Fundus Photography-Based Distribution of Retinal Hemorrhages in Newborns: Implications for Underlying Mechanisms
Abstract
1. Introduction
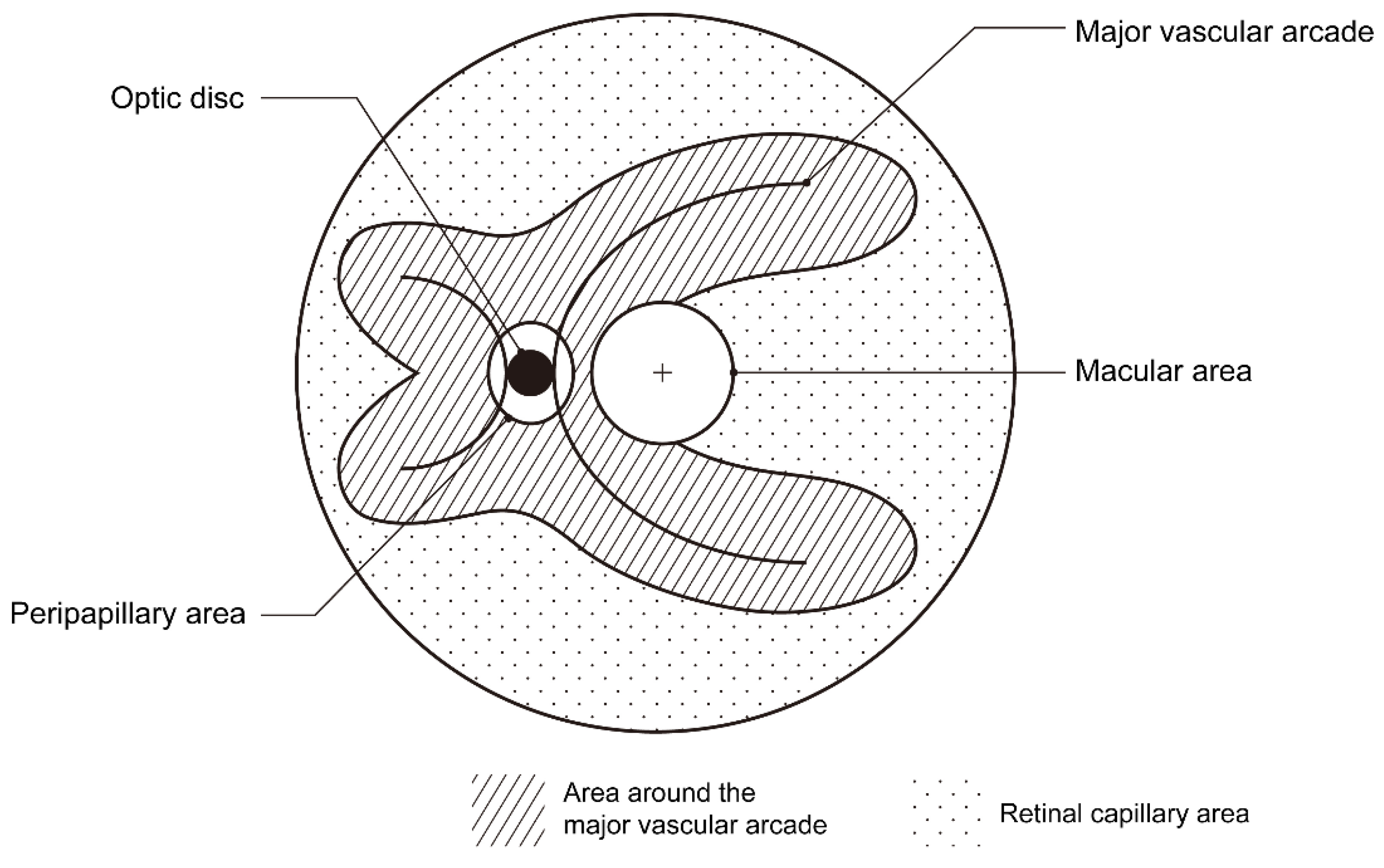
2. Methods
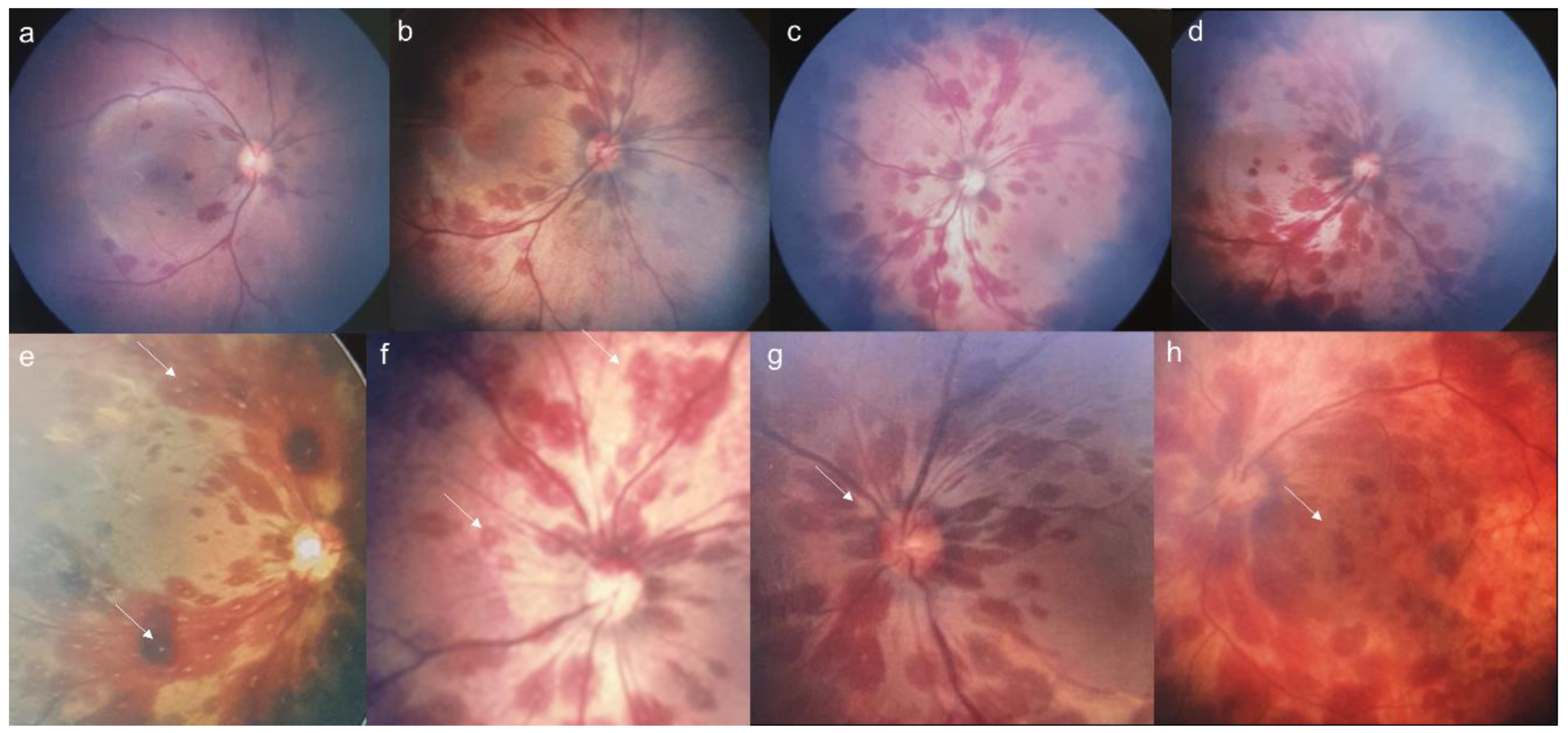
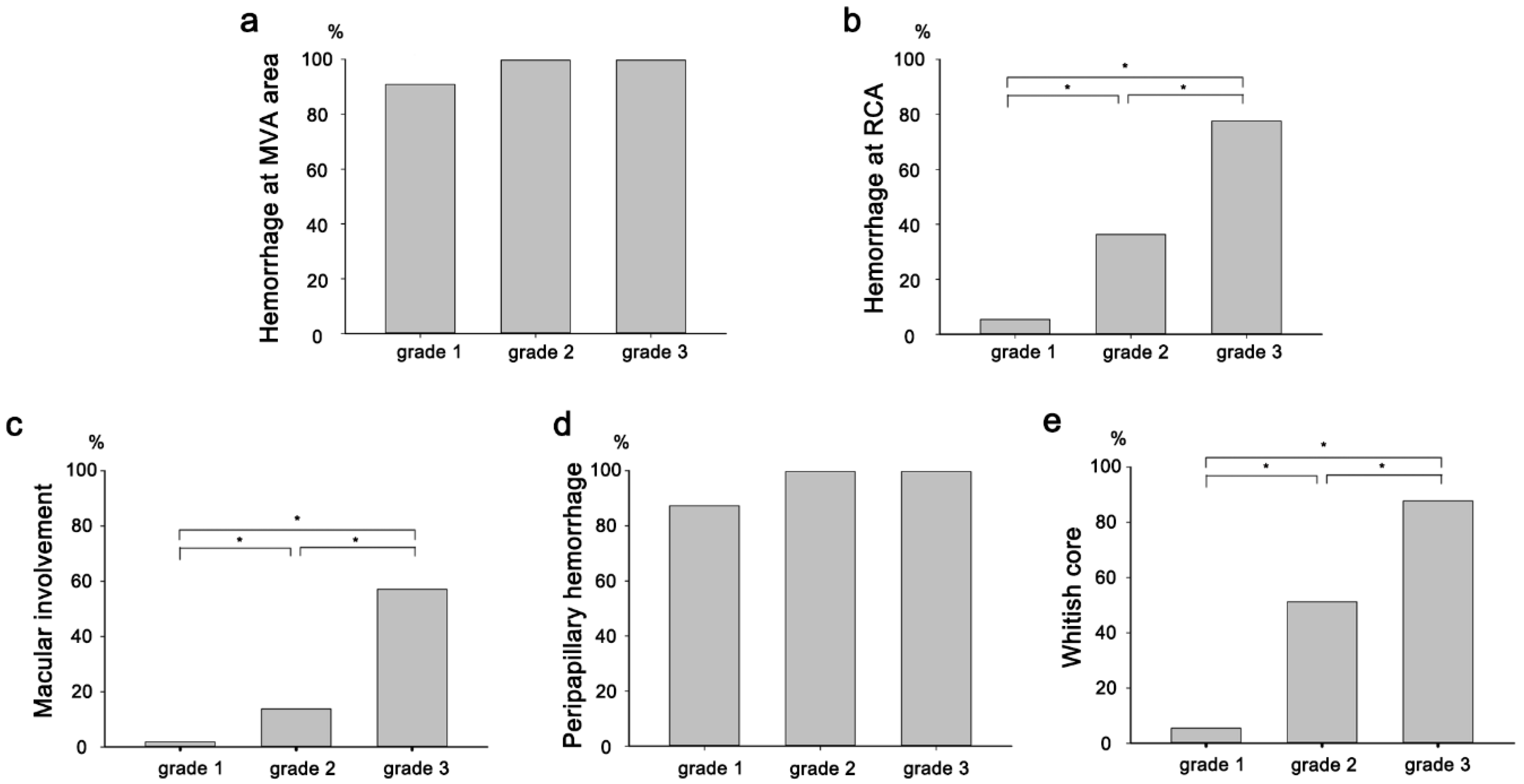
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emerson, M.V.; Pieramici, D.J.; Stoessel, K.M.; Berreen, J.P.; Gariano, R.F. Incidence and rate of disappearance of retinal hemorrhage in newborns. Ophthalmology 2001, 108, 36–39. [Google Scholar] [CrossRef]
- Baum, J.D.; Bulpitt, C.J. Retinal and conjunctival haemorrhage in the newborn. Arch. Dis. Child. 1970, 45, 344–349. [Google Scholar] [CrossRef][Green Version]
- Besio, R.; Caballero, C.; Meerhoff, E.; Schwarcz, R. Neonatal retinal hemorrhages and influence of perinatal factors. Am. J. Ophthalmol. 1979, 87, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.; May, K.; Talbot, J.F.; Parsons, M.A. Incidence, distribution, and duration of birth-related retinal hemorrhages: A prospective study. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2006, 10, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Li, S. A meta-analysis of prognostic biomarkers in neonatal retinal hemorrhage. Int. Ophthalmol. 2022, 42, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jiang, Z.; Li, S.; Liu, J.; Su, M.; Lu, Y.; Li, Z.; Ding, X. What Is Left After Resolution of Neonatal Retinal Hemorrhage: The Longitudinal Long-term Outcome in Foveal Structure and Visual Function. Am. J. Ophthalmol. 2021, 226, 182–190. [Google Scholar] [CrossRef] [PubMed]
- AlShaker, S.; Jivraj, I.; Chen, H.; Muthusami, P.; Phillips, J.; DeAngelis, D. Management of a Large Congenital Hemangioma Obstructing Visual Axis: A Case Report and Review of Literature. Ophthalmic Plast. Reconstr. Surg. 2019, 35, e154–e157. [Google Scholar] [CrossRef]
