Multimodality Imaging in the Diagnosis of Coronary Microvascular Disease: An Update
Abstract
:1. Introduction
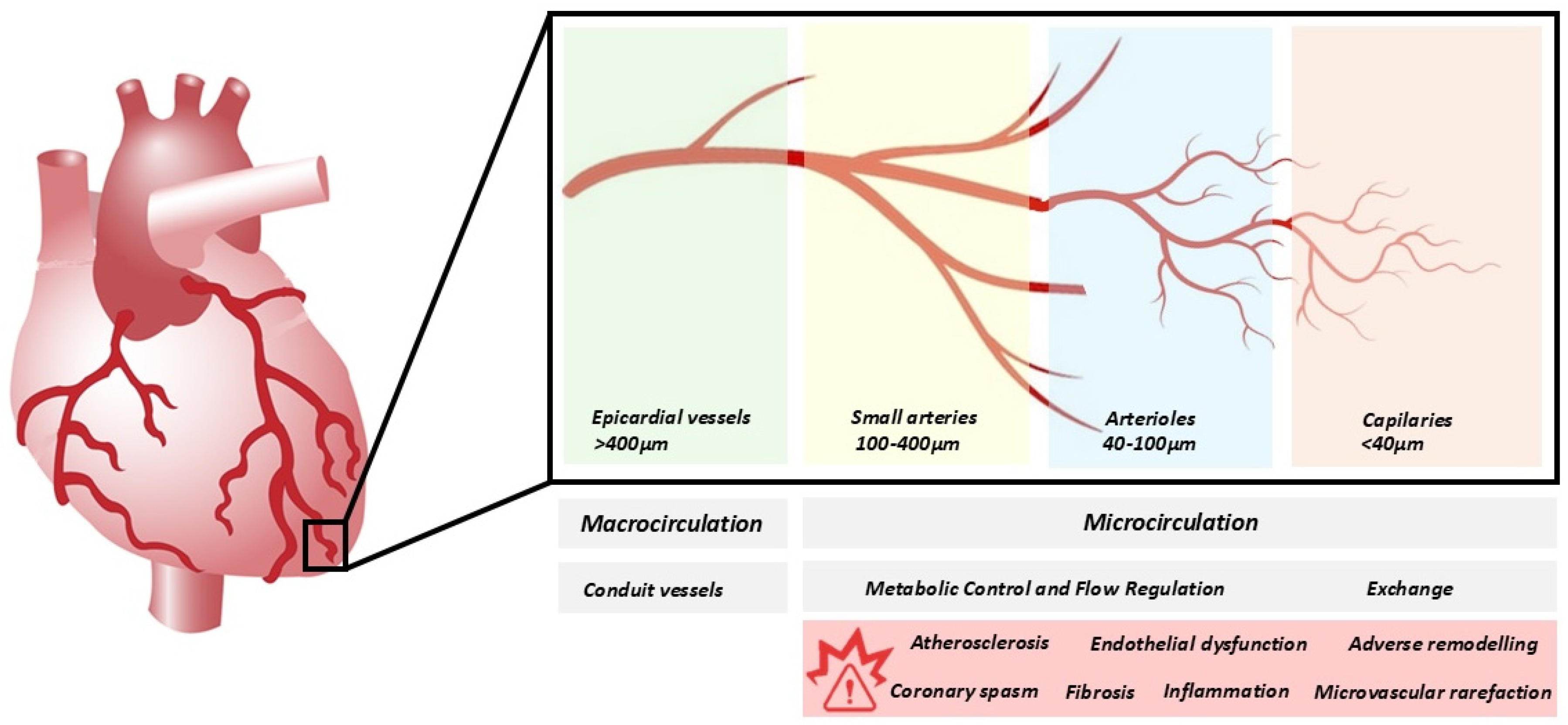
2. Overview of the Coronary Microvascular Circulation
3. Clinical Presentation and Diagnosis
3.1. Clinical Presentation
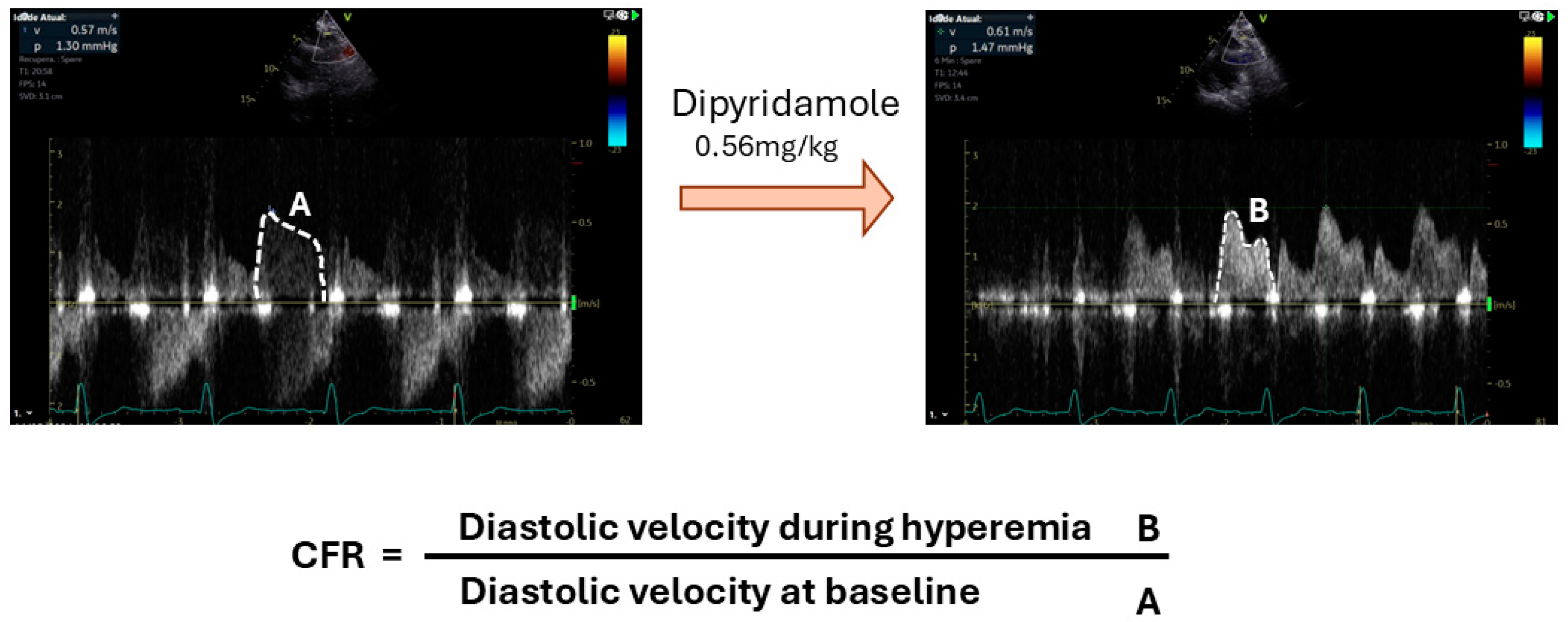
3.2. Assessment of Microvascular Blood Flow
- -
- Invasive testing
- -
- Non invasive techniques
- Echocardiography
- 2.
- Cardiac Computed Tomography (CT)
- 3.
- Nuclear cardiac imaging
- 4.
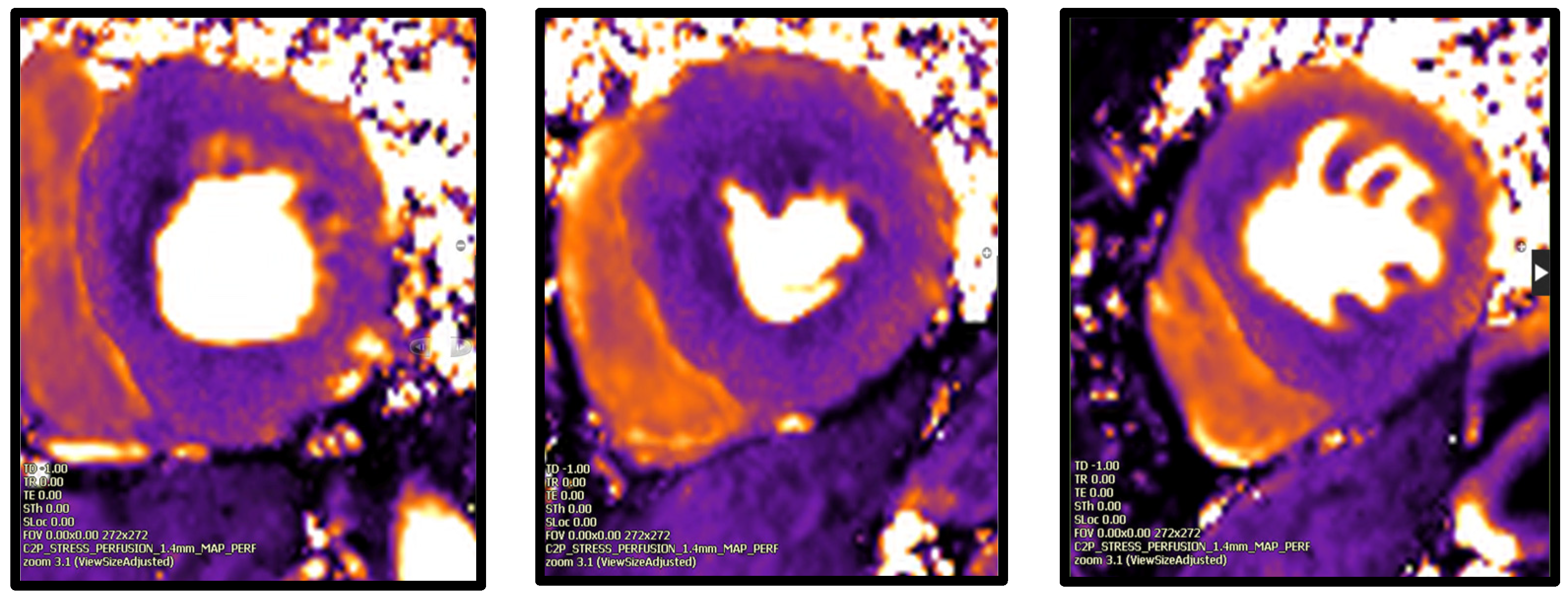
- Cardiac Magnetic Resonance
4. Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIF | arterial input function |
| ASL | arterial spin labelling |
| CAD | coronary artery disease |
| CFR | coronary flow reserve |
| CFVR | coronary flow velocity reserve |
| CMD | coronary microvascular dysfunction |
| CMR | cardiac magnetic resonance |
| CT | computed tomography |
| CTA | computed tomography angiography |
| CT-MPI | computed tomography myocardial perfusion imaging |
| CZT | cadmium-zinc-telluride |
| FFR | fractional flow reserve |
| FFRCT | computed tomography angiography derived fractional flow reserve |
| GBCA | gadolinium-based contrast age |
| iFR | instantaneous wave-free ratio |
| IMR | index of microvascular resistance |
| MACE | major adverse cardiovascular events |
| MBF | myocardial blood flow |
| MPR | myocardial perfusion reserve |
| OS-CMR | oxygenation-sensitive cardiac magnetic resonance |
| PET | Positron Emission Tomography |
| SPECT | Single-Photon-Emission Computed Tomography |
References
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijić, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2023, 44, 142–158. [Google Scholar] [CrossRef]
- Cannon, R.O.; Epstein, S.E. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am. J. Cardiol. 1988, 61, 1338–1343. [Google Scholar] [CrossRef]
- Kutty, S.; Moukagna, K.S.B.; Craft, M.; Shostrom, V.; Xie, F.; Porter, T.R. Clinical Outcome of Patients With Inducible Capillary Blood Flow Abnormalities During Demand Stress in the Presence or Absence of Angiographic Coronary Disease. Circ. Cardiovasc. Imaging 2018, 11, e007483. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Crea, F. Microvascular Angina. Circ. Cardiovasc. Imaging 2015, 8, e003252. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Pepine, C.J. Heart Failure with Preserved Ejection Fraction: Is Ischemia Due to Coronary Microvascular Dysfunction a Mechanistic Factor? Am. J. Med. 2019, 132, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Y-Hassan, S. Coronary microvascular dysfunction in Takotsubo syndrome: Cause or consequence. Am. J. Cardiovasc. Dis. 2021, 11, 184–193. [Google Scholar]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ. J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Chilian, W.M. Coronary Microcirculation in Health and Disease. Circulation 1997, 95, 522–528. [Google Scholar] [CrossRef]
- Kuo, L.; Chilian, W.M.; Davis, M.J. Coronary arteriolar myogenic response is independent of endothelium. Circ. Res. 1990, 66, 860–866. [Google Scholar] [CrossRef]
