Pharmacogenomics in Diabetes: Population-Specific Insights from Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
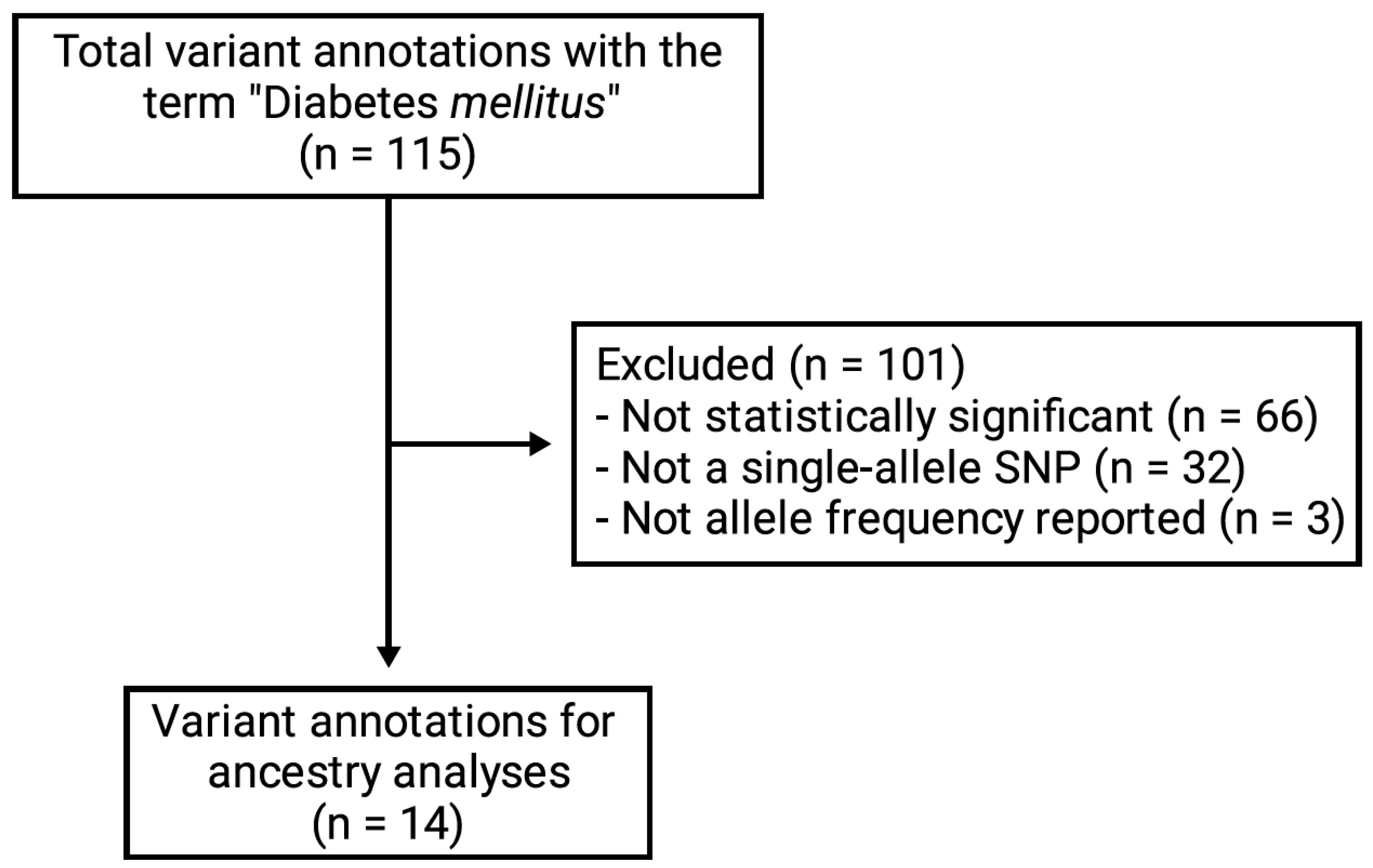
2.2. Data Collection
2.3. Statistical Analysis
2.4. Bibliometric Analysis
3. Results
3.1. Bibliometric Landscape
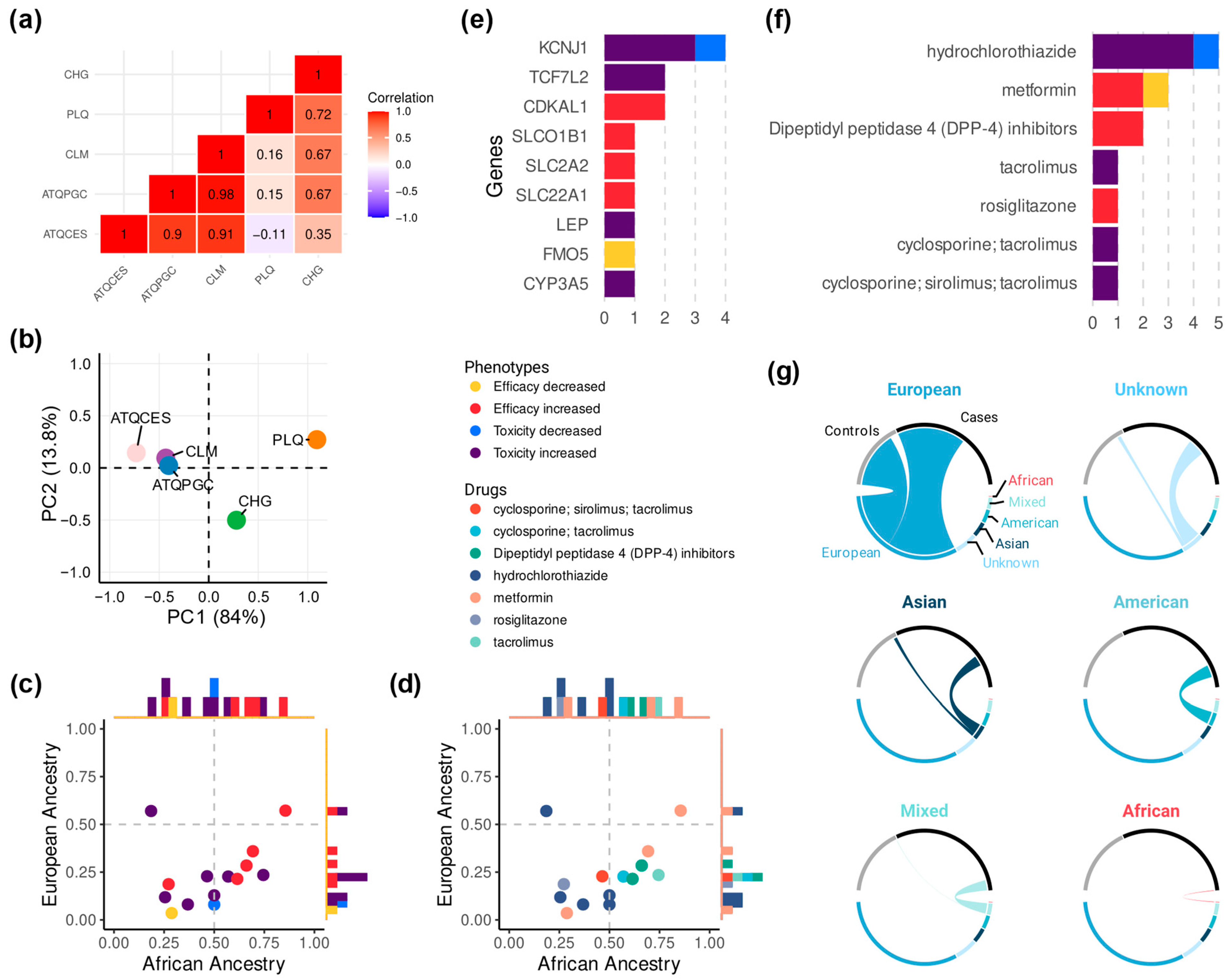
3.2. The Role of Genetic Ancestry in Colombia
3.3. A Summary of Drugs and Genes with the Most Reported Associations
3.4. What We Overlook: The European Bias
4. Discussion
4.1. Gene–Variant Context and Mechanistic Links to Phenotypes
4.2. Knowledge Gaps as Research Opportunities
4.3. Contribution to Precision Medicine, Endocrinology, and Diabetes Care
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATQCES | European ancestry from Antioquia #1 |
| ATQPGC | European ancestry from Antioquia #2 |
| CHG | Chocó |
| CLM | Colombians in Medellin |
| CÓDIGO | Consortium for Genomic Diversity, Ancestry, and Health in Colombia |
| DM | Diabetes mellitus |
| DPP-4 | Dipeptidyl peptidase-4 |
| LMICs | Low- and middle-income countries |
| PLQ | Palenque |
| SNPs | Single-nucleotide polymorphisms |
| WHO | World Health Organization |
References
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Welday Kahssay, S.; Demeke, N.F. Pharmacotherapy problems and associated factors among type 2 adult diabetic patients on follow up at Mizan-Tepi University Teaching Hospital, Southwest Ethiopia. PLoS ONE 2023, 18, e0288093. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ibarra, H.E.; Reyes-Cortes, L.M.; Jiang, X.L.; Luna-Aguirre, C.M.; Aguirre-Trevino, D.; Morales-Alvarado, I.A.; Leon-Cachon, R.B.; Lavalle-Gonzalez, F.; Morcos, F.; Barrera-Saldaña, H.A. Genotypic and Phenotypic Factors Influencing Drug Response in Mexican Patients with Type 2 Diabetes Mellitus. Front. Pharmacol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Mannino, G.C.; Andreozzi, F.; Sesti, G. Pharmacogenetics of type 2 diabetes mellitus, the route toward tailored medicine. Diabetes Metab. Res. Rev. 2019, 35, e3109. [Google Scholar] [CrossRef]
- Guo, Z.; Priefer, R. Current progress in pharmacogenomics of Type 2 diabetes: A systemic overview. Diabetes Metab. Syndr. 2021, 15, 102239. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, S.Y.; Sun, T. Pharmacogenomic Studies of Current Antidiabetic Agents and Potential New Drug Targets for Precision Medicine of Diabetes. Diabetes Ther. 2020, 11, 2521–2538. [Google Scholar] [CrossRef] [PubMed]
- Corpas, M.; Siddiqui, M.K.; Soremekun, O.; Mathur, R.; Gill, D.; Fatumo, S. Addressing Ancestry and Sex Bias in Pharmacogenomics. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 53–64. [Google Scholar] [CrossRef]
- Norris, E.T.; Wang, L.; Conley, A.B.; Rishishwar, L.; Mariño-Ramírez, L.; Valderrama-Aguirre, A.; Jordan, I.K. Genetic ancestry, admixture and health determinants in Latin America. BMC Genom. 2018, 19 (Suppl. 8), 861. [Google Scholar] [CrossRef]