- Chen, F.; Cheng, D.; Pan, J.; Huang, C.; Cai, X.; Tian, Z.; Lu, F.; Shen, L. The efficacy and safety of Retcam in detecting neonatal retinal hemorrhages. BMC Ophthalmol. 2018, 18, 202. [Google Scholar] [CrossRef]
- Svenningsen, L.; Lindemann, R.; Eidal, K.; Jensen, O. Neonatal retinal hemorrhages and neurobehavior related to tractive force in vacuum extraction. Acta Obstet. Gynecol. Scand. 1987, 66, 165–169. [Google Scholar] [CrossRef]
- Chung, C.W.; Levin, A.V.; Forbes, B.J.; Binenbaum, G. Retinal hemorrhage after pediatric neurosurgical procedures. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2022, 26, 74.e1–74.e5. [Google Scholar] [CrossRef]
- Yenice, E.K.; Petriçli, İ.S.; Kara, C. Findings of ocular examinations in healthy full-term newborns. Arq. Bras. Oftalmol. 2022, 87, 0536. [Google Scholar] [CrossRef] [PubMed]
- Jolobe, O.M.P. Cardiac and extracardiac side effects of eye drops. Am. J. Emerg. Med. 2021, 46, 731. [Google Scholar] [CrossRef]
- Goldenberg, D.; Shahar, J.; Loewenstein, A.; Goldstein, M. Diameters of retinal blood vessels in a healthy cohort as measured by spectral domain optical coherence tomography. Retina 2013, 33, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Abtahi, M.; Adejumo, T.; Ebrahimi, B.; Dadzie, A.K.; Son, T.; Yao, X. Deep learning for artery-vein classification in optical coherence tomography angiography. Exp. Biol. Med. 2023, 248, 747–761. [Google Scholar] [CrossRef]
- Son, T.; Alam, M.; Kim, T.H.; Liu, C.; Toslak, D.; Yao, X. Near infrared oximetry-guided artery-vein classification in optical coherence tomography angiography. Exp. Biol. Med. 2019, 244, 813–818. [Google Scholar] [CrossRef]
- Bergen, R.; Margolis, S. Retinal hemorrhages in the newborn. Ann. Ophthalmol. 1976, 8, 53–56. [Google Scholar] [PubMed]