- Di Carli, M.F.; Charytan, D.; McMahon, G.T.; Ganz, P.; Dorbala, S.; Schelbert, H.R. Coronary Circulatory Function in Patients with the Metabolic Syndrome. J. Nucl. Med. 2011, 52, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Feher, A.; Sinusas, A.J. Quantitative Assessment of Coronary Microvascular Function. Circ. Cardiovasc. Imaging 2017, 10, e006427. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.A.; Löffler, A.I.; Ouellette, M.; Smith, L.; Kramer, C.M.; Bourque, J.M. Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. JACC Cardiovasc. Imaging 2015, 8, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Rooks, C.; Faber, T.; Votaw, J.; Veledar, E.; Goldberg, J.; Raggi, P.; Quyyumi, A.A.; Bremner, J.D.; Vaccarino, V. Effects of smoking on coronary microcirculatory function: A twin study. Atherosclerosis 2011, 215, 500–506. [Google Scholar] [CrossRef]
- A Kaufmann, P.; Gnecchi-Ruscone, T.; Schäfers, K.P.; Lüscher, T.F.; Camici, P.G. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 36, 103–109. [Google Scholar] [CrossRef]
- Yang, Y.; Hwang, E.; Lee, S.-A.; Lee, S.; Kim, D.-H.; Song, J.-M.; Kang, D.-H. Effect of Rosuvastatin on Coronary Flow Reserve in Hypertensive Patients at Cardiovascular Risk. J. Cardiovasc. Imaging 2021, 29, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Olivotto, I.; Rimoldi, O.E. The coronary circulation and blood flow in left ventricular hypertrophy. J. Mol. Cell. Cardiol. 2011, 52, 857–864. [Google Scholar] [CrossRef]
- Zanatta, E.; Colombo, C.; D’amico, G.; D’humières, T.; Lin, C.D.; Tona, F. Inflammation and Coronary Microvascular Dysfunction in Autoimmune Rheumatic Diseases. Int. J. Mol. Sci. 2019, 20, 5563. [Google Scholar] [CrossRef]
- Weber, B.N.; Stevens, E.; Perez-Chada, L.M.; Brown, J.M.; Divakaran, S.; Bay, C.; Bibbo, C.; Hainer, J.; Dorbala, S.; Blankstein, R.; et al. Impaired Coronary Vasodilator Reserve and Adverse Prognosis in Patients With Systemic Inflammatory Disorders. JACC Cardiovasc. Imaging 2021, 14, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- E Konst, R.; E Elias-Smale, S.; Lier, A.; Bode, C.; Maas, A.H. Different cardiovascular risk factors and psychosocial burden in symptomatic women with and without obstructive coronary artery disease. Eur. J. Prev. Cardiol. 2019, 26, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Mygind, N.D.; Michelsen, M.M.; Pena, A.; Frestad, D.; Dose, N.; Aziz, A.; Faber, R.; Høst, N.; Gustafsson, I.; Hansen, P.R.; et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women With Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. J. Am. Heart Assoc. 2016, 5, e003064. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of Sex on Coronary Microvascular Dysfunction and Cardiac Outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.D.; Wei, J.; Bairey Merz, C.N. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: The chicken or the egg? Eur. Heart J. 2018, 39, 850–852. [Google Scholar] [CrossRef]