- Karamperis, K.; Katz, S.; Melograna, F.; Ganau, F.P.; Van Steen, K.; Patrinos, G.P.; Lao, O. Genetic ancestry in population pharmacogenomics unravels distinct geographical patterns related to drug toxicity. iScience 2024, 27, 110916. [Google Scholar] [CrossRef] [PubMed]
- Salas-Hernández, A.; Galleguillos, M.; Carrasco, M.; López-Cortés, A.; Redal, M.A.; Fonseca-Mendoza, D.; Esperón, P.; González-Martínez, F.; Lares-Asseff, I.; Lazarowski, A.; et al. An updated examination of the perception of barriers for pharmacogenomics implementation and the usefulness of drug/gene pairs in Latin America and the Caribbean. Front. Pharmacol. 2023, 14, 1175737. [Google Scholar] [CrossRef]
- Klein, M.E.; Parvez, M.M.; Shin, J.G. Clinical Implementation of Pharmacogenomics for Personalized Precision Medicine: Barriers and Solutions. J. Pharm. Sci. 2017, 106, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Roman, Y. Bridging the United States population diversity gaps in clinical research: Roadmap to precision health and reducing health disparities. Pers. Med. 2025, 22, 193–203. [Google Scholar] [CrossRef]
- Díaz-Peña, R.; Adelowo, O. Advancing equity in genomic medicine for rheumatology. Nat. Rev. Rheumatol. 2024, 20, 595–596. [Google Scholar] [CrossRef]
- Stanford University. PharmGKB. Available online: https://www.pharmgkb.org/ (accessed on 10 April 2025).