- Bist, H.K.; Singh, M.; Satsangi, S.K.; Mishra, B.; Singh, R.S.; Pandey, D.N.; Singh, Y.P.; Prasad, R. Retinal hemorrhages in newborn--fetal causative factors. Indian Pediatr. 1989, 26, 558–565. [Google Scholar] [PubMed]
- Zhao, Q.; Zhang, Y.; Yang, Y.; Li, Z.; Lin, Y.; Liu, R.; Wei, C.; Ding, X. Birth-related retinal hemorrhages in healthy full-term newborns and their relationship to maternal, obstetric, and neonatal risk factors. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1021–1025. [Google Scholar] [CrossRef]
- Sitorus, R.S.; Pambudy, I.M.; Rohsiswatmo, R.; Barliana, J.D.; Yulia, D.E.; Widyahening, I.S. Retinal abnormalities in universal eye screening of healthy, full-term newborn infants in Jakarta. The incidence and its risk factors: A pilot study. Int. J. Retin. Vitr. 2021, 7, 67. [Google Scholar] [CrossRef]
- Lam, M.R.; Yang, C.D.; Colmenarez, J.A.; Dong, P.; Gu, L.; Suh, D.W. The role of intrapartum fetal head compression in neonatal retinal hemorrhage. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2023, 27, 267.e1–267.e7. [Google Scholar] [CrossRef]
- Yang, T.; Hu, R.; Chen, J.; Lu, Y.; Guo, Y.; Liu, Y.; Yu, R.; Jin, G. Prevalence, Characteristics, and Risk Factors of Retinal Hemorrhage among Full-Term Neonates in Southern China. Int. J. Environ. Res. Public Health 2022, 19, 13927. [Google Scholar] [CrossRef]
- Yanli, Z.; Qi, Z.; Yu, L.; Haike, G. Risk Factors Affecting the Severity of Full-Term Neonatal Retinal Hemorrhage. J. Ophthalmol. 2017, 2017, 4231489. [Google Scholar] [CrossRef]
- Pu, Q.; Li, P.; Jiang, H.; Wang, H.; Zhou, Q.; Liu, J.; Zhong, W.; Huang, H. Factors related to retinal haemorrhage in infants born at high risk. Acta Ophthalmol. 2017, 95, e477–e480. [Google Scholar] [CrossRef]