- Almeida, A.G.; Grapsa, J.; Gemelli, A.; Bucciarelli-Ducci, C.; Gerber, B.; Ajmone-Marsan, N.; Bernard, A.; Donal, E.; Dweck, M.R.; Haugaa, K.H.; et al. Cardiovascular multimodality imaging in women: A scientific statement of the European Association of Cardiovascular Imaging of the European Society of Cardiology. Eur. Heart J.-Cardiovasc. Imaging 2024, 25, e116–e136. [Google Scholar] [CrossRef] [PubMed]
- Calamante, F. Arterial input function in perfusion MRI: A comprehensive review. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 74, 1–32. [Google Scholar] [CrossRef]
- Waller, A.H.; Blankstein, R.; Kwong, R.Y.; Di Carli, M.F. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr. Cardiol. Rep. 2014, 16, 483. [Google Scholar] [CrossRef]
- Saraste, M.; Koskenvuo, J.W.; Knuuti, J.; Toikka, J.O.; Laine, H.; Niemi, P.; Sakuma, H.; Hartiala, J.J. Coronary flow reserve: Measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin. Physiol. Funct. Imaging 2001, 21, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Montaldo, C.; Zedda, N.; Montisci, R.; Ruscazio, M.; Lai, G.; Cadeddu, M.; Meloni, L.; Iliceto, S. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve. J. Am. Coll. Cardiol. 1999, 34, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, L.; Di Serafino, L.; Mitrano, L.; De Rosa, M.L.; Carbone, C.; Rea, F.S.; Monaco, S.; Scalamogna, M.; Cirillo, P.; Esposito, G. Invasive Assessment of Coronary Microcirculation: A State-of-the-Art Review. Diagnostics 2023, 14, 86. [Google Scholar] [CrossRef]
- Sueda, S.; Sakaue, T. The Need for Separate Testing with Acetylcholine for the Assessment of Endothelial Dysfunction and Coronary Artery Spasm. Eur. Cardiol. Rev. 2024, 19, e17. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Götberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.-E.; Öhagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Jayaweera, A.R.; Firoozan, S.; Linka, A.; Skyba, D.M.; Kaul, S. Quantification of Myocardial Blood Flow With Ultrasound-Induced Destruction of Microbubbles Administered as a Constant Venous Infusion. Circulation 1998, 97, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Firschke, C.; Lindner, J.R.; Wei, K.; Goodman, N.C.; Skyba, D.M.; Kaul, S. Myocardial perfusion imaging in the setting of coronary artery stenosis and acute myocardial infarction using venous injection of a second-generation echocardiographic contrast agent. Circulation 1997, 96, 959–967. [Google Scholar]
- Vogel, R.; Indermühle, A.; Reinhardt, J.; Meier, P.; Siegrist, P.T.; Namdar, M.; Kaufmann, P.A.; Seiler, C. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: Algorithm and validation. J. Am. Coll. Cardiol. 2005, 45, 754–762. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, M.; Li, H.; Pu, Z.; Liu, H.; Huang, T.; Cheng, H.; Gong, Y.; Chu, Y.; Wang, Z.; et al. Early diagnosis of coronary microvascular dysfunction by myocardial contrast stress echocardiography. Math. Biosci. Eng. 2023, 20, 7845–7858. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Zhong, L.; Wu, J. Assessment and Treatment for Coronary Microvascular Dysfunction by Contrast Enhanced Ultrasound. Front. Cardiovasc. Med. 2022, 9, 899099. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Yoshida, K.; Akasaka, T.; Asami, Y.; Ogata, Y.; Takagi, T.; Kaji, S.; Kawamoto, T.; Ueda, Y.; Morioka, S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography. J. Am. Coll. Cardiol. 1998, 32, 1251–1259. [Google Scholar] [CrossRef]