- Mariño-Ramírez, L.; Sharma, S.; Hamilton, J.M.; Nguyen, T.L.; Gupta, S.; Natarajan, A.V.; Nagar, S.D.; Menuey, J.L.; Chen, W.A.; Sánchez-Gómez, A.; et al. The Consortium for Genomic Diversity, Ancestry, and Health in Colombia (CÓDIGO): Building local capacity in genomics and bioinformatics. Commun. Biol. 2025, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Georgia Institute of Technology. CÓDIGO. Available online: https://codigo.biosci.gatech.edu/ (accessed on 10 April 2025).
- World Health Organization. Countries/Areas by WHO Region. Available online: https://apps.who.int/violence-info/Countries%20and%20areas%20by%20WHO%20region%20-%2012bfe12.pdf (accessed on 10 April 2025).
- World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 10 April 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Parving, H.H.; Jacobsen, P.; Tarnow, L.; Rossing, P.; Lecerf, L.; Poirier, O.; Cambien, F. Effect of deletion polymorphism of angiotensin converting enzyme gene on progression of diabetic nephropathy during inhibition of angiotensin converting enzyme: Observational follow up study. BMJ 1996, 313, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; De, T.; Zhong, Y.; Perera, M.A. The Advantages and Challenges of Diversity in Pharmacogenomics: Can Minority Populations Bring Us Closer to Implementation? Clin. Pharmacol. Ther. 2019, 106, 338–349. [Google Scholar] [CrossRef]
- Osada, U.N.; Sunagawa, H.; Terauchi, Y.; Ueda, S. A Common Susceptibility Gene for Type 2 Diabetes Is Associated with Drug Response to a DPP-4 Inhibitor: Pharmacogenomic Cohort in Okinawa Japan. PLoS ONE 2016, 11, e0154821. [Google Scholar] [CrossRef]
- Zhou, K.; Yee, S.W.; Seiser, E.L.; van Leeuwen, N.; Tavendale, R.; Bennett, A.J.; Groves, C.J.; Coleman, R.L.; van der Heijden, A.A.; Beulens, J.W.; et al. Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat. Genet. 2016, 48, 1055–1059. [Google Scholar] [CrossRef]
- Karnes, J.H.; Gong, Y.; Pacanowski, M.A.; McDonough, C.W.; Arwood, M.J.; Langaee, T.Y.; Pepine, C.J.; Johnson, J.A.; Cooper-Dehoff, R.M. Impact of TCF7L2 single nucleotide polymorphisms on hydrochlorothiazide-induced diabetes. Pharmacogenet. Genom. 2013, 23, 697–705. [Google Scholar] [CrossRef]