- Naderian, G.; Fesharaki, H.; Sajjadi, V.; Naderian, M.A. Retinal Hemorrhages in a Neonate following Vacuum Extraction. J. Ophthalmic Vis. Res. 2013, 8, 179–181. [Google Scholar]
- Wood, E.H.; Capone, A., Jr.; Drenser, K.A.; Berrocal, A.; Hubbard, G.B.; Callaway, N.F.; Kychenthal, A.; Ells, A.; Harper, C.A., 3rd; Besirli, C.G.; et al. Referable Macular Hemorrhage-A Clinically Meaningful Screening Target in Newborn Infants. Position Statement of the Association of Pediatric Retina Surgeons. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.P.; McMillan, K.R.; Coats, B. Morphological Analysis of Retinal Microvasculature to Improve Understanding of Retinal Hemorrhage Mechanics in Infants. Investig. Ophthalmol. Vis. Sci. 2020, 61, 16. [Google Scholar] [CrossRef] [PubMed]
- Odogu, V.K.; Hayat MBBS, U. Infective Endocarditis Presenting as Roth Spots. Niger. J. Vitr. Dis. 2020, 3, 15–18. [Google Scholar] [CrossRef]
- Kaur, B.; Taylor, D. Fundus hemorrhages in infancy. Surv. Ophthalmol. 1992, 37, 1–17. [Google Scholar] [CrossRef]
- Ling, R.; James, B. White-centred retinal haemorrhages (Roth spots). Postgrad. Med. J. 1998, 74, 581–582. [Google Scholar] [CrossRef]
- Kaur, S.; Sharda, S.; Aggarwal, H.; Dadeya, S. Comprehensive review of amblyopia: Types and management. Indian J. Ophthalmol. 2023, 71, 2677–2686. [Google Scholar] [CrossRef]
- Greenberg, S.; Plummer, C.; Maisenbacher, H.; Friary, J.; Berg, A. The effect of topical ophthalmic 1% atropine on heart rate and rhythm in normal dogs. Vet. Ophthalmol. 