Xu, D.; Hu, Y. Thinking on the Informatization Development of China’s Healthcare System in the Post-COVID-19 Era. Intell. Med. 2021, 1, 24–28. [Google Scholar] [CrossRef]
- Lambert, T.E.; Harvey, L.A.; Avdalis, C.; Chen, L.W.; Jeyalingam, S.; Pratt, C.A.; Tatum, H.J.; Bowden, J.L.; Lucas, B.R. An App with Remote Support Achieves Better Adherence to Home Exercise Programs than Paper Handouts in People with Musculoskeletal Conditions: A Randomised Trial. J. Physiother. 2017, 63, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Takedani, H.; Haga, N.; Kubota, M.; Ishiyama, M.; Ito, S.; Nitta, O. Self-Monitoring Has Potential for Home Exercise Programmes in Patients with Haemophilia. Haemophilia 2014, 20, e121–e127. [Google Scholar] [CrossRef] [PubMed]
- Davidovitch, R.I.; Anoushiravani, A.A.; Feng, J.E.; Chen, K.K.; Karia, R.; Schwarzkopf, R.; Iorio, R. Home Health Services Are Not Required for Select Total Hip Arthroplasty Candidates: Assessment and Supplementation with an Electronic Recovery Application. J. Arthroplast. 2018, 33, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Memarzadeh, K. The Rise of Artificial Intelligence in Healthcare Applications. Artif. Intell. Healthc. 2020, 25–60. [Google Scholar] [CrossRef]
- Wearable Technology: Innovation, Adherence, and Clinical Outcomes. Available online: https://www.pharmasalmanac.com/articles/wearable-technology-innovation-adherence-and-clinical-outcomes (accessed on 20 December 2024).
- Argent, R.; Daly, A.; Caulfield, B. Patient Involvement with Home-Based Exercise Programs: Can Connected Health Interventions Influence Adherence? JMIR Mhealth Uhealth 2018, 6, e47. [Google Scholar] [CrossRef] [PubMed]
- Peek, K.; Sanson-Fisher, R.; Mackenzie, L.; Carey, M. Interventions to Aid Patient Adherence to Physiotherapist Prescribed Self-Management Strategies: A Systematic Review. Physiotherapy 2016, 102, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, E.M.; Kok, G.J.; van der Zee, J. Correlates of Exercise Compliance in Physical Therapy. Phys. Ther. 1993, 73, 771–782; discussion 783–786. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.K.; Palo, N.; Arora, G.; Chandel, S.S.; Kumar, M. Effects of Preoperative Walking Ability and Patient’s Surgical Education on Quality of Life and Functional Outcomes after Total Knee Arthroplasty. Rev. Bras. Ortop. 2016, 52, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Pastora-Bernal, J.-M.; Martín-Valero, R.; Barón-López, F.J.; García-Gómez, O. Effectiveness of Telerehabilitation Programme Following Surgery in Shoulder Impingement Syndrome (SIS): Study Protocol for a Randomized Controlled Non-Inferiority Trial. Trials 2017, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, G.; D’Angelo, D.; Piredda, M.; Petitti, T.; Matarese, M.; Oliveti, A.; De Marinis, M.G. Continuity of Care Interventions for Preventing Hospital Readmission of Older People with Chronic Diseases: A Meta-Analysis. Int. J. Nurs. Stud. 2020, 101, 103396. [Google Scholar] [CrossRef] [PubMed]
- The Ethics of Artificial Intelligence: Issues and Initiatives|Think Tank|European Parliament. Available online: https://www.europarl.europa.eu/thinktank/en/document/EPRS_STU(2020)634452 (accessed on 20 December 2024).
- Cabitza, F.; Rasoini, R.; Gensini, G.F. Unintended Consequences of Machine Learning in Medicine. JAMA 2017, 318, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.; Turchetti, G. Telerehabilitation after Total Knee Replacement in Italy: Cost-Effectiveness and Cost-Utility Analysis of a Mixed Telerehabilitation-Standard Rehabilitation Programme Compared with Usual Care. BMJ Open 2016, 6, e009964. [Google Scholar] [CrossRef]
- Nelson, M.; Russell, T.; Crossley, K.; Bourke, M.; McPhail, S. Cost-Effectiveness of Telerehabilitation versus Traditional Care after Total Hip Replacement: A Trial-Based Economic Evaluation. J. Telemed. Telecare 2021, 27, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Malloy, K.M.; Milling, L.S. The Effectiveness of Virtual Reality Distraction for Pain Reduction: A Systematic Review. Clin. Psychol. Rev. 2010, 30, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, V.C.; Mosadeghi, S.; Howard, A.R.; Lopez, M.; Dupuy, T.; Reid, M.; Martinez, B.; Ahmed, S.; Dailey, F.; Robbins, K.; et al. Virtual Reality for Management of Pain in Hospitalized Patients: Results of a Controlled Trial. JMIR Ment. Health 2017, 4, e9. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.; Kostova, Z.; Nakamoto, K.; Schulz, P.J. The Effect of Social Support Features and Gamification on a Web-Based Intervention for Rheumatoid Arthritis Patients: Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e14. [Google Scholar] [CrossRef] [PubMed]
- Negrillo-Cárdenas, J.; Jiménez-Pérez, J.-R.; Feito, F.R. The Role of Virtual and Augmented Reality in Orthopedic Trauma Surgery: From Diagnosis to Rehabilitation. Comput. Methods Programs Biomed. 2020, 191, 105407. [Google Scholar] [CrossRef]