- Sicari, R.; Rigo, F.; Cortigiani, L.; Gherardi, S.; Galderisi, M.; Picano, E. Additive Prognostic Value of Coronary Flow Reserve in Patients With Chest Pain Syndrome and Normal or Near-Normal Coronary Arteries. Am. J. Cardiol. 2009, 103, 626–631. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Faxén, U.L.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, M.M.; Mygind, N.D.; Pena, A.; Olsen, R.H.; Christensen, T.E.; Ghotbi, A.A.; Hasbak, P.; Kjaer, A.; Gustafsson, I.; Hansen, P.R.; et al. Transthoracic Doppler echocardiography compared with positron emission tomography for assessment of coronary microvascular dysfunction: The iPOWER study. Int. J. Cardiol. 2017, 228, 435–443. [Google Scholar] [CrossRef]
- Nieman, K.; Chandrashekhar, Y. Myocardial CT Perfusion Imaging in 2023. JACC Cardiovasc. Imaging 2023, 16, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- George, R.T.; Arbab-Zadeh, A.; Miller, J.M.; Vavere, A.L.; Bengel, F.M.; Lardo, A.C.; Lima, J.A. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ. Cardiovasc. Imaging 2012, 5, 333–340. [Google Scholar] [CrossRef]
- Mushtaq, S.; Conte, E.; Pontone, G.; Baggiano, A.; Annoni, A.; Formenti, A.; Mancini, M.E.; Guglielmo, M.; Muscogiuri, G.; Tanzilli, A.; et al. State-of-the-art-myocardial perfusion stress testing: Static CT perfusion. J. Cardiovasc. Comput. Tomogr. 2020, 14, 294–302. [Google Scholar] [CrossRef]
- Bischoff, B.; Bamberg, F.; Marcus, R.; Schwarz, F.; Becker, H.-C.; Becker, A.; Reiser, M.; Nikolaou, K. Optimal timing for first-pass stress CT myocardial perfusion imaging. Int. J. Cardiovasc. Imaging 2013, 29, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Sliwicka, O.; Sechopoulos, I.; Baggiano, A.; Pontone, G.; Nijveldt, R.; Habets, J. Dynamic myocardial CT perfusion imaging—State of the art. Eur. Radiol. 2023, 33, 5509–5525. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, S.; Sirajuddin, A.; Arai, A.E.; Zhao, S. Dynamic stress computed tomography myocardial perfusion for detecting myocardial ischemia: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 258, 325–331. [Google Scholar] [CrossRef]
- Valdiviezo, C.; Ambrose, M.; Mehra, V.; Lardo, A.C.; Lima, J.A.C.; George, R.T. Quantitative and qualitative analysis and interpretation of CT perfusion imaging. J. Nucl. Cardiol. 2010, 17, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ling, R.; Yu, L.; Lu, Z.; Li, Y.; Zhang, J. A Novel Computed Tomography-Based Imaging Approach for Etiology Evaluation in Patients With Acute Coronary Syndrome and Non-obstructive Coronary Angiography. Front. Cardiovasc. Med. 2021, 8, 735118. [Google Scholar] [CrossRef]