2015, 18, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Twelker, J.D.; Mutti, D.O. Retinoscopy in infants using a near noncycloplegic technique, cycloplegia with tropicamide 1%, and cycloplegia with cyclopentolate 1%. Optom. Vis. Sci. 2001, 78, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sengo, D.B.; Dos Santos, I.; Faquihe, M.F.; Tomo, H.; Muaprato, A.M.; Puchar, S.; Lôbo, G.; López-Izquierdo, I.; Caballero, P. The Prevalence of Visual Impairment and Refractive Errors among a Youth Population in Mozambique: Evidence of the Need for Intervention. Children 2021, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- Paudel, N.; Thompson, B.; Chakraborty, A.; Harding, J.; Jacobs, R.J.; Wouldes, T.A.; Yu, S.T.; Anstice, N.S. Relationship between visual and neurodevelopmental measures at 2 years with visual acuity and stereopsis at 4.5 years in children born at risk of neonatal hypoglycaemia. Ophthalmic Physiol. Opt. 2022, 42, 195–204. [Google Scholar] [CrossRef] [PubMed]
| Retinal Hemorrhage | Control | p Value | |
|---|---|---|---|
| Total number of infants | 98 | 30 | |
| Sex (male/female) | 57/41 | 20/10 | 0.523 * |
| Gestational age (weeks) | 39.3 ± 1.1 | 39.6 ± 1.3 | 0.628 † |
| Birth weight (kg) | 3.29 ± 0.39 | 3.36 ± 0.34 | 0.344 † |
| Method of delivery (infants) | 0.004 * | ||
| NSVD | 90 (91.8%) | 21 (70.0%) | |
| Cesarean section | 8 (8.2%) | 9 (30.0%) | |
| Laterality (infants) | |||
| Bilateral | 86 (87.8%) | ||
| Unilateral | 12 (12.2%) | ||
| Time intervals (days) | |||
| Birth–Exam 1 | 1.0 ± 0.8 (range 1–5) | 1.1 ± 0.7 (range 1–5) | |
| Exam 1–Exam 2 | 45.6 ± 15.9 | ||
| Birth–Exam 3 | 234.5 ± 39.3 | 231.9 ± 32.0 | 0.841 † |
| Refractive errors in Exam 3 (D) | 0.53 ± 0.70 | 0.69 ± 1.34 | 0.538 † |
| Total Number of Eyes | 196 |
|---|---|
| Grade (eyes) | |
| Grade 0 * | 12 (6.1%) |
| Grade 1 | 55 (28.1%) |
| Grade 2 | 80 (40.8%) |
| Grade 3 | 49 (25.0%) |
| Grade difference between both eyes (newborns) | |
| 0 (same grade) | 38 (38.8%) |
| 1 | 45 (45.9%) |
| 2 | 14 (14.3%) |
| 3 | 1 (1.0%) |