- Hippman, C.; Nislow, C. Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges. J. Pers. Med. 2019, 9, 40. [Google Scholar] [CrossRef]
- Severin, A.; Egger, M.; Eve, M.P.; Hürlimann, D. Discipline-specific open access publishing practices and barriers to change: An evidence-based review. F1000Research 2018, 7, 1925. [Google Scholar] [CrossRef]
- Jordan, I.K.; Sharma, S.; Mariño-Ramírez, L. Population Pharmacogenomics for Health Equity. Genes 2023, 14, 1840. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.H.; Limdi, N.A. Translational Pharmacogenomics: Discovery, Evidence Synthesis and Delivery of Race-Conscious Medicine. Clin. Pharmacol. Ther. 2021, 110, 909–925. [Google Scholar] [CrossRef]
- Goodman, C.W.; Brett, A.S. Race and Pharmacogenomics-Personalized Medicine or Misguided Practice? JAMA 2021, 325, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Paetznick, C.; Okoro, O. The Intersection between Pharmacogenomics and Health Equity: A Case Example. Pharmacy 2023, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Ji, Y. Pharmacogenomics and health disparities, are we helping? Front. Genet. 2023, 14, 1099541. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, X.; Cai, R.; Yan, B.; Liang, W.; Cai, M.; Yang, P. Tacrolimus and Diabetes in Kidney Transplantation: The Impact of Cyp3a5 Gene Polymorphism. Transplant. Proc. 2023, 55, 2398–2402. [Google Scholar] [CrossRef]
- Shaman, J.A. The Future of Pharmacogenomics: Integrating Epigenetics, Nutrigenomics, and Beyond. J. Pers. Med. 2024, 14, 1121. [Google Scholar] [CrossRef]
- Lozada-Martinez, I.D.; Neira-Rodado, D.; Martinez-Guevara, D.; Cruz-Soto, H.S.; Sanchez-Echeverry, M.P.; Liscano, Y. Why is it important to implement meta-research in universities and institutes with medical research activities? Front. Res. Metr. Anal. 2025, 10, 1497280. [Google Scholar] [CrossRef]
- Lozada-Martinez, I.D.; Lozada-Martinez, L.M.; Fiorillo-Moreno, O. Leiden manifesto and evidence-based research: Are the appropriate standards being used for the correct evaluation of pluralism, gaps and relevance in medical research? J. R. Coll. Physicians Edinb. 2024, 54, 4–6. [Google Scholar] [CrossRef]