- Mahnken, A.H.M.; Klotz, E.D.-P.; Pietsch, H.; Schmidt, B.; Allmendinger, T.; Haberland, U.; Kalender, W.A.; Flohr, T. Quantitative Whole Heart Stress Perfusion CT Imaging as Noninvasive Assessment of Hemodynamics in Coronary Artery Stenosis. Investig. Radiol. 2010, 45, 298–305. [Google Scholar] [CrossRef]
- Rossi, A.; Merkus, D.; Klotz, E.; Mollet, N.; de Feyter, P.J.; Krestin, G.P. Stress Myocardial Perfusion: Imaging with Multidetector CT. Radiology 2014, 270, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; Erglis, A.; Doh, J.-H.; Daniels, D.V.; Jegere, S.; Kim, H.-S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of Ischemia-Causing Coronary Stenoses by Noninvasive Fractional Flow Reserve Computed From Coronary Computed Tomographic Angiograms. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [CrossRef]
- Min, J.K.; Leipsic, J.; Pencina, M.J.; Berman, D.S.; Koo, B.-K.; van Mieghem, C.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Apruzzese, P.; et al. Diagnostic Accuracy of Fractional Flow Reserve From Anatomic CT Angiography. JAMA 2012, 308, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic Performance of Noninvasive Fractional Flow Reserve Derived From Coronary Computed Tomography Angiography in Suspected Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef]
- Grover, R.; Leipsic, J.A.; Mooney, J.; Kueh, S.-H.; Ohana, M.; Nørgaard, B.L.; Eftekhari, A.; Bax, J.J.; Murphy, D.T.; Hague, C.J.; et al. Coronary lumen volume to myocardial mass ratio in primary microvascular angina. J. Cardiovasc. Comput. Tomogr. 2017, 11, 423–428. [Google Scholar] [CrossRef]
- Prokšelj, K.; Brida, M. Cardiovascular imaging in pregnancy. Int. J. Cardiol. Congenit. Heart Dis. 2021, 5, 100235. [Google Scholar] [CrossRef]
- Slomka, P.; Berman, D.S.; Alexanderson, E.; Germano, G. The role of PET quantification in cardiovascular imaging. Clin. Transl. Imaging 2014, 2, 343–358. [Google Scholar] [CrossRef]
- Kaufmann, P.; Camici, P. Myocardial blood flow measurement by PET: Technical aspects and clinical applications. J. Nucl. Med. 2005, 46, 75–88. [Google Scholar]
- Nagamachi, S.; Czernin, J.; Kim, A.S.; Sun, K.T.; Böttcher, M.; E Phelps, M.; Schelbert, H.R. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J. Nucl. Med. 1996, 37, 1626–1631. [Google Scholar] [PubMed]
- Einstein, A.J.; Moser, K.W.; Thompson, R.C.; Cerqueira, M.D.; Henzlova, M.J. Radiation Dose to Patients From Cardiac Diagnostic Imaging. Circulation 2007, 116, 1290–1305. [Google Scholar] [CrossRef]
- Everaars, H.; de Waard, G.A.; Driessen, R.S.; Danad, I.; van de Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; van Rossum, A.C.; Knaapen, P.; van Royen, N. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve: A Head-to-Head Comparison With [15O]H2O PET. JACC Cardiovasc. Interv. 2018, 11, 2044–2054. [Google Scholar] [CrossRef]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse Cardiovascular Outcomes in Women With Nonobstructive Coronary Artery Disease. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef]