| Characteristics (eyes) | |
| Whitish core | 87 (44.4%) |
| Macular involvement | 40 (20.4%) |
| Peripapillary hemorrhage | 177 (90.3%) |
| Location (eyes) | |
| Around the major vascular arcades | 180 (91.8%) |
| In retinal capillary area | 70 (35.7%) |
| Present | Absent | p-Value | |
|---|---|---|---|
| Retinal hemorrhage (D) | +0.53 ± 0.70 (184) | +0.60 ± 0.78 (12) | 0.787 |
| Macular involvement (D) | +0.48 ± 0.53 (40) | +0.55 ± 0.75 (156) | 0.679 |
| Whitish core (D) | +0.47 ± 0.71 (87) | +0.58 ± 0.70 (109) | 0.411 |
| Retinal capillary area hemorrhage (D) | +0.49 ± 0.79 (70) | +0.56 ± 0.66 (126) | 0.627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, G.H.; Choi, M.Y.; Lee, K.; Kim, K.T.; Kim, D.Y.; Chae, J.B.; Seo, E.J. Fundus Photography-Based Distribution of Retinal Hemorrhages in Newborns: Implications for Underlying Mechanisms. J. Pers. Med. 2025, 15, 38. https://doi.org/10.3390/jpm15010038
Jo GH, Choi MY, Lee K, Kim KT, Kim DY, Chae JB, Seo EJ. Fundus Photography-Based Distribution of Retinal Hemorrhages in Newborns: Implications for Underlying Mechanisms. Journal of Personalized Medicine. 2025; 15(1):38. https://doi.org/10.3390/jpm15010038
Chicago/Turabian StyleJo, Gwon Hui, Mi Young Choi, Kibum Lee, Kyung Tae Kim, Dong Yoon Kim, Ju Byung Chae, and Eoi Jong Seo. 2025. "Fundus Photography-Based Distribution of Retinal Hemorrhages in Newborns: Implications for Underlying Mechanisms" Journal of Personalized Medicine 15, no. 1: 38. https://doi.org/10.3390/jpm15010038
APA StyleJo, G. H., Choi, M. Y., Lee, K., Kim, K. T., Kim, D. Y., Chae, J. B., & Seo, E. J. (2025). Fundus Photography-Based Distribution of Retinal Hemorrhages in Newborns: Implications for Underlying Mechanisms. Journal of Personalized Medicine, 15(1), 38. https://doi.org/10.3390/jpm15010038