- Lozada-Martinez, I.D.; Hernandez-Paz, D.A.; Fiorillo-Moreno, O.; Picón-Jaimes, Y.A.; Bermúdez, V. Meta-Research in Biomedical Investigation: Gaps and Opportunities Based on Meta-Research Publications and Global Indicators in Health, Science, and Human Development. Publications 2025, 13, 7. [Google Scholar] [CrossRef]
- Nieh, H.E.V.; Roman, Y.M. Major Allele Frequencies in CYP2C9 and CYP2C19 in Asian and European Populations: A Case Study to Disaggregate Data Among Large Racial Categories. J. Pers. Med. 2025, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Martinez, I.D.; Bolaño-Romero, M.P.; Picón-Jaimes, Y.A.; Moscote-Salazar, L.R.; Narvaez-Rojas, A.R. Quality or quantity? Questions on the growth of global scientific production. Int. J. Surg. 2022, 105, 106862. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Martinez, I.D.; Ealo-Cardona, C.I.; Marrugo-Ortiz, A.C.; Picón-Jaimes, Y.A.; Cabrera-Vargas, L.F.; Narvaez-Rojas, A.R. Meta-research studies in surgery: A field that should be encouraged to assess and improve the quality of surgical evidence. Int. J. Surg. 2023, 109, 1823–1824. [Google Scholar] [CrossRef]
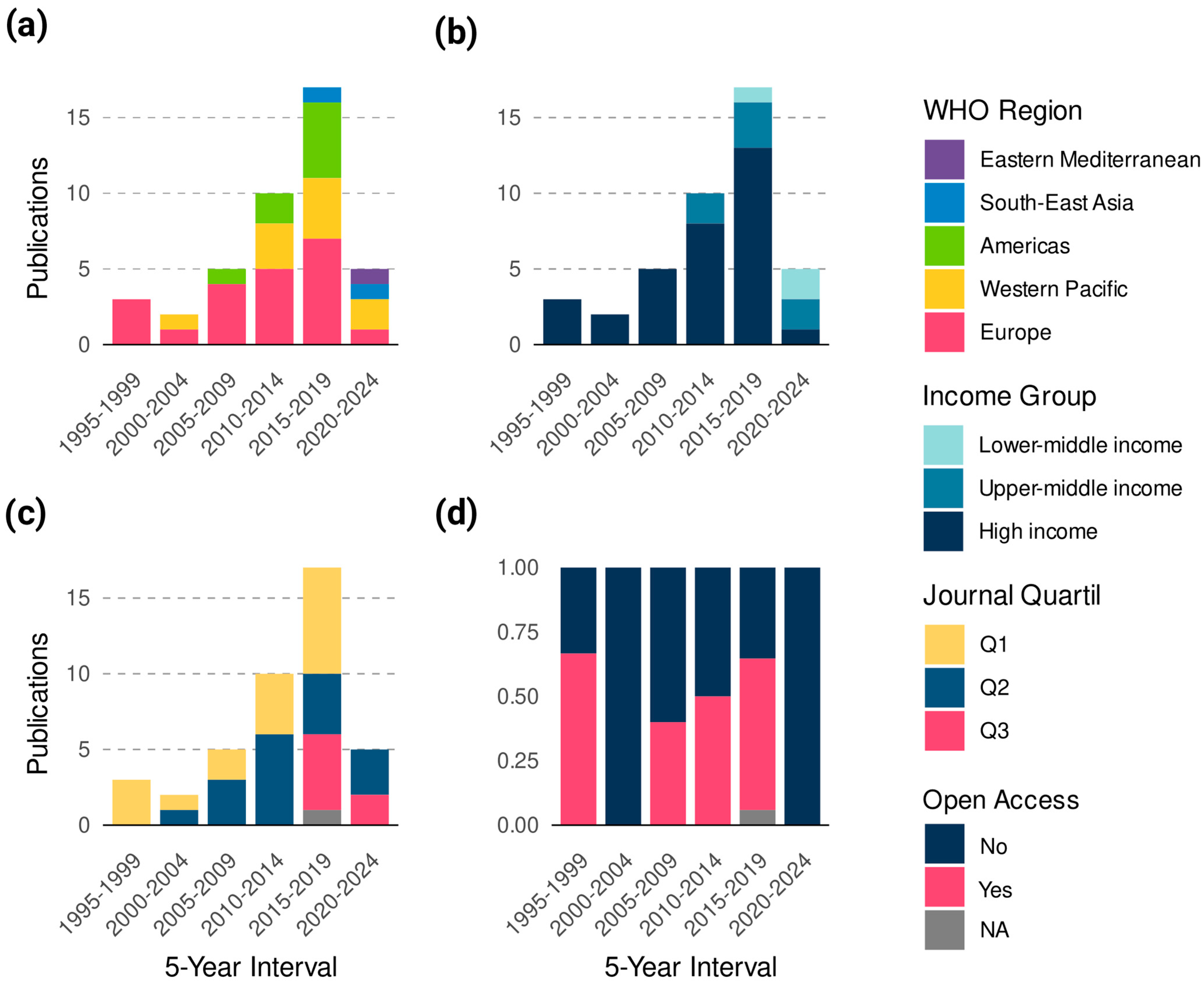
| Americas | Europe | Western Pacific | South-East Asia | Eastern Mediterranean | Africa | p-Value | |
|---|---|---|---|---|---|---|---|
| Publications (%) | 8 (19) | 21 (50) | 10 (23.8) | 2 (0.04) | 1 (0.02) | 0 | |
| Total Citations (Avg per Paper) | 106 (13.2) | 1046 (49.8) | 303 (30.3) | 19 (9.5) | 0 | 0 | 0.04 a |
| Avg H-index (SD) | 143 (45.1) | 203.7 (159.3) | 220.9 (147.9) | 73.5 (27.5) | 99 (0) | 0 | 0.2 a |
| Open Access/No Open Access (ratio) | 6/2 (3) | 9/12 (0.75) | 3/7 (2.4) | 1/1 (1) | 0/1 (0) | 0 | 0.18 b |
| Journal quartile (%) (n = 41) | 0.14 b | ||||||
| Q1 | 2 (28.5) | 9 (42.8) | 6 (60) | 0 | 0 | 0 | |
| Q2 | 4 (57.1) | 10 (47.6) | 2 (20) | 0 | 1 (0) | 0 | |
| Q3 | 1 (14.2) | 2 (0.9) | 2 (20) | 2 (100) | 0 | 0 | |
| Q4 | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | |
| High Income | Upper-Middle Income | Lower-Middle Income | Low-Income | p-Value | |
|---|---|---|---|---|---|