- Ziadi, M.C.; Dekemp, R.A.; Williams, K.A.; Guo, A.; Chow, B.J.; Renaud, J.M.; Ruddy, T.D.; Sarveswaran, N.; Tee, R.E.; Beanlands, R.S. Impaired Myocardial Flow Reserve on Rubidium-82 Positron Emission Tomography Imaging Predicts Adverse Outcomes in Patients Assessed for Myocardial Ischemia. J. Am. Coll. Cardiol. 2011, 58, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R.; et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 2017, 39, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Shaw, L.J.; Cook, N.R.; Murthy, V.L.; Shah, N.R.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Di Carli, M.F. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation 2017, 135, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Lortie, M.; Beanlands, R.S.B.; Yoshinaga, K.; Klein, R.; DaSilva, J.N.; Dekemp, R.A. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur. J. Nucl. Med. 2007, 34, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.; Hu, L.-H.; Yang, B.-H.; Chen, L.-C.; Chen, Y.-K.; Ting, C.-H.; Hung, G.-U.; Huang, W.-S.; Wu, T.-C. SPECT myocardial blood flow quantitation toward clinical use: A comparative study with 13N-Ammonia PET myocardial blood flow quantitation. Eur. J. Nucl. Med. 2017, 44, 117–128. [Google Scholar] [CrossRef]
- Liu, C.; Sinusas, A.J. Is Assessment of Absolute Myocardial Perfusion with SPECT Ready for Prime Time? J. Nucl. Med. 2014, 55, 1573–1575. [Google Scholar] [CrossRef]
- Axel, L. Tissue Mean Transit Time from Dynamic Computed Tomography by a Simple Deconvolution Technique. Investig. Radiol. 1983, 18, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Jerosch-Herold, M.; Wilke, N.; Stillman, A.E.; Wilson, R.F. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med. Phys. 1998, 25, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.F.; Rettmann, D.W.; Aletras, A.H.; Liao, S.L.; Taylor, J.L.; Balaban, R.S.; Arai, A.E. Absolute Myocardial Perfusion in Canines Measured by Using Dual-Bolus First-Pass MR Imaging. Radiology 2004, 232, 677–684. [Google Scholar] [CrossRef]
- Schuster, A.; Zarinabad, N.; Ishida, M.; Sinclair, M.; Wijngaard, J.P.v.D.; Morton, G.; Hautvast, G.L.; Bigalke, B.; van Horssen, P.; Smith, N.; et al. Quantitative assessment of magnetic resonance derived myocardial perfusion measurements using advanced techniques: Microsphere validation in an explanted pig heart system. J. Cardiovasc. Magn. Reson. 2014, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Engblom, H.; Xue, H.; Akil, S.; Carlsson, M.; Hindorf, C.; Oddstig, J.; Hedeer, F.; Hansen, M.S.; Aletras, A.H.; Kellman, P.; et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: A comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J. Cardiovasc. Magn. Reson. 2016, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Mygind, N.D.; Pena, A.; Michelsen, M.M.; Qayyum, A.A.; Frestad, D.; Christensen, T.E.; Ghotbi, A.A.; Hasbak, P.; Kjaer, A.; Vejlstrup, N.; et al. Myocardial first pass perfusion assessed by cardiac magnetic resonance and coronary microvascular dysfunction in women with angina and no obstructive coronary artery disease. Scand. J. Clin. Lab. Investig. 