- Albahri, A.S.; Duhaim, A.M.; Fadhel, M.A.; Alnoor, A.; Baqer, N.S.; Alzubaidi, L.; Albahri, O.S.; Alamoodi, A.H.; Bai, J.; Salhi, A.; et al. A Systematic Review of Trustworthy and Explainable Artificial Intelligence in Healthcare: Assessment of Quality, Bias Risk, and Data Fusion. Inf. Fusion. 2023, 96, 156–191. [Google Scholar] [CrossRef]
- Mehta, M.; Palade, V.; Chatterjee, I. (Eds.) Explainable AI: Foundations, Methodologies and Applications; Intelligent Systems Reference Library; Springer International Publishing: Cham, Switzerland, 2023; Volume 232, ISBN 978-3-031-12806-6. [Google Scholar]
- Bicer, E.K.; Fangerau, H.; Sur, H. Artifical Intelligence Use in Orthopedics: An Ethical Point of View. EFORT Open Rev. 2023, 8, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.G.; Ramkumar, P.N.; Ricciardi, B.F.; Urish, K.L.; Kipper, J.; Ketonis, C. Artificial Intelligence and Orthopaedics: An Introduction for Clinicians. J. Bone Jt. Surg. Am. 2020, 102, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Balaji, S.; Jeyaraman, N.; Yadav, S. Unraveling the Ethical Enigma: Artificial Intelligence in Healthcare. Cureus 2023, 15, e43262. [Google Scholar] [CrossRef]
- Pesapane, F.; Volonté, C.; Codari, M.; Sardanelli, F. Artificial Intelligence as a Medical Device in Radiology: Ethical and Regulatory Issues in Europe and the United States. Insights Imaging 2018, 9, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, S.; Yang, H.; Guo, J.; Wu, Y.; Liu, J. Ethical Considerations of Using ChatGPT in Health Care. J. Med. Internet Res. 2023, 25, e48009. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Spooner, B.; Isherwood, J.; Lane, M.; Orrock, E.; Dennison, A. A Systematic Review of the Barriers to the Implementation of Artificial Intelligence in Healthcare. Cureus 2023, 15, e46454. [Google Scholar] [CrossRef] [PubMed]
- Mithany, R.H.; Aslam, S.; Abdallah, S.; Abdelmaseeh, M.; Gerges, F.; Mohamed, M.S.; Manasseh, M.; Wanees, A.; Shahid, M.H.; Khalil, M.S.; et al. Advancements and Challenges in the Application of Artificial Intelligence in Surgical Arena: A Literature Review. Cureus 2023, 15, e47924. [Google Scholar] [CrossRef] [PubMed]
- Bullock, E.K.C.; Brown, M.J.; Clark, G.; Plant, J.G.A.; Blakeney, W.G. Robotics in Total Hip Arthroplasty: Current Concepts. J. Clin. Med. 2022, 11, 6674. [Google Scholar] [CrossRef] [PubMed]
| Application Area | Specific AI Use | Impact | Key Examples |
|---|---|---|---|
| Diagnosis | Automated grading of hip OA severity | Improved diagnostic accuracy and reliability | DL models (e.g., DRR-based systems) [9,10,11] |
| Preoperative Planning | Predicting hip joint center; implant positioning | Reduced variability in planning; enhanced precision | AI models predicting hip joint center, 3D imaging [12,13,14,15] |
| Surgical Assistance | Real-time intraoperative feedback during robotic-assisted THA | Enhanced implant placement precision; reduced complications | Robotic systems (image-based vs. imageless) [16,17,18,19,20,21] |
| Risk Stratification | Predicting postoperative complications based on patient-specific data | Personalized risk assessment; improved decision-making | ML algorithms [22,23] |
| Implant Identification | Automated recognition of implant designs from imaging | Reduced planning time; streamlined revision | CNN-based implant recognition tools [24,25,26,27,28,29] |
| Rehabilitation | VR and AR for guided exercises and patient engagement | Improved adherence to recovery programs; enhanced patient satisfaction | AR-guided systems (e.g., HoloLens) and VR platforms [30,31,32,33,34,35,36] |
| Outcome Prediction | Forecasting long-term implant success and risk of revision | Proactive patient management; improved long-term outcomes | AI models analyzing radiological and clinical data [37,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriollo, L.; Picchi, A.; Iademarco, G.; Fidanza, A.; Perticarini, L.; Rossi, S.M.P.; Logroscino, G.; Benazzo, F. The Role of Artificial Intelligence and Emerging Technologies in Advancing Total Hip Arthroplasty. J. Pers. Med. 2025, 15, 21. https://doi.org/10.3390/jpm15010021
Andriollo L, Picchi A, Iademarco G, Fidanza A, Perticarini L, Rossi SMP, Logroscino G, Benazzo F. The Role of Artificial Intelligence and Emerging Technologies in Advancing Total Hip Arthroplasty. Journal of Personalized Medicine. 2025; 15(1):21. https://doi.org/10.3390/jpm15010021
Chicago/Turabian StyleAndriollo, Luca, Aurelio Picchi, Giulio Iademarco, Andrea Fidanza, Loris Perticarini, Stefano Marco Paolo Rossi, Giandomenico Logroscino, and Francesco Benazzo. 2025. "The Role of Artificial Intelligence and Emerging Technologies in Advancing Total Hip Arthroplasty" Journal of Personalized Medicine 15, no. 1: 21. https://doi.org/10.3390/jpm15010021
APA StyleAndriollo, L., Picchi, A., Iademarco, G., Fidanza, A., Perticarini, L., Rossi, S. M. P., Logroscino, G., & Benazzo, F. (2025). The Role of Artificial Intelligence and Emerging Technologies in Advancing Total Hip Arthroplasty. Journal of Personalized Medicine, 15(1), 21. https://doi.org/10.3390/jpm15010021