| Publications (%) | 32 (76.1) | 7 (16.6) | 3 (0.71) | 0 | |
| Total Citations (Avg per Paper) | 1322 (41.3) | 133 (19) | 19 (6.3) | 0 | 0.03 a |
| Avg H-index (SD H-index) | 196.1 (144.4) | 201.2 (140.1) | 82 (24.4) | 0 | 0.09 a |
| Open Access/No Open Access (ratio) | 17/15 (1.13) | 5/2 (2.5) | 1/2 (0.5) | 0 | 1 b |
| Journal quartile (%) (n = 41) | 0.04 b | ||||
| Q1 | 14 (45.1) | 3 (42.8) | 0 | 0 | |
| Q2 | 14 (45.1) | 2 (28.5) | 1 (33.3) | 0 | |
| Q3 | 3 (0.96) | 2 (28.5) | 2 (66.6) | 0 | |
| Q4 | 0 | 0 | 0 | 0 | |
| Gene | SNP | Drug | Mean African AF (%) | Mean European AF (%) | Phenotype | Reference |
|---|---|---|---|---|---|---|
| Efficacy, increased | ||||||
| CDKAL1 | rs7754840-C | DPP-4 inhibitors | 66.1 | 28.4 | ↑ HbA1c reduction | [22] |
| CDKAL1 | rs7756992-G | DPP-4 inhibitors | 61.5 | 21.4 | ↑ HbA1c reduction | [22] |
| SLC2A2 | rs8192675-C | Metformin | 69.3 | 35.9 | ↑ HbA1c reduction | [23] |
| Toxicity, increased | ||||||
| TCF7L2 | rs7917983-T | Hydrochlorothiazide | 18.5 | 57 | ↑ NODM risk | [24] |
| CYP3A5 | rs776746-T | Tacrolimus | 74.5 | 23.5 | ↑ PTDM risk | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Paez, D.A.; Galván-Barrios, J.; Montoya-Quintero, K.F.; Torres, I.L.R. Pharmacogenomics in Diabetes: Population-Specific Insights from Colombia. J. Pers. Med. 2025, 15, 481. https://doi.org/10.3390/jpm15100481
Hernandez-Paez DA, Galván-Barrios J, Montoya-Quintero KF, Torres ILR. Pharmacogenomics in Diabetes: Population-Specific Insights from Colombia. Journal of Personalized Medicine. 2025; 15(10):481. https://doi.org/10.3390/jpm15100481
Chicago/Turabian StyleHernandez-Paez, David A., Johana Galván-Barrios, Kevin Fernando Montoya-Quintero, and Indiana Luz Rojas Torres. 2025. "Pharmacogenomics in Diabetes: Population-Specific Insights from Colombia" Journal of Personalized Medicine 15, no. 10: 481. https://doi.org/10.3390/jpm15100481
APA StyleHernandez-Paez, D. A., Galván-Barrios, J., Montoya-Quintero, K. F., & Torres, I. L. R. (2025). Pharmacogenomics in Diabetes: Population-Specific Insights from Colombia. Journal of Personalized Medicine, 15(10), 481. https://doi.org/10.3390/jpm15100481