2019, 79, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Levelt, E.; Piechnik, S.K.; Liu, A.; Wijesurendra, R.S.; Mahmod, M.; Ariga, R.; Francis, J.M.; Greiser, A.; Clarke, K.; Neubauer, S.; et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J. Cardiovasc. Magn. Reson. 2016, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, M.; Piechnik, S.K.; Levelt, E.; Ferreira, V.M.; Francis, J.M.; Lewis, A.; Pal, N.; Dass, S.; Ashrafian, H.; Neubauer, S.; et al. Adenosine stress native T1 mapping in severe aortic stenosis: Evidence for a role of the intravascular compartment on myocardial T1 values. J. Cardiovasc. Magn. Reson. 2014, 16, 92. [Google Scholar] [CrossRef]
- Mathew, R.C.; Kramer, C.M. Recent advances in magnetic resonance imaging for peripheral artery disease. Vasc. Med. 2018, 23, 143–152. [Google Scholar] [CrossRef]
- Zun, Z.; Varadarajan, P.; Pai, R.G.; Wong, E.C.; Nayak, K.S. Arterial Spin Labeled CMR Detects Clinically Relevant Increase in Myocardial Blood Flow With Vasodilation. JACC Cardiovasc. Imaging 2011, 4, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Hillier, E.; Covone, J.; Friedrich, M.G. Oxygenation-sensitive Cardiac MRI with Vasoactive Breathing Maneuvers for the Non-invasive Assessment of Coronary Microvascular Dysfunction. J. Vis. Exp. 2022, 186, e64149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lee, J.C.Y.; Leung, S.T.; Lai, A.; Lee, T.-F.; Chiang, J.B.; Cheng, Y.W.; Chan, H.-L.; Yiu, K.-H.; Goh, V.K.-M.; et al. Long-Term Prognosis of Patients With Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2021, 14, 602–611. [Google Scholar] [CrossRef]
- Rocco, M.; Flavia, N.; Margherita, L.; Monaco, M.L.; Collaku, E.; Nudi, A.; Gad, A.; Procopio, C.; Ioppolo, A.; Bertella, E. Coronary Microvascular Dysfunction: Searching the Strongest Imaging Modality in Different Scenarios. Echocardiography 2024, 41, e70022. [Google Scholar] [CrossRef]
- Watson, R., Jr.; McKinney, A.; Stafford, J. ACR Manual on MR Safety ACR Committee on MR Safety. Am. J. Radiol. 2024. [Google Scholar]
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.J.; Hayes, S.W.; Hartigan, P.M.; Weintraub, W.S.; O’rourke, R.A.; Dada, M.; Spertus, J.A.; et al. Optimal Medical Therapy With or Without Percutaneous Coronary Intervention to Reduce Ischemic Burden. Circulation 2008, 117, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L. Changes in Myocardial Perfusion Abnormalities by Positron Emission Tomography After Long-term, Intense Risk Factor Modification. JAMA J. Am. Med. Assoc. 1995, 274, 894. [Google Scholar] [CrossRef] [PubMed]
- Quercioli, A.; Montecucco, F.; Pataky, Z.; Thomas, A.; Ambrosio, G.; Staub, C.; Di Marzo, V.; Ratib, O.; Mach, F.; Golay, A.; et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: Relation to alterations in endocannabinoids and adipocytokines. Eur. Heart J. 2013, 34, 2063–2073. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Rahman, H.; Douiri, A.; Demir, O.M.; De Silva, K.; Clapp, B.; Webb, I.; Gulati, A.; Pinho, P.; Dutta, U.; et al. ChaMP-CMD: A Phenotype-Blinded, Randomized Controlled, Cross-Over Trial. Circulation 2024, 149, 36–47. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. WARRIOR: Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD (NCT03417388). 2018. Available online: https://clinicaltrials.gov/ (accessed on 18 January 2025).
- National Heart, Lung, and Blood Institute. PRIZE: Prednisone in Duchenne Muscular Dystrophy (NCT04097314). 2019. Available online: https://clinicaltrials.gov/ (accessed on 18 January 2025).
- Al-Mohaissen, M.A. Echocardiographic assessment of primary microvascular angina and primary coronary microvascular dysfunction. Trends Cardiovasc. Med. 2022, 33, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H.; Fearon, W.F.; Pelletier-Galarneau, M.; Ambrosio, G.; Sechtem, U.; Ruddy, T.D.; Patel, K.K.; Bhatt, D.L.; Bateman, T.M.; Gewirtz, H.; et al. Myocardial Perfusion PET for the Detection and Reporting of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2023, 16, 536–548. [Google Scholar] [CrossRef] [PubMed]
| Imaging Modality | Advantages | Drawbacks | Sensitivity/Specificity |
|---|---|---|---|
| Echocardiography | Easy access Low risk Low cost | Significant intraobserver and interobserver variability. Artifacts. Acoustic window limitations | +/++ [94] |
| Cardiac CT | Anatomic and functional data in the same study | Potential nephrotoxicity Exposure to radiation Potential for overestimating MBF | ++/+++ [84] |
| PET | Most extensively validated technique Strong prognostic value High accuracy and reproducibility Not constrained by renal function | High cost Radiation exposure Limited accessibility Time-consuming process | +++/+++ [95] |
| SPECT | More widely available than CMR or PET | Requires new generation cameras Radiation exposure Limited data | +/++ [73,74] |
| CMR | High spatial resolution and tissue characterization No radiation exposure Validated and compared with PET and invasive techniques | High costs Limited by renal function Limited accessibility Time consuming | +++/++ [77,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.M.; Nobre Menezes, M.; Alves da Silva, P.; Almeida, A.G. Multimodality Imaging in the Diagnosis of Coronary Microvascular Disease: An Update. J. Pers. Med. 2025, 15, 75. https://doi.org/10.3390/jpm15020075
Martins AM, Nobre Menezes M, Alves da Silva P, Almeida AG. Multimodality Imaging in the Diagnosis of Coronary Microvascular Disease: An Update. Journal of Personalized Medicine. 2025; 15(2):75. https://doi.org/10.3390/jpm15020075
Chicago/Turabian StyleMartins, Ana Margarida, Miguel Nobre Menezes, Pedro Alves da Silva, and Ana G. Almeida. 2025. "Multimodality Imaging in the Diagnosis of Coronary Microvascular Disease: An Update" Journal of Personalized Medicine 15, no. 2: 75. https://doi.org/10.3390/jpm15020075
APA StyleMartins, A. M., Nobre Menezes, M., Alves da Silva, P., & Almeida, A. G. (2025). Multimodality Imaging in the Diagnosis of Coronary Microvascular Disease: An Update. Journal of Personalized Medicine, 15(2), 75. https://doi.org/10.3390/jpm15020075